Abstract
Purpose
XXXX is the first NCI-sponsored trial to treat multiple (2–4) extracranial metastases with SBRT. Benchmark credentialing is required to ensure adherence to this complex protocol, particularly for metastases in close proximity. This report summarizes dosimetric results and approval rates.
Materials/Methods
The benchmark used anonymized data from a patient with bilateral adrenal metastases, separated by <5 cm of normal tissue. Because the PTV overlaps with organs-at-risk (OARs), institutions must utilize the planning priority guidelines to balance PTV coverage (45Gy in 3 fractions) against OAR sparing. Submitted plans were processed by IROC and assessed by protocol co-chairs by comparing doses to targets, OARs, and conformity metrics using non-parametric tests.
Results
Of 63 benchmarks submitted through October, 2015, 94% were approved, with 51% at first attempt. The majority used VMAT (78%), a single plan for both PTVs (90%), and prioritized PTV over stomach (75%). Median D95% was 44.8 ± 1.0 Gy and 44.9 ± 1.0 Gy for right and left PTVs, respectively. Median dose to 0.03 cc was 14.2 ± 2.2 Gy to spinal cord and 46.5 ± 3.1 Gy to stomach. Plans that spared stomach significantly reduced dose to the left PTV and stomach. Conformity metrics were significantly better for single plans that simultaneously treated both PTVs with VMAT/IMRT/3DCRT compared to separate plans. No significant differences existed in dose at 2 cm from PTVs.
Conclusions
While the majority of plans utilized VMAT, the range of conformity and dose falloff was large. The decision to prioritize either OAR or PTV coverage varied considerably suggesting toxicity outcomes in the trial could be affected. Several benchmarks met DVH metrics while producing unacceptable plans due to low conformity. Dissemination of a frequently-asked-questions document improved the approval rate at first attempt. Benchmark credentialing was found to be a valuable tool for educating institutions about protocol requirements.
Introduction
Oligometastases defines the clinical state (1), in which cancer patients with few metastases might be cured if all macroscopic tumors were ablated. Coupled with technological advances enabling stereotactic body radiotherapy (SBRT) (2), several institutional trials have used ablative radiotherapy to treat oligometastases (3–7). In 2014, XXXX initiated the first NCI-sponsored, phase I trial to determine safe SBRT doses for the treatment of 3–4 metastases, or 2 metastases in close proximity, in multiple organs (XXXX; NCTXXXXXXXX). Trial XXXX aims to test the safety of SBRT with or without surgery for the treatment of 2 – 4 metastases, originating from primary breast, non-small cell lung, or prostate cancer, distributed throughout the body. Because of the technical challenges inherent when treating multiple metastases, particularly as their spatial separation decreases, there is an emphasis on enrolling patients with two metastases within close proximity (≤ 5 cm). Data collected from the trial will be used to develop dosimetric guidelines using current treatment delivery techniques as a secondary endpoint.
To ensure that institutions meet the minimum technical standards to implement all aspects of any protocol, the NCI Imaging and Radiation Oncology Core (IROC) has developed various pre-enrollment credentialing steps. While only one of several steps required for Trial XXXX, benchmark credentialing provides a planning exercise to familiarize participating institutions with the specific planning goals and compliance criteria prior to enrolling their first patient. Feedback can be given to an institution without the pressure of a patient urgently waiting to begin treatment, which has been demonstrated to decrease plan quality (8). This exercise may be particularly beneficial for protocols with technically challenging radiation techniques (8). Our goal is to summarize the benchmark credentialing results for Trial XXXX.
Methods/Materials
Benchmark Case
A challenging, complex clinical scenario was chosen for the benchmark planning case, consisting of bilateral adrenal metastases < 5 cm apart. Expansion using protocol-mandated margins resulted in a combined planning target volume (PTV) of 103 cc that partially overlapped with liver (11.2 cc), the left kidney (3.0 cc), and the stomach (4.4 cc) (see Figures 1–2). The simulation CT scan acquired without motion management and 3-mm slice thickness containing all target and organ-at-risk (OAR) contours was provided to participating institutions to enable direct comparison of plans.
