Abstract
Objective
To identify relationships between complex and simple clinical measures of reaction time (RTclin), and indicators of balance in older subjects with and without diabetic peripheral neuropathy (DPN).
Design
Prospective cohort design. Complex RTclin Accuracy, Simple RTclin Latency, and their ratio were determined using a novel device in 42 subjects (age = 69.1 ± 8.3 yrs), 26 with DPN and 16 without. Dependent variables included unipedal stance time (UST), step width variability and range on an uneven surface, and major fall-related injury over 12 months.
Results
In the DPN subjects the ratio of Complex RTclin Accuracy:Simple RTclin Latency was strongly associated with longer UST (r/p = .653/.004), and decreased step width variability and range (r/p = −.696/.001 and −.782/<.001, respectively) on an uneven surface. Additionally, the two DPN subjects sustaining major injuries had lower Complex RTclin Accuracy:Simple: RTclin Latency than those without.
Conclusions
The ratio of Complex RTclin Accuracy:Simple RTclin Latency is a potent predictor of UST and frontal plane gait variability in response to perturbations, and may predict major fall injury in older subjects with DPN. These short latency neurocognitive measures may compensate for lower limb neuromuscular impairments, and provide a more comprehensive understanding of balance and fall risk.
INTRODUCTION
Accidental falls are a public health priority, with the estimated lifetime costs of over $37 billion in 2010.1 The risk of fall-related injury in older people with diabetic peripheral neuropathy (DPN) is at least twice that in older people without,2–4 and this increased risk impedes the engagement of this population in meaningful life experiences5 as well as walking exercise programs which are the foundation for management of Type II diabetes mellitus.6–7 Moreover, a distal symmetric polyneuropathy is common in older age groups, with about 2/3 related to diabetes mellitus,8,9 so that an estimated 20 million Americans aged 55 to 85 are affected.
Falls occur most frequently while older adults are walking on uneven or irregular surfaces10,11,12, with lateral falls appearing to have greater injury potential than other falls.13,14 Therefore the ability to withstand (or reject) a frontal plane perturbation is necessary for safe ambulation.15 Prior work confirms that frontal plane hip strength is essential to maintain control of the head/arms/trunk during single limb stance, and allow appropriate swing limb foot placement,16,17 Accordingly, our prior research in older subjects with varying degrees of DPN found that frontal plane neuromuscular factors (laboratory measured hip strength as normalized rate of torque in the frontal plane and ankle inversion/eversion proprioceptive thresholds as a ratio; HipSTR:AnkPRO) strongly predicted unipedal stance time (UST)18, sagittal plane responses to perturbation while walking,19,20 and prospectively recorded falls and fall-related injury.21 However, these frontal plane neuromuscular attributes did not predict frontal plane responses to perturbation while walking or identify the subjects who sustained a major injury during the 12 months prospective follow up.
An increasing volume of research links cognitive functions and gait/fall risk. Of the cognitive domains evidence suggests that executive function, specifically inhibitory executive function, is the most important with reference to gait, balance, and fall risk.22 Intact inhibitory executive function provides the ability to attend selectively to a specific stimulus while diminishing others, and the ability to “withhold pre-potent (automatic) responses”.23 In contrast, impairments in executive function lead to the inability to rapidly selectively focus on relevant afferent stimuli and/or alter routine motor patterns when they become inappropriate or unnecessary. As such older subjects with inhibitory executive impairments demonstrate decreased balance in the setting of multiple afferent stimuli24 and are at increased risk for falls and injury,22,25
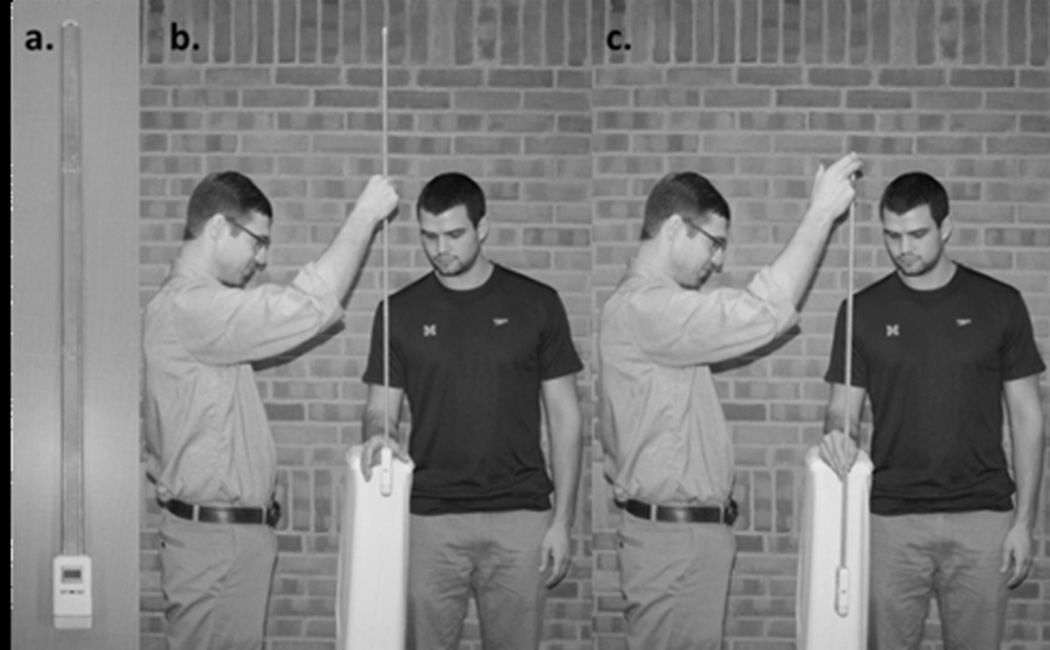
We developed and validated a clinical reaction time device and method (RTclinDev; Figure 1) that allows measurement of simple and complex reaction times in a clinical setting. The clinical reaction time device (RTclinDev) is employed in a ruler-drop testing paradigm where Simple Clinical Reaction Time (RTclin) Latency is determined by the time a vertically oriented rod falls before it is caught by subject hand closure.26 Complex RTclin Accuracy requires the subject to catch the same device solely on the randomly-determined 50% of trials in which lights affixed to it illuminate.27 Simple RTclin Latency is measured in ms, and Complex RTclin Accuracy is measured as the percentage of trials in which the subject responds correctly. Simple RTclin Latency requires sustained attention and rapid reaction. A high level of Complex RTclin Accuracy requires rapid processing and response as the subject must interpret the signal and make a decision whether to catch the RTclinDev within the approximately 400 ms prior to it striking the floor. The challenging component of Complex RTclin testing is to selectively attend to the lights on the RTclinDev and to withhold catching the rapidly descending device when the lights do not illuminate, requiring the ability “to inhibit a pre-potent (automatic) response”.23 We, and others, have found this action-based technique of determining simple and recognition reaction times to be superior (faster and less variable) than perception/computer-based techniques.28–30 Therefore, the RTclinDev allows the measurement of short latency inhibitory executive function by measuring the frequency with which subjects can withhold a pre-potent/automatic response. Complex RTclin Accuracy and Simple RTclin Latency are of interest separately, and also as a ratio (Complex RTclin Accuracy:Simple RTclin Latency) which reflects both accuracy of decision-making and speed of response. The ratio allows the greatest (best) scores to occur in subjects who were accurate as well as quick, while the lowest (worst) scores would occur in subjects who were neither.