Figure 1.

An axial slice through the PTVs (dark blue and green colorwash) which overlaps with stomach (brown colorwash) with dose overlaid from two benchmarks plans. Both VMAT plans passed credentialing although one prioritized PTV (left) while the other prioritized stomach (right). The distance at which D2cm is assessed is depicted in magenta. Even when the stomach is spared, the PTV is covered by the 70% dose (orange).
Figure 2.

A. Dose from separate plans is shown simultaneously on the same axial slice treating PTVs (dark blue and green colorwash). The 50% (purple line) is limited to the left side for the plan treating the left PTV and to the right side for the plan treating the right PTV. The distance at which D2cm is assessed is depicted in magenta.
B. The accumulated dose for these two separate plans is shown on the same slice. The 50% line (purple) from Figure 2A corresponds approximately to the 70% (orange line) on the dose composite. Although no high doses (> 80%) exist between the two PTVs, large volumes of 120% (salmon line) exist within the PTVs.
Treatment Planning and Delivery Techniques
Trial XXXX allows all photon (≥ 6MV) SBRT delivery systems and planning techniques (e.g., 3DCRT, IMRT/VMAT). Furthermore, planning with either a single isocenter for both targets or a separate isocenter localized to each target is allowed as long as institutions satisfy additional phantom credentialing demonstrating accurate dose delivery to two separate targets using a single isocenter (9, 10). Isocenter selection is independent of whether a single inclusive plan or separate plans are developed for each metastasis.
Per Trial XXXX, abdominal/pelvic metastases are to be planned to 45Gy in 3 fractions using IROC approved dose algorithms. The benchmark case requires use of protocol-mandated parameters such as a dose calculation grid size of ≤ 2 × 2 × 3 mm and reporting dose-to-water. Each institution used their own CT-to-density conversion table to mimic clinical cases.
Planning Priorities
Planning priorities were specified in the protocol, which is particularly important in the case of the benchmark wherein PTVs overlap with OARs. A hard constraint always applies to the spinal cord. For all other OARs, the physician chooses either to reduce the PTV coverage to 70% of the prescribed dose in volumes overlapping with OAR or to cover the PTV as long as overlapping OARs do not receive >105% of the prescribed dose (10). For this SBRT trial, conformity was also prioritized by requiring that the ratio of the prescription dose to the PTV volume < 1.2–1.5 and specifying dose fall-off guidelines away from the PTV. The protocol lists the order of the planning priorities as: 1) spinal cord constraint 2) plan conformity 3) OAR constraints. Within 6 months of the first benchmark submission, additional planning guidance in the form of a frequently-asked-questions (FAQ) document and sample passing plans was posted on XXXX under “Credentialing.”
Compliance Criteria and Plan Evaluation
Protocol co-chairs assessed benchmark plans by comparing the OAR and PTV dose volume histograms (DVHs) to the protocol dose constraints as well as reviewing dose in all axial planes. The benchmark was assessed in a manner identical to that used for protocol cases. Passing plans were required to strictly meet the spinal cord constraint while all other constraints for PTV, OAR, and conformity could be within the variation acceptable range (Table 2). Conformity metrics and dose fall-off metrics were collected: the maximum dose at 2cm away from the PTVs (D2cm) and the ratio of the volumes of various dose levels to the PTV volume (i.e., R50%, R70%, R80%, R90%, R95%). Trial XXXX recommends limits for R50% and D2cm based upon previous SBRT protocols in which a single target is treated; these are provided in tables as a function of PTV volume. Guidance for calculating D2cm and R50% are also described in the protocol. Multiple targets with different anatomical distributions with respect to each other and OARs may be treated on Trial XXXX and low dose lines may be deliberately elongated in order to avoid OARs. Therefore, R50% and D2cm could not be explicitly included in the DVA forms but were assessed by reviewing dose in all planes, similarly to protocol cases. Because of the implicit nature of the conformity constraint, it was leniently scored such that plans passed if doses ≥ 90% disconnected between the PTVs. As specified by Trial XXXX, composite dose is used to assess OAR dose but PTV coverage is assessed on individual plans intended to treat each metastasis separately (10).
Table 2.
Summary of median dose to targets and organs-at-risk for credentialed plans and the applicable protocol constraints and acceptable variation doses. Statistically significant differences caused by prioritizing the stomach are indicated by an asterisk (*). The volumes of kidney and liver are reported as ranges.
| Passing Plans (n = 59) | Plans Prioritizing Stomach (n = 14) | Protocol Constraint | Variation Acceptable | |
|---|---|---|---|---|
| D95% (Gy) | ||||
| PTV_RT (32cc) | 44.8 ± 1.0 | 44.7 ± 0.8 | = 45 | ≥42.5 and < 45.5 |
| PTV_LT (71cc) | 44.9 ± 1.0 | 36.0 ± 3.3* | = 45 | ≥31.5 and < 45.5 |
| GTV_RT (6.3cc) | 45.7 ± 1.6 | 46.7 ± 1.9 | = 45 | |
| GTV_LT (24cc) | 45.6 ± 1.7 | 44.9 ± 2.7 | = 45 | |
| V42.5Gy (%) | ||||
| GTV_RT (6.3cc) | 100.0 ± 0.0 | 100.0 ± 0.0 | = 100 | |
| GTV_LT (24cc) | 100.0 ± 2.0 | 97.0 ± 2.9 | = 100 | |
| Dose to 0.03 cc of OAR (Gy) | ||||
| Spinal Cord | 14.2 ± 2.2 | 14.7 ± 2.1 | < 22.5 | < 22.5 |
| Stomach | 46.5 ± 3.1 | 42.4 ± 4.3* | < 30 | < 47.25 |
| Dose to 10 cc of OAR (Gy) | ||||
| Stomach | 34.2 ± 5.3 | 28.3 ± 4.1* | < 22.5 | < 47.25 |
| V105% (cc) | ||||
| Kidney_LT | 0.0–4.9 | 0.0–2.6 | = 0 | |
| Liver | 0.0–8.1 | 0.0–17.8 | = 0 | |
Abbreviations:
PTV = planning target volume
GTV = gross tumor volume
PTV_RT = right-sided PTV
PTV_LT = left-sided PTV
GTV_RT = right-sided GTV
GTV_LT = left-sided GTV
OAR = organ-at-risk
D95% = Dose to 95% of the volume
V42.5Gy = Volume of an structure receiving 42.5Gy
V105% = Volume of an structure receiving 105% of the prescribed dose
Centralized Review
The infrastructure for conducting centralized review was developed through AAA, BBB and the CCC. Data including the planning CT image set with contours and the associated treatment plan were submitted by each site using the Transfer of Images and Data (TRIAD) system developed by the American College of Radiology (11). Centralized reviews were performed through secure remote Citrix access. Communication between the trial investigators and the treating sites was facilitated electronically by AAA staff.
Statistical Analysis
Dose conformity metrics created by various planning techniques were compared using a nonparametric Kruskal-Wallis H-test at an α = 0.01 level. To correct for multiple comparisons of 4 dose metrics from the same plan, p-values for individual tests were modified using a Bonferroni correction (p < 0.0025). If significant differences were found for a dose metric among different plans, the Mann-Whitney U-test was used to compare two groups at a time to determine which groups accounted for the difference. Mann-Whitney tests were used to assess if stomach sparing decreased dose coverage to PTVs or GTVs or whether it affected R50%, R70%, R80%, R90%, with Bonferroni corrections (p < 0.0025). Pearson’s correlation coefficients were calculated to study the relationship between R50%, R70%, R80%, R90%, and D2cm.