Figure 1.
The Clinical Reaction Time Device (RTclinDev) used to determine Simple RTclin Latency and Complex RTclin Accuracy.
Given the inability of frontal plane lower limb neuromuscular attributes (HipSTR:AnkPRO) to predict frontal plane responses to perturbation and identify the subjects who sustained a major injury during follow up,21 we performed a secondary analysis to determine whether the short latency neurocognitive attributes Complex RTclin Accuracy and Simple RTclin Latency as determined by RTclinDev were related to these important outcomes. More specifically, we hypothesized that decreased/inaccurate baseline Complex RTclin Accuracy and/or increased/slow Simple RTclin Latency would be associated with: 1) Decreased unipedal stance time (UST); 2) Increased frontal plane gait variability in older subjects with and without DPN while walking on smooth and uneven surfaces; and 3) Major fall-related injuries prospectively determined over one year follow up.
METHODS
Design
We performed a prospective cohort study, with baseline evaluations of independent variables followed by UST testing, gait evaluation, and then one year prospective follow-up for falls and fall-related injuries. The work presented here is a secondary analysis to determine whether Complex RTclin Accuracy and Simple RTclin Latency as determined by RTclinDev were related to UST, frontal plane gait variability and major fall-related injuries, outcomes which had not been predicted by lower limb neuromuscular factors. To minimize bias, the study team member evaluating UST and recording falls and fall-related injuries, was blinded with regard to baseline lower limb neuromuscular and RTclin variables. The original sample size of 42 subjects, about 2/3 with DPN of varying severity, was recruited so as to create a group of older adults with a spectrum of lower limb peripheral neuromuscular function that would provide a high likelihood of significant correlation coefficients between lower limb neuromuscular variables and UST and perturbed gait measures. Therefore, sample size represents a study limitation with regard to the falls and fall-related injury outcomes.
Subjects
The research protocol was approved by the Institutional Review Board, and all participants provided informed written consent. Subjects were recruited between July of 2009 and December of 2011, and prospectively followed until January of 2013. Ninety-nine subjects were screened by phone, with 23 failing to meet inclusion/exclusion criteria and 20 not interested in participating, usually due to the time commitment or not staying in the area. Of the remaining 56, six did not pass the physical examination screen and four did not show for scheduled appointments. Of the remaining 46, 4 cancelled before the onset of testing due to medical concerns within their families, to provide the final cohort of 42 subjects. Of these, 26 subjects had varying degrees of DPN and 16 were without. (Table 1) All 42 were included in the UST analyses. However, 10 subjects (seven with DPN and three without) were preferred not to participate in the gait, falls, and fall-related injury portion of the study citing concerns with the associated time commitment, and so those analyses include 32 subjects. The 10 subjects who dropped out after baseline and UST testing were not significantly different from those who remained with regard to age, gender, body mass index, or neuropathy severity.
Table 1.
Study subject characteristics. The only significant group differences are subjects with DPN having greater MDNS scores (worse neuropathy) than subjects without DPN (p < .001) and a trend toward greater BMI (p = .052).
| All Subjects (n = 42) |
Subjects without DPN (n = 16) |
Subjects with DPN (n = 26) |
|
|---|---|---|---|
| Gender (%women) |
21 (50%) | 10 (63%) | 11 (42%) |
| Age (years) | 69.0 ± 8.4 | 67.8 ± 8.9 | 69.8 ± 8.1 |
| BMI | 31.0 ± 6.9 | 28.3 ± 7.2 | 32.6 ± 6.4 |
| MDNS | 9.2 ± 8.0 | 1.7 ± 3.8 | 13.8 ± 6.1 |
Subjects were recruited from the University of Michigan Orthotics and Prosthetics Clinic, Endocrinology Clinic, and the Older Americans Independence Center Human Subjects Core between November of 2010 and January of 2012. Written informed consent was obtained from all subjects after review from the Institutional Review Board. Eligible subjects were between the ages of 50 and 85 years, weighed < 136 kg (as required for harness support system during gait evaluation), were free of central neurologic disease, including Parkinson’s Disease or Parkinsonism, vestibular disorders, symptomatic coronary artery disease, plantar skin sores or joint replacement within the previous year, symptomatic postural hypotension, severe musculoskeletal deformity (e.g., amputation or Charcot changes), lower extremity or back pain that limited standing to <10 minutes, were able to walk 1 block or more, had greater than anti-gravity ankle strength (> grade 3/5 by manual muscle testing), and corrected vision not worse than 20/50. Subjects with DPN had a history of type 2 diabetes mellitus confirmed by review of records and the ongoing use of oral hypoglycemic agents or insulin. The presence of DPN was confirmed by: (a) symptoms (subject reported altered sensation in the distal lower limbs); (b) signs XXXXXXXXXXXs Neuropathy Score (MDNS; a 46 point scale with increasing score relating to more severe DPN) >10;31 and (c) bilaterally abnormal fibular motor nerve conduction studies (recording over the extensor digitorum brevis, defined as amplitude <2.0 mV, latency >6.2 ms, and/or conduction velocity <41.0 m/s, using Nicolet Viking 4). Subjects without DPN had no history of diabetes mellitus, no symptoms or signs of DPN (MDNS <10), and normal fibular nerve conduction studies. Subjects were excluded if they reported a fall within one month of testing.
Independent Variables
Simple RTclin Latency
Simple RTclin Latency was determined using the RTclinDev. This is a custom-built device that consists of a 107 cm collapsible, rigid, lightweight dumbbell-shaped shaft affixed to an 11×6×2.5 cm spacer box housing a linear accelerometer, timing circuit, microprocessor, battery, liquid crystal display, and two light emitting diodes. Using a “ruler drop” test paradigm participants stood with their dominant forearm resting on an adjustable table surface so that the hand was positioned at the edge. The examiner suspended the device vertically so that the spacer box rested between the thumb and other digits. (Figure 1) After pre-determined random delay times ranging from 2 to 5 seconds, the examiner released the device. The participants were instructed to catch the falling device as quickly as possible every trial. Participants were given 2 Simple RTclin practice trials before 10 data collection trials. Simple RTclin Latency was defined as the mean of the 10 trials and reported in ms.
Complex RTclin Accuracy
Subjects were positioned in the same manner as for determining Simple RTclin Latency. To determine Complex RTclin Accuracy, the light emitting diodes on the device illuminated randomly during 50% of the trials at the instant the device accelerated upon release. Participants were instructed to catch the falling RTclinDev only during those trials when the light emitting diodes illuminated, and to resist catching it when the light emitting diodes did not turn on, consistent with a standard go/no-go testing paradigm. Verbal instructions emphasized response accuracy, not speed. Participants completed 6 practice trials prior to 20 data collection trials. Complex RTclin Accuracy was recorded as the percentage of correctly performed trials/total number of trials.
Complex RTclin Accuracy:Simple RTclin Latency
The ratio of Complex RTclin Accuracy to Simple RTclin Latency was also of interest, given that it reflects both accuracy of decision-making and speed of response, so that the greatest (best) scores occur in subjects who were both accurate and quick, while the lowest (worst) scores occur in subjects who were neither. The resulting value was multiplied by 1000 given that Simple RTclin Latency was reported as msec.
Dependent Variables
Unipedal Stance Time (UST)
As per prior protocol, subjects were performed 3 trials on the foot of choice, and then 3 trials on the opposite foot.18 One practice trial was allowed for each foot prior to data collection. The mean UST in seconds of all 6 trials was the outcome of interest.
Gait Analysis of Frontal Plane Gait Variability
As illustrated in prior reports subjects were fitted in a safety harness that housed a cable fastened to an overhead track (Figure 1).19,20 The cable was secured high enough to catch subjects should they experience an accidental fall. Subjects wore flat-soled, standard athletic shoes. Kinematic data were collected through two optoelectronic markers (infrared-emitted diodes) positioned 5 cm apart on an aluminum strip (10 cm × 1.5 cm) that was bent at a 90 degree angle and inserted at the under the laces of each shoe at the midline. The top marker was located anterior to the center of the malleoli. The subjects also wore a waist marker positioned on a belt at the level of the umbilicus.
The smooth surface was constructed of flat, linoleum tile. The uneven surface, which has been described in prior reports,19 was created by placing a 1.5 × 10 m piece of dark industrial carpet over randomly distributed prism-shaped blocks of wood (height = 1.5 cm, width = 3.5 cm, length = 6–16 cm). The blocks of wood were located within the mid 6.5 m section of carpet and were not changed between trials. For trials on both the smooth and uneven surfaces, subjects were instructed to walk down the runway at their own pace, as if they were “walking to mail a letter.” Subjects completed 10 trials on each walkway, with the first 2 used for accommodation and the last 8 for data collection. The subjects ambulated down the walkway toward an optoelectronic camera system (Optotrak 3020, Northern Digital Corp., Waterloo, Ontario) which recorded marker positions at 100 Hz. To detect heel strike and toe off, each subject wore rearfoot and forefoot foot switches in each shoe (force sensing resistors made by FlexiForce, Tekscan Inc., South Boston, MA). These sensors were connected to the data acquisition hardware. A custom C++ program operating in conjunction with the Optotrak Application Programming Interface was then used to track the timing of heel strike and toe off for each step. Once the heel strike and toe off information were known, then the timing of double support was known and step width and step length was taken from the kinematic marker data as previously described.32 Kinematic data were quantified by using a custom algorithm written in MATLAB. Frontal plane gait variability was measured using the standard deviation and range of step width and referred to as step width variability and step width range, respectively. The latter is of interest given that falls are unusual events as compared to the number of steps most people take each day, and therefore “outlier” steps in the frontal plane likely have clinical relevance.
Recording falls and fall-related injuries
Falls and fall-related injuries were recorded through one year of follow-up using methods described by Tinetti et al.33 Twenty-six calendars (each spanning a two-week period) were provided to each of the 32 subjects so that data could be collected prospectively for each subject for one year. Subjects assessed themselves daily, and if a fall or fall-related injury occurred, they checked a box on the calendar and recorded a description of the circumstances. Subjects returned the calendars every two weeks, and in the few cases where a subject did not return a calendar the study coordinator contacted the subject to determine the occurrence of a fall or fall-related injury during the missed time period. Falls were defined as unintentional changes in body posture that resulted in the subject coming to rest on the ground or other lower level that was not a consequence of a physical blow or loss of consciousness.
Fall-related injuries were separated into two groups: major and minor. Major injuries were defined as an Abbreviated Injury Scale Score greater than two,34 and minor injuries were defined as abrasions, bruises, and lacerations that did not require sutures but interfered with the subject’s activities of daily living for at least 24 hours.35
Statistical Analyses
Descriptive statistics were generated for the independent variables, Complex RTclin Accuracy and Simple RTclin Latency, and also for the laboratory-based dependent variables, UST, step width variability and range on the smooth and uneven surfaces, and inspected for normality. Bivariate relationships between these were determined using Pearson correlation coefficients. These relationships were evaluated, separately, for subjects with and without DPN. Significance was set at p = .05/3 or .016, and a trend set at p = .032, to adjust for three correlations being performed for each group.
To determine the independence of the relationships identified between Complex RTclin Accuracy, Simple RTclin Latency and their ratio (referred to collectively as “Short Latency Neurocognitive Attributes”) and the outcomes UST, step width variability, and range, multiple regression analyses were performed by singly entering other variables with known relationships to the outcome variables. More specifically, age, Michigan Diabetes Neuropathy Score, and the composite measure of lower limb neuromuscular function previously demonstrated to predict UST and falls (HipSTR:AnkPRO) were evaluated.18,21
Major fall-related injury group differences in Simple RTclin Latency, Complex RTclin Accuracy, and their ratio among the subjects with DPN were not subjected to statistical analyses given the small number of subjects sustaining such an injury.
RESULTS
Subjects
Study subject characteristics are provided in Table 1. Forty-two subjects were available for the UST testing. Ten subjects had barriers to continuing participation and so 32 subjects are included in the gait testing and prospective evaluation of fall-related injuries.
UST
When all subjects were considered, UST demonstrated significant correlations with Simple RTclin Latency (r/p = −.421/.005) and Complex RTclin Accuracy:Simple RTclin Latency (.386/.011) but not Complex RTclin Accuracy (.215/.172). However, when considered separately, the subjects without DPN demonstrated no significant relationships between Complex RTclin Accuracy, Simple RTclin Latency, and their ratio, and UST (Table 2.b.). The limitation of UST to a maximum of 30 seconds resulted in a ceiling effect for this group and the resulting skewing of the data may have obscured an association between the two variables. In contrast, the subjects with DPN demonstrated robust relationships with UST, with decreased (quicker) Simple RTclin Latency and increased (more accurate) Complex RTclin Accuracy, and greater Complex RTclin Accuracy:Simple RTclin Latency ratio significantly related to longer UST. (Table 2.a.)