Results
The protocol opened in August 2014 with the first benchmark case submitted shortly thereafter. As of October 1, 2015, 63 institutions initiated the benchmark process, with the majority succeeding although some required multiple attempts (Table 1). Reasons for not passing are listed in Table 1. Although 7 institutions developed separate plans as recommended by Trial XXXX, the resulting plans did not pass benchmark credentialing indicating the degree of difficulty in meeting protocol constraints for two nearby targets with separate plans. Metrics were explicitly listed in the DVA/protocol to avoid violating all reasons except for the conformity constraint, which affected a minority of submissions (16%). The passing rate at first attempt increased from 37% to 51% once additional guidance for benchmark planning was posted to IROC Houston’s website (e.g., FAQ and sample plans).
Table 1.
Summary of benchmark credentialing attempts, reasons for not passing, and planning techniques used in the 59 passing benchmarks.
| No. | Percentage | |
|---|---|---|
| Number of attempts at passing benchmark (n = 63) | ||
| 1 | 32 | 51% |
| 2 | 24 | 38% |
| 3 | 3 | 5% |
| No further attempts | 4 | 6% |
| Reasons for not passing benchmark at first attempt (n = 31) | ||
| Separate plans did not meet OAR criteria | 7 | 23% |
| PTV coverage unacceptably high or low | 7 | 23% |
| Stomach dose constraints exceeded | 3 | 10% |
| Spinal dose constraints exceeded | 7 | 23% |
| Conformity does not meet SBRT guideline | 5 | 16% |
| Other | 2 | 6% |
| Planning technique used to create passing benchmark (n = 59) | ||
| VMAT | 46 | 78% |
| IMRT | 8 | 14% |
| 3DCRT | 1 | 2% |
| Robotic Radiosurgery | 4 | 7% |
| Number of plans and isocenters utilized (n = 59) | ||
| 1 plan/1 isocenter | 39 | 66% |
| 1 plan/2 isocenters | 14 | 24% |
| 2 plans | 2 | 3% |
| Robotic Radiosurgery | 4 | 7% |
| Structures prioritized (n = 59) | ||
| PTV | 44 | 75% |
| Stomach | 15 | 25 % |
Since institutions could re-attempt to pass the benchmark, data from the 59 passing plans was compiled (Table 1) and further analyzed. All passing plans met the variation acceptable constraint for 0.03cc of the stomach as it was not possible to meet the protocol constraint due to the intersection of stomach and PTV; 3 institutions achieved the protocol constraint to 10cc of stomach (<22.5 Gy). Of the 59 passing plans, the majority were developed using VMAT and a single plan with a single isocenter; the majority also prioritized PTV over stomach (Figure 1). VMAT plans used 2–10 arcs with 178–1424 control points; IMRT plans used 9–14 beams with 180–1833 control points.
Figure 2 depicts dose from separate plans shown simultaneously on the same axial slice and their accumulated dose used by an institution to credential successfully. For each separate plan (Figure 2A), the 50% dose is limited to the lateral side treating the metastasis such that when composite dose is accumulated (Figure 2B), high doses are not created in the normal tissue between the PTVs. Note that the 50% from individual plans sums to approximately 70% on the composite plan. Dose accumulation causes large hot spot volumes within the PTVs as assessed on the composite plan (Figure 2B) compared to the individual plans (Figure 2A). For the two institutions that successfully credentialed with separate plans and isocenters, large volumes of the PTVs (68–77%) contained doses ≥ 120% (Figure 2B) in comparison to single plans used to simultaneously treat both PTVs in which only 0–8.3% of the PTVs contained ≥ 120%. Because these plans intended to treat each PTV on a separate day, these large volumes of 120% dose will not be delivered to the PTV in a single calendar day.