Table 2.
| a. Relationships (Pearson correlation coefficients, R, and p values) between Simple RTclin Latency (SRT), Complex RTclin Accuracy (RRTAcc), and their ratio (RRTAcc:SRT), with UST in subjects with and without DPN | ||
|---|---|---|
| Unipedal Stance Time | ||
| Subjects without DPN (R/p values) |
Subjects with DPN (R/p values) |
|
| SRT | −.473/.064 | −.520/.008 |
| RRTAcc | .026/.924 | .472/.017 |
| RRTAcc:SRT | .319/.229 | .653/.004 |
| b. Results of multivariate analyses with UST as the dependent variable | ||||
|---|---|---|---|---|
| Subjects without DPN | Subjects with DPN | |||
| RRTAcc/SRT | HipSTR/AnkPRO | RRTAcc/SRT | HipSTR/AnkPRO | |
| Beta | NS | 3.82 | .505 | .525 |
| P value | NS | .002 | .001 | .001 |
| R2 | .580 | .680 | ||
Given prior work strongly correlating the ratio of HipSTR:AnkPRO and UST in the same cohort,18 this variable was included with Complex RTclin Accuracy:Simple RTclin Latency in a regression model. When subjects without DPN were considered, the ratio of Hip Strength to Ankle Proprioceptive precision was the only significant predictor. However when subjects with DPN were analyzed, Complex RTclin Accuracy:Simple RTclin Latency and HipSTR:AnkPRO were both significant predictors, with a resultant R2 of .680. (Table 2.b.) No other variables contributed to the model.
Frontal plane gait variability
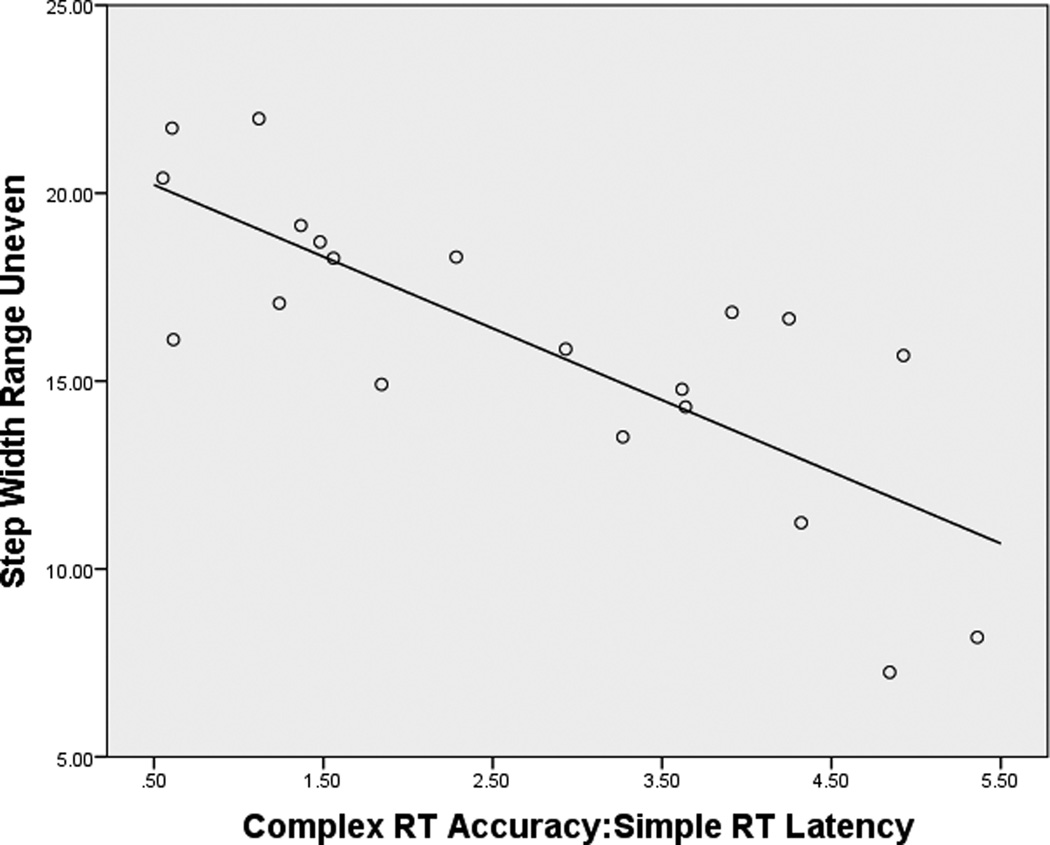
When all subjects were considered, step width range demonstrated significant correlations with Simple RTclin Latency, Complex RTclin Accuracy and Complex RTclin Accuracy:Simple RTclin Latency on the uneven surface (r/p = .541/.001, −.523/.002, and −.709/.001, respectively) but not the even surface (r/p = .137/.441, −.183/.300, and −.201/.255, respectively). However, when subjects without DPN were considered separately there were no significant relationships between the Short Latency Neurocognitive Attributes and frontal plane gait variability or range on the even or uneven surfaces. (Table 3) The subjects with DPN demonstrated a trend between Complex RTclin Accuracy:Simple RTclin Latency and step width variability on the even surface. However, the subjects with DPN demonstrated strong relationships between Complex RTclin Accuracy and Complex RTclin Accuracy:Simple RTclin Latency for both step width variability and range on the uneven surface. (Table 3) The relationship between the ratio of neurocognitive attributes and step width range on the uneven surface was particularly robust, with an R2 of .611. (Figure 3) Notably, none of the demographic variables or Hip Strength:Ankle Proprioceptive Precision demonstrated significant or near significant relationships with step width variability or range on the uneven surface.
Table 3.
Relationships (Pearson correlation coefficients, R, and p values) between Simple RTclin Latency (SRT), Complex RTclin Accuracy (RRTAcc), and their ratio RRTAcc:SRT), and step width variability and range on even and uneven surfaces.
| Step Width Variability Even Surface | Step Width Variability Uneven Surface | |||
|---|---|---|---|---|
| Subjects without DPN R/p values |
Subjects with DPN (R/p values) |
Subjects without DPN R/p values |
Subjects with DPN R/p value |
|
| SRT | −.025/.936 | .331/.143 | .485/.093 | .443/.050 |
| RRTAcc | −0.197/.519 | −.397/.075 | −.213/.485 | −.562/.010 |
| RRTAcc:SRT | −0.119/.698 | −.481/.027 | −.459/.114 | −.696/.001 |
| Step Width Range Even Surface | Step Width Range Uneven Surface | |||
|---|---|---|---|---|
| Subjects without DPN R/p values |
Subjects with DPN R/p values |
Subjects without DPN R/p values |
Subjects with DPN R/p values |
|
| SRT | −.134/.662 | .232/.311 | .570/.042 | .484/.030 |
| RRTAcc | −0.037/.905 | −.245/.285 | −.059/.849 | −.696/.001 |
| RRTAcc:SRT | −0.040/.896 | −.308/.174 | −.427/.146 | −.782/<.001 |
Figure 3.