Unless specified, inverse planning algorithms do not limit dose to non-target volumes. Figure 3 demonstrates that benchmark plans that met all DVH criteria could be produced with unacceptable conformity (first row) using VMAT. Even plans with acceptable conformity (second row) had wide variability in the shape and location of low dose lines such as the 70% and 50%. This is in contrast to 3DCRT in which the high dose volumes are limited to the intersection of the beam apertures.
Figure 3.
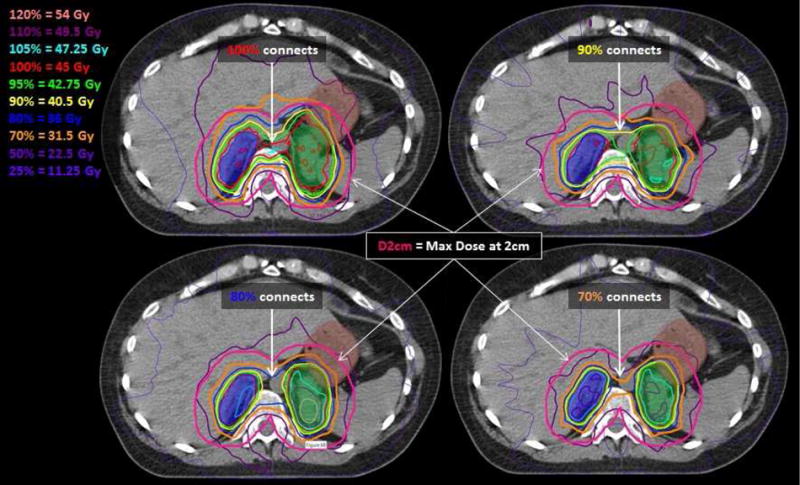
Dose from 4 benchmark plans developed with VMAT are depicted on the same axial slices through PTVs (dark blue and green colorwash) which overlaps with the stomach (brown colorwash). Plans in the first row met DVH metrics but not conformity metrics with high doses (100% and 90%) connecting in normal tissue between the PTVs. Even plans (second row) that met both DVH and conformity metrics produced low doses (70% and 50%) which varied in shape and location. The distance at which D2cm is assessed is depicted in magenta; plans in the top row have D2cm ≥ 70% while plans in the bottom row have D2cm ≥ 50%.
Table 2 summarizes the median doses and standard deviations to PTV and OAR for passing plans. Target volumes are well covered by 45 Gy except for the left-sided PTV, for which the dose is significantly lower for plans that prioritized the stomach compared to plans that did not. In fact, both GTVs are still covered by the “variation acceptable” dose of 42.5 Gy even when prioritizing stomach (i.e., GTV coverage is not significantly reduced for plans sparing the stomach). Because the stomach overlaps with PTV, it was not possible to meet the maximum dose constraint. However, 20% of plans prioritizing stomach were able to meet the dose to 10cc constraint. Plans sparing stomach resulted in significant dose reductions to 0.03cc and 10cc of the stomach but no significant differences to the maximum spinal dose. These plans are still considered “variation acceptable” because OAR volumes overlapping PTV can receive up to 105% of the prescription dose. Small volumes of the kidney and liver (i.e., median of < 2cc) received doses ≥ 105%, which was considered “variation acceptable” for these parallel organs. All plans met the partial volumes constraints for both kidney and liver.
Figure 4 summarizes plan conformity metrics. Statistical comparisons show that single plans simultaneously treating both PTVs with VMAT, IMRT, or 3D provided improved conformity as assessed by R50%, R70%, R80%, and R90% compared to plans treating each target with a separate plan (Figure 4A). In addition, R50% trended towards significance (p = 0.007) for single plans developed with VMAT that used 2 separate isocenters compared to a single isocenter. While most plans reduced dose to 69 ± 8% at a distance 2cm away from the PTV (Figure 4B), those developed to treat each PTV with a separate plan and isocenter delivered higher median doses of 79 ± 2%. However no statistical difference was found in the D2cm metric among the various planning techniques. Furthermore, correlation between D2cm and R50%, R70%, R80%, R90% was low (Pearson’s r = 0.52 – 0.65), particularly when considering that correlation among R70%, R80%, R90% was as high as 0.9 – 0.97. Only the high dose volume (i.e., R90%) was statistically different in plans that spared the stomach compared to those prioritizing PTV coverage.