Relationship between Complex RTclin Accuracy:Simple RTclin Latency and step width range on the uneven surface in subjects with DPN. (R2 = .611)
Major Injury
There were no significant differences in Complex RTclin Accuracy or Simple RTclin Latency, or their ratio, between subjects sustaining minor injury as compared to those who did not, with all p values > .40. (Table 4) One subjects without DPN sustained a major injury, an 80 year old woman with Complex RTclin Accuracy Simple RTclin of .76 and 192 ms, respectively with a ratio of 3.95, all of which were within 1 standard deviation of the means for all subjects without DPN (.72 ± .09; 173 ± 22 and 4.2 ± .8, respectively). Two of the subjects with DPN sustained a major injury in the one year follow up period. These two subjects demonstrated increased (worse) Simple RTclin Latency and decreased Complex RTclin Accuracy, as well as decreased Complex RTclin Accuracy:Simple RTclin Latency as compared to subjects with DPN not sustaining major injury. The mean Complex RTclin Accuracy:Simple RTclin Latency of the two DPN subjects with major injuries were approximately 2 standard deviations less than those subjects without major injury. (Table 4)
Table 4.
Simple RTclin Latency (SRT), Complex RTclin Accuracy (RRTAcc), and their ratio RRTAcc:SRT) in subjects with and without major and minor injuries
| Subjects With DPN | ||||
|---|---|---|---|---|
| +Major Injury (n = 2) |
−Major Injury (n = 17) |
+Minor Injury (n = 9) |
−Minor Injury (n = 8) |
|
| SRT | 229 ± 68 | 178 ± 23 | 185 ± 38 | 182 ± 20 |
| RRTAcc | .55 ± .14 | .76 ± .13 | .72 ± .14 | .77 ± .14 |
| *RRTAcc:SRT | 2.5 ± .8 | 4.4 ± 1.0 | 4.1 ± 1.4 | 4.3 ± .1 |
Multiplied by 1000
DISCUSSION
The results of this research demonstrate that in older adults with DPN there appear to be relationships between the Short Latency Neurocognitive Attributes, Complex RTclin Accuracy, Simple RTclin Latency, and their ratio, and three important mobility-related outcomes: UST, frontal plane gait variability on an uneven surface, and major prospective fall-related injuries. These findings support at least three novel, clinically relevant concepts: 1) When the data reported here are considered with prior work27 RTclin appears to be an innovative, clinically accessible method for measuring inhibitory executive function over a brief time interval; 2) The data link RTclin based Short Latency Neurocognitive Attributes to UST and frontal plane responses to sequential perturbations during walking, and in so doing provide a mechanism by which poor inhibitory executive function increases fall risk; and 3) The relationships between Complex RTclin Accuracy:Simple RTclin Latency and measures of balance, frontal plane gait control, and major injuries were identified predominantly in the subjects with DPN suggesting that short latency neurocognitive capability is of greater importance in the setting of lower limb neuromuscular impairment. These points are addressed in sequence below.
Complex RTclin Accuracy as a measure of short latency inhibitory executive function
There appear to be two inhibitory challenges to the patient or subject attempting to achieve high Complex RT Accuracy, one afferent in nature and the other efferent. The afferent task is to selectively attend to the lights on RTclinDev while ignoring the dominant visual stimulus, the rapidly descending device. This requires the ability to quickly prioritize and/or suppress incoming stimuli. The efferent task is to withhold the urge to catch the falling device. Accordingly, Giordani and Persad describe inhibitory executive function as the ability to: “prevent distracting information from …causing interference,” and “prevent pre-potent (automatic) responses that may not be appropriate…”23 and both appear present when Complex RT is tested. There are a variety other methods for evaluating executive function, most commonly through Stroop or Trails B testing, but neither requires the subject to make a decision within approximately 420 msec or evaluates visuo-motor pathways. This may represent an advantage for RTclinDev when evaluating fall risk as falls are events that occur, or are prevented, over a brief time interval and often include visuo-motor responses. Further, the time available for decision-making is similar to the time interval available for altering swing limb trajectory (435 and 480 msec for our subjects on smooth and uneven surfaces, respectively) possibly making RTclinDev particularly suitable for gait evaluation. Another potential advantage over standard methods of measuring executive function is that RTclinDev is a three dimensional object rather than a screen image. We, along with Montare,29,30 have found that simple and complex reaction time tests using falling objects yield quicker responses and decreased variability as compared to screen-based measurements, possibly due to the fact that moving objects activate the visuo-motor pathways while the latter works through visuo-perceptual pathways.29 In one of the only other studies to evaluate short latency inhibitory executive function and prospective falls, Schoene et al evaluated a large group of older subjects with respect to their ability to perform a Stroop-like stepping test on a computer-controlled mat which provided cues.36 Consistent with our findings, subjects with prolonged and/or inaccurate stepping responses were more likely to report a history of accidental falls.