Figure 4.


Plots of conformity metrics including: (A) the dose at a 3D distance of 2cm away from the PTVs (D2cm), and (B) the ratio of various isodose volumes to the total PTV volume (R50%, R70%, R80%, R90%) demonstrate that plans generated with two separate plans and multiple isocenters intended to be treated separately produced lower conformity compared to other types of plans including VMAT, IMRT, 3D.
Discussion
In this report we detail the outcomes of benchmark credentialing for trial XXXX. While the majority (93.7%) of institutions successfully passed benchmark credentialing, the initial passing rate was lower than expected. This may indicate the utility of benchmark planning to educate institutions about this complex protocol.
When faced with the challenge of either covering targets and overlapping abdominal organs fully or reducing coverage to meet OAR tolerances, most centers delivered full dose to targets and organs at risk. The preference of institutions to cover PTV rather than spare stomach implies that toxicity outcomes in the trial could potentially be affected. On the other hand, permitting lower PTV coverage could compromise tumor control. Alternatively, some may have planned the benchmark case differently than an actual patient. The benchmark data indicate that, with a 5–7mm expansion margin, the GTV is still well-covered by the minimum prescription dose. This does not account for daily setup variations or organ motion, which could further compromise tumor coverage. Benchmark plans prioritizing stomach did not meet the maximum protocol dose constraint and rarely (20%) achieved the 10cc constraint, indicating that current planning techniques encountered significant challenges for this case. Sparing stomach only affected plan conformity at the highest doses (≥90%), which are closest to the target.
Achieving ideal SBRT dose fall-off was challenging for the benchmark case. Standard constraints could not be mandated in this protocol as no guidance is available in the literature for this situation. Thus, benchmark plans were submitted that could meet DVH criteria but with a wide range of conformity. According to Trial XXXX, conformity metric recommendations vary with PTV volume (32cc – 71 cc for individual PTVs, 103cc for the composite): R50% < 4.3 – 5.3 and D2cm < 68 – 89.5%. While some plans achieved metrics in the lower end of this range, benchmarks using separate plans for each PTV as recommended by Trial XXXX yielded significantly lower conformity (i.e., R50%, R70%, R80%, R90%). These differences were not reflected in D2cm among the various planning techniques. D2cm may not be a good measure of conformity for multiple nearby targets because it does not correlate with dose fall-off as would be expected for a single target in which dose is expected to fall off symmetrically away from the target. In fact, high dose at 2cm may be tolerated when treating two nearby targets in order to preferentially spare another OAR (i.e., spinal cord) in between them, as demonstrated by the benchmark.
Because benchmark credentialing is a planning exercise, extrapolation of these results to patient treatments developed with these various treatment delivery systems is limited. Institutions may have spent less time developing the benchmark plan with the knowledge that it would not be used for treatment. Some borderline plans (i.e., due to low conformity or 105% of the prescribed dose in the kidney/liver) were allowed to pass with feedback given to the institution, particularly on the second or third attempt. Once the FAQ and sample plans were posted, some institutions may have been biased to use VMAT or prioritize the PTV when they originally would not have done so. Robotic radiosurgery systems may have technical disadvantages in treating posterior targets as it does not allow posterior beams. Finally, benchmark planning does not provide any information about the deliverability of the plans developed by each institution. Since this planning exercise could have been approached differently than a clinical case, it could have caused variations in plan quality that might not otherwise exist.