The relationships between Complex RTclin Accuracy:Simple RTclin Latency and mobility outcomes provide a mechanism by which poor executive function increases fall risk
The second clinically relevant aspect of this research is that the data link Complex RTclin Accuracy, Simple RTclin Latency and their ratio to UST and frontal plane responses to sequential walking surface perturbations in older subjects with DPN. Although prior work has linked executive function with gait speed and gait variability,37 a recent review22 found minimal research investigating the influence of executive function on balance and response to perturbation as reported here. One potential explanation for this relationship is to suggest that the subjects with poor Complex RTclin Accuracy:Simple RTclin Latency are unable to rapidly inhibit attention to irrelevant external stimuli and distracting internal cognitive processes within the 420 msec available, and so are similarly unable to inhibit pre-planned lower limb responses while walking and cannot quickly adjust swing limb trajectories within the time available. This uneven surface would then lead to intermittent mis-steps in relation to the center of mass with the subsequent development of lateral momentum and the need for a lateral recovery step. In support of this reasoning Asai et al. found that lateral, but not sagittal, momentum was less effectively attenuated during a distracting task while walking, suggesting that lateral control is most heavily reliant upon attention.38 Perhaps most compelling, Sturnieks et al. found that executive functioning was an independent predictor of the need to take a step after lateral waist pull perturbations, while standing sway and lower limb strength predicted anterior perturbations.39 This mirrors our findings that HipSTR:AnkPRO predict sagittal plane characteristics of step length and speed on the uneven surface,19 whereas Complex RTclin Accuracy:Simple RTclin Latency predict frontal plane gait variability. Finally, the increase in extreme lateral step placement on the uneven surface associated with poor Complex RTclin Accuracy:Simple RTclin Latency provide a mechanism by which poor or slow inhibitory executive function can lead to frontal plane instability, and predispose to more severely injurious falls.22
The relationships between Complex RTclin Accuracy:Simple RTclin Latency and mobility were prominent only in subjects with DPN suggesting greater importance in the setting of neuromuscular impairments
The third novel feature of this research is that the relationships between the RTclinDev derived short latency neurocognitive attributes and UST, response to gait perturbations on the uneven surface, and major injuries were identified solely in the subjects with DPN. These relationships did not appear to be present in the older subjects with normal lower limb neuromuscular function and minimally in subjects with DPN on a smooth surface. One possible explanation for this may be that subjects with DPN and its associated lower limb neuromuscular limitations are more reliant on cortical control of balance and gait and posture in challenging situations than subjects without DPN. If so, the ability to quickly inhibit attention to less relevant stimuli and internal cognitive processes as measured by Complex RTclin Accuracy:Simple RTclin Latency would offer an advantage in terms of immediacy of response to perturbation. In support of this the prefrontal cortex, the region associated with executive cognitive functions, shows increased activity during challenging activities such as adapting to different walking speeds on a treadmill,40 maintaining balance while standing on a suddenly translating surface,41 and in patients with cerebellar/brainstem strokes while walking at a uniform speed as compared to controls.42 Other supportive work finds that the effect of executive function on mobility is greatest in older adults with lower limb sensorimotor changes43 and that distraction, a sign of executive impairment, interferes with the calibration of ankle muscle response to perturbation in older subjects about 400 msec after perturbation and so increases the likelihood of taking multiple steps.44 Together these studies suggest that the prefrontal cortex and its associated executive functions are most essential during challenges to balance, particularly when sub-cortical systems are disrupted or desynchronized by altered peripheral sensory function or weakness, as is the case for the DPN subjects reported here.
The results may have clinical utility. The ability to respond quickly and the capacity to rapidly suppress irrelevant stimuli are difficult for the clinician to quantify. However, RTclinDev may offer a clinically accessible method for doing so. If so, measurement of Simple RTclin Latency and Complex RTclin Accuracy may be useful in the evaluation of patients with lower limb neuromuscular impairments which increase their fall risk. The presence of neuromuscular as well as neurocognitive impairments may then mark the patient as high risk for falls and injury. Potential interventions would target proximal strengthening as well as cognitive procedures that enhance attention and mental focus. There is evidence that interventions such as mindfulness training, discontinuing of sedating medications, and/or the addition of activating medications, may lead to laudable changes in gait that suggest reduced fall risk.45 If neither is possible, then environmental modification with reduction in surface irregularities and distracters while walking is indicated.
Although the study’s strengths and innovative features have been described, enthusiasm must be reserved given the study’s limitations. The greatest weakness is the limited subject numbers, asymmetry of subjects with and without DPN, and the absence of power calculations, consistent with this being a secondary analysis of data obtained previously for other purposes. Further, Complex RTclin Accuracy and Simple RTclin Latency have not been validated specifically within older people with DPN. However, it seems unlikely that the presence of DPN had influence as post-hoc testing revealed no significant or near-significant DPN/non-DPN group differences for any of the three measures (p values of .992, .129 and .464 for, respectively, Complex RTclin Accuracy, Simple RTclin Latency and their ratio). Formal neuropsychologic testing was not performed and so the inclusion of subjects with mild cognitive impairment is possible. Additionally, subject moods and personality traits were not evaluated, and so these may have influenced the results. Despite these, the strength of the associations between UST and step width variability, and Complex RTclin Accuracy and Simple RTclin Latency and their ratio, are so strong in the subjects with DPN that the likelihood of a spurious relationship appears small. In contrast, with only two DPN group major injuries during the one year of prospective follow-up the possibility of a chance association with respect to that outcome is clearly present, and the results need to be replicated before acceptance is considered. Weak associations between short latency neurocognitive attributes and the three mobility outcomes may have been missed in the non-DPN group, a problem likely accentuated by the limiting of UST to a maximum of 30 seconds which could have obscured correlational analyses for these subjects. However, it should be noted that HipSTR:AnkPRO demonstrated a significant relationship with UST in this group despite the same limitations of this outcome. (Table 2.b.)
In summary, this preliminary study suggests that Complex RTclin Accuracy, Simple RTclin Latency and their ratio were potent predictors of UST, frontal plane gait variability in response to perturbations while walking, and associated with major fall injury in older subjects with DPN. The findings suggest a plausible mechanism by which impairments in inhibitory executive function can increase fall and injury risk. These neurocognitive variables, which can be obtained at the bedside or in the clinic using RTclinDev, may be combined with evaluation of critical lower limb neuromuscular attributes46 so as to allow the clinician a more comprehensive understanding of fall risk.