Recent studies have acknowledged the difficulty of assessing patients on randomized trials when they are treated using varying technology and rapidly improving platforms (12). This study provides a unique opportunity to compare/contrast treatment delivery systems in a multi-institutional setting for a challenging planning case. Gallo et al (13) showed that various treatment systems could provide accurate SBRT targeting but with wide variability in treatment time. The current study demonstrates that plans could successfully credential while providing similar conformity using a variety of treatment planning and delivery systems. Although the majority of plans used intensity modulation, a single institution utilized 3DCRT to successfully credential with comparable conformity metrics. The infrequent use of 3DCRT may have been limited by the significant time commitment required to produce a complex treatment plan without an inverse optimization algorithm (13).
The fact that most institutions eventually passed indicates that education to the institutions was achieved, particularly once additional planning guidance was disseminated on IROC’s website. The considerable time spent educating centers on the benchmark was intended to achieve a higher passing rate for pre-treatment review of cases enrolled onto this complex protocol, as pre-treatment review passing rates have been reported to be lower than benchmark passing rates (8). It remains to be determined how the passing rate for pre-treatment reviews of two nearby metastases enrolled onto Trial XXXX will compare to those of the benchmark passing rates.
Conclusions
In conclusion, the low first attempt passing rate (51%) indicates that benchmark credentialing was necessary to educate institutions about the technical requirements of treating two nearby targets according to Trial XXXX. Because SBRT conformity criteria were not mandated, plans were submitted which met DVH criteria but not the conformity criteria. Since dose fall-off for treating two separate PTVs was not symmetric, D2cm was not a good indicator of dose conformity. Data from Trial XXXX should be used to provide more detailed constraints for dose gradients surrounding multiple nearby targets.
Summary.
This study summarizes the benchmark credentialing results for Trial XXXX. While the majority of plans utilized VMAT and a single isocenter to target two nearby metastases, there was wide variability in conformity metrics. Moreover, low dose volumes were significantly larger in those intending to treat each metastasis using a separate plan. The choice by most institutions to prioritize PTV over organs-at-risk could impact toxicity outcomes in this Phase I trial.
Acknowledgments
This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U24CA180803 (IROC) from the National Cancer Institute (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Followill reports grants from NCI, during the conduct of the study. Dr. Robinson reports grants, personal fees and non-financial support from Varian, grants from Elekta, personal fees and non-financial support from ViewRay, non-financial support from DFINE, other from Radialogica, outside the submitted work. Ms. Winter reports grants from NCI, during the conduct of the study. Dr. Matuszak reports grants from Varian Medical Systems, outside the submitted work.
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol Off J Am Soc Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 3.XXXX
- 4.Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol Stockh Swed. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 5.Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838–846. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:3824–3830. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 8.Gondi V, Cui Y, Mehta MP, et al. Real-time pretreatment review limits unacceptable deviations on a cooperative group radiation therapy technique trial: quality assurance results of RTOG 0933. Int J Radiat Oncol Biol Phys. 2015;91:564–570. doi: 10.1016/j.ijrobp.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.XXXX
- 10.XXXX
- 11.Yu J, Straube W, Mayo C, et al. Radiation Therapy Digital Data Submission Process for National Clinical Trials Network. Int J Radiat Oncol. 2014;90:466–467. doi: 10.1016/j.ijrobp.2014.05.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin AGR, Thomas SJ, Harden SV, et al. Evaluating competing and emerging technologies for stereotactic body radiotherapy and other advanced radiotherapy techniques. Clin Oncol R Coll Radiol G B. 2015;27:251–259. doi: 10.1016/j.clon.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Gallo JJ, Kaufman I, Powell R, et al. Single-fraction spine SBRT end-to-end testing on TomoTherapy, Vero, TrueBeam, and CyberKnife treatment platforms using a novel anthropomorphic phantom. J Appl Clin Med Phys Am Coll Med Phys. 2015;16:5120. doi: 10.1120/jacmp.v16i1.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]