Supplementary Material
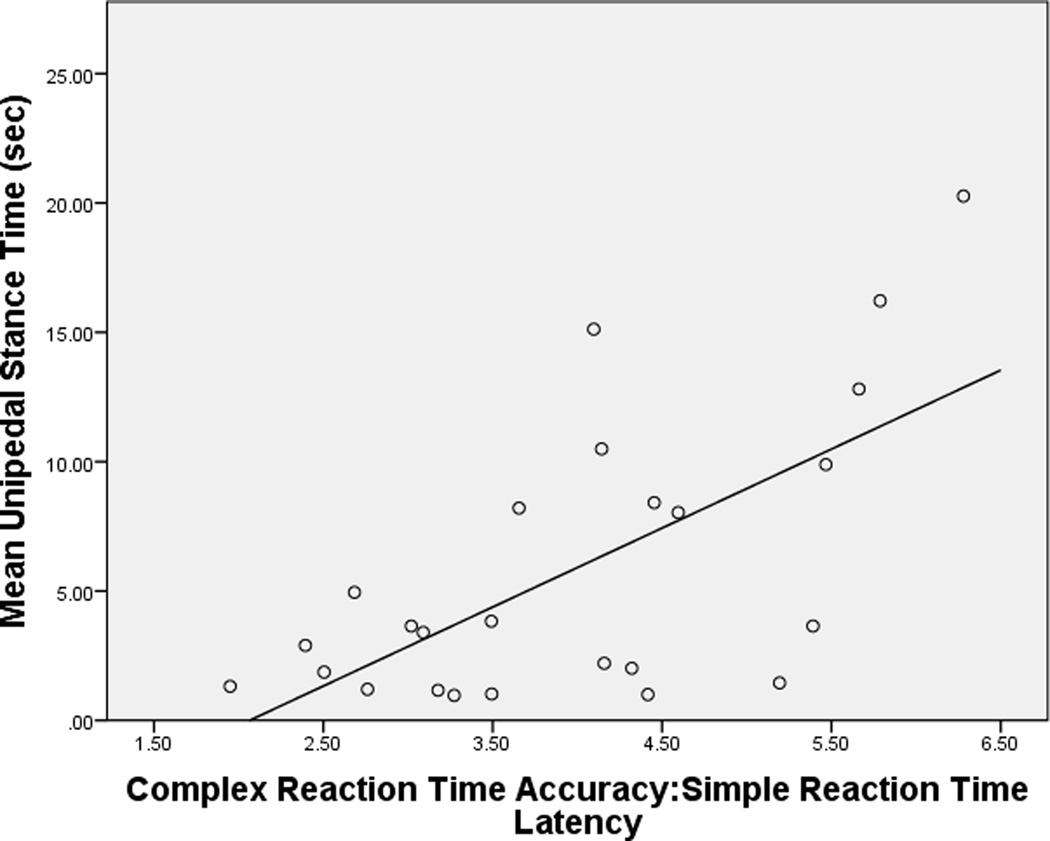
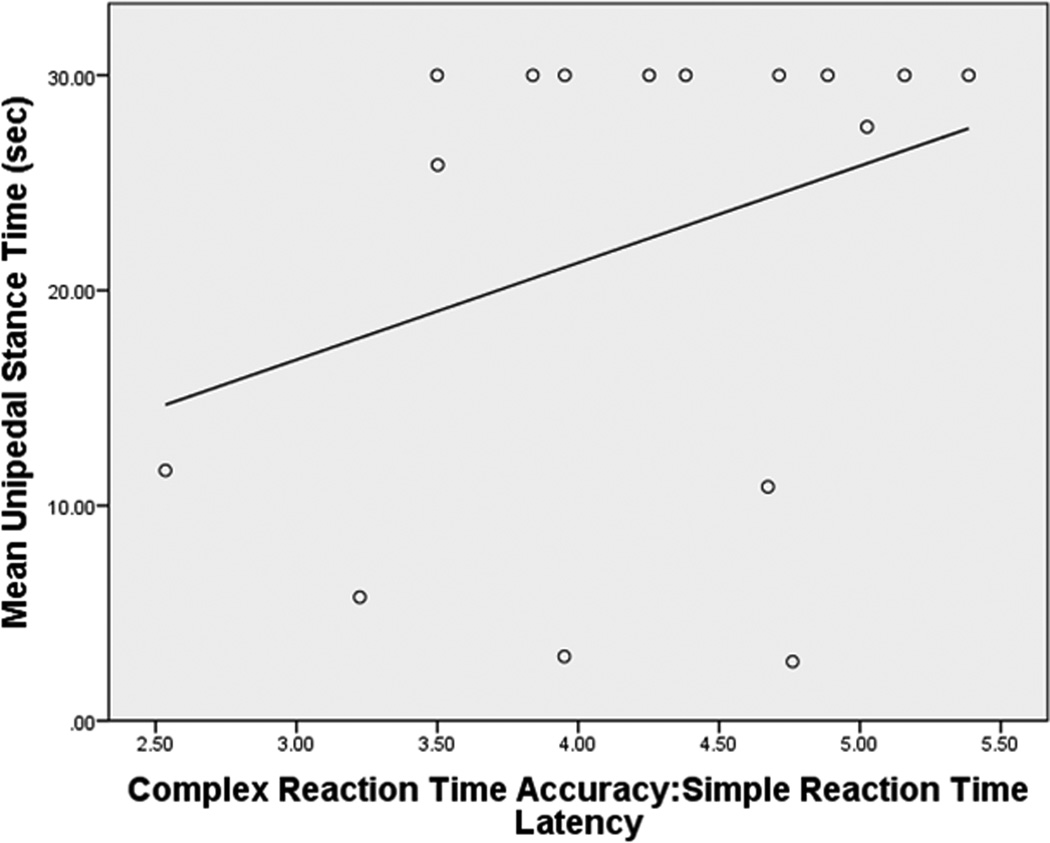
Figure 2.
Relationships between Complex RTclin Accuracy/Simple RTclin Latency and UST in subjects with, and without, DPN
a. Subjects with DPN
b. Subjects without DPN
Footnotes
Supplementary Checklist: STROBE Checklist
REFERENCES
- 1.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) [Google Scholar]
- 2.Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992 Oct;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 3.Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995 Jul;50(4):M211–M215. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patient with insulin-dependent diabetes mellitus. Diabet Med. 1992 Jun;9(5):469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 5.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005 Oct;28(1):2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 6.Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, Zhang Q Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012 Mar 29;366(13):1209–1217. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sluik D, Buijsee B, Muckelbauer R, Kaaks R, Teucher B, et al. Physical activity and mortality in individuals with diabetes mellitus: a prospective study and meta-analysis. doi: 10.1001/archinternmed.2012.3130. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. 1999–2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004 Jul;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 9.Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D ILSA Working Group. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007 May 1;68(18):1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- 10.Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing. 1997 Jul;26(4):261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 11.DeMott TK, Richardson JK, Thies SB, Ashton-Miller JA. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med Rehabil. 2007 Feb;86(2):125–132. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 12.Koski K, Luukinen H, Laippala P, Kivela SL. Physiological factors and medications as predictors of injurious falls by elderly people: a prospective population-base study. Age Ageing. 1996 Jan;25(1):29–38. doi: 10.1093/ageing/25.1.29. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan SL, Myers ER, Maitland LA, Resnick NM, Hayes WC. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA. 1994 Jan 12;271(2):128–133. [PubMed] [Google Scholar]
- 14.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of Osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 1993 Nov;41(11):1226–1234. doi: 10.1111/j.1532-5415.1993.tb07307.x. [DOI] [PubMed] [Google Scholar]
- 15.Maki BE, Sibley KM, Jaglal SB, Bayley M, Brooks D, Fernie GR, Flint AJ, Gage W, Liu BA, McIlroy WE, Mihailidis A, Perry SD, Popovic MR, Pratt J, Zettel JL. Reducing fall risk by improving balance control: development, evaluation and knowledge-translation of new approaches. J Safety Res. 2011 Dec;42(6):473–485. doi: 10.1016/j.jsr.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plan during human walking. J Biomech. 1993 Jun;26(6):633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 17.Rietdyk S, Patla AE, Winter DA, Ishac MG, Little CE NACOB presentation CSB New Investigator Award. Balance recovery from medio-lateral perturbations of the upper body during standing. North American Congress on Biomechanics. J Biomech. 1999 Nov;32(11):1149–1158. doi: 10.1016/s0021-9290(99)00116-5. [DOI] [PubMed] [Google Scholar]
- 18.Allet LA, Kim H, Ashton-Miller JA, De Mott T, Richardson JK. Frontal plane hip and ankle sensorimotor function, not age, predicts unipedal stance time. Muscle Nerve. 2012 Apr;45(4):578–585. doi: 10.1002/mus.22325. PMCID: PMC3313445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allet LA, Kim H, Ashton-Miller JA, Richardson JK. Which lower limb frontal plane sensory and motor functions predict gait speed and efficiency on uneven surfaces in older persons with diabetic neuropathy? PM&R. 2012 Oct;4(10):726–733. doi: 10.1016/j.pmrj.2012.05.002. PMCID: PMC3477498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allet L, Kim H, Ashton-Miller JA, DeMott T, Richardson JK. Step length after discrete perturbation predicts accidental falls and fall-related injury in elderly people with a range of peripheral neuropathy. J Diabetes Complications. 2014 Jan-Feb;28(1):79–84. doi: 10.1016/j.jdiacomp.2013.09.001. PMCID: PMC3895931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson JK, DeMott T, Allet L, Kim H, Ashton-Miller JA. Hip strength: Ankle proprioceptive threshold ratio predicts falls and injury in diabetic neuropathy. Muscle Nerve. 2014 Sep;50(3):437–442. doi: 10.1002/mus.24134. PMCID: PMC4033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: a systematic review. Dement Geriatr Cogn Disord. 2013;36(1–2):20–35. doi: 10.1159/000350031. [DOI] [PubMed] [Google Scholar]
- 23.Giordani B, Persad C. Chapter 6 Neuropsychological influences on gait in the Elderly. Gait Disorders Evaluation and Management – Taylor and Francis Group. 2005 [Google Scholar]
- 24.Mendelson DN, Redfern MS, Nebes RD, Richard Jennings J. Inhibitory processes relate differently to balance/reaction time dual tasks in young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2010 Jan;17(1):1–18. doi: 10.1080/13825580902914040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology. 2009 Jul;23(4):500–508. doi: 10.1037/a0015389. [DOI] [PubMed] [Google Scholar]
- 26.Eckner JT, Whitacre RD, Kirsch NL, Richardson JK. Evaluating a clinical measure of reaction time: an observational study. Percept Mot Skills. 2009 Jun;108(3):717–720. doi: 10.2466/PMS.108.3.717-720. [DOI] [PubMed] [Google Scholar]
- 27.Eckner JT, Richardson JK, Kim H, Lipps DB, Ashton-Miller JA. A novel clinical test of recognition reaction time in healthy adults. Psychol Assess. 2012 Mar;24(1):249–254. doi: 10.1037/a0025042. PMCID: PMC3643808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckner JT, Kutcher JS, Richardson JK. Between-seasons test-retest reliability of clinically measured reaction time in National Collegiate Athletic Association Division I athletes. J Athl Train. 2011 Jul-Aug;46(4):409–414. doi: 10.4085/1062-6050-46.4.409. PMCID: PMC3419153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montare A. Simplest chronoscope III Further comparisons between reaction times obtained by meterstick versus machine. Percept Mot Skills. 2013 Jun;116(3):796–805. doi: 10.2466/27.24.PMS.116.3.796-805. [DOI] [PubMed] [Google Scholar]
- 30.Montare A. The simplest chronoscope II: reaction time measured by meterstick versus machine. Percept Mot Skills. 2010 Dec;111(3):819–828. doi: 10.2466/03.15.25.PMS.111.6.819-828. [DOI] [PubMed] [Google Scholar]
- 31.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994 Nov;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 32.Thies SB, Richardson JK, Ashton-Miller JA. Effects of surface irregularity and lighting on step variability during gait: a study in healthy young and older women. Gait Posture. 2005 Aug;22(1):26–31. doi: 10.1016/j.gaitpost.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 34.MacKenzie EJ, Shapiro S, Eastham JN. The Abbreviated Injury Scale and Injury Severity Score. Levels of inter- and intrarater reliability. Med Care. 1985 Jun;23(6):823–835. doi: 10.1097/00005650-198506000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher PC, Hirdes JP. Risk factor for accidental injuries within senior citizens’ homes: analysis of the Canadian Survey on Ageing and Independence. J Gerontol Nurs. 2005 Feb;31(2):49–57. doi: 10.3928/0098-9134-20050201-10. [DOI] [PubMed] [Google Scholar]
- 36.Schoene D, Smith St, Davies TA, Delbaerr K, Lord SR. A stroop stepping test (SST) using low-cost computer game technology discriminates between older fallers and non-fallers. Age and Aging. 2013;0:1–5. doi: 10.1093/ageing/aft157. [DOI] [PubMed] [Google Scholar]
- 37.Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, Callisaya ML. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol: Med Sci. 2013;68(6):726–732. doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- 38.Asai T, Doe T, Hirata S, Ando H. Dual tasking affects lateral trunk control in healthy younger and older adults. Gait & Posture. 2013;83:830–836. doi: 10.1016/j.gaitpost.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 39.NEW. Sturnieks DL, Menant J, Vanrenterghem J, Delbaere K, Fitzpatrick RC, Lord SR. Sensorimotor and neuropsychological correlates of force perturbations that induce stepping in older adults. Gait & Posture. 2012;36:356–360. doi: 10.1016/j.gaitpost.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 40.NEW. Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, Kubota K. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imagint study. Neuroimage. 2004;23:1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuoimage. 2008;43:329–336. doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? Neuro Image. 2007;37:1338–1345. doi: 10.1016/j.neuroimage.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Martin KL, Blizzard L, Srikanth VK, Wood A, Thomson R, Sanders LM, Callisaya ML. Cognitive function modifies the effect of physiologic function on the risk of multiple falls—a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68(9):1091–1097. doi: 10.1093/gerona/glt010. [DOI] [PubMed] [Google Scholar]
- 44.Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA. Cognitive influence on postural stability: A neuromuscular analysis in young and older adults. J Gerontol: Med Sci. 2000;55A(3):M112–M119. doi: 10.1093/gerona/55.3.m112. [DOI] [PubMed] [Google Scholar]
- 45.Segen-Jacubovski O, Herman T, Yogev-Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition; can cognitive therapy reduce fall risk? Expert Rev. 2011;11:1057–1075. doi: 10.1586/ern.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donaghy A, DeMott T, Allett L, Kim H, Ashton-Miller JA, Richardson JK. Accuracy of clinical techniques for evaluating lower limb sensorimotor functions associated with increased fall risk. PM&R Journal. 2016;8:331–339. doi: 10.1016/j.pmrj.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.