Abstract
Purpose
The delivery of high quality prostate cancer care is increasingly important for health systems, physicians and patients. Integrated delivery systems may have the greatest ability to deliver high quality, efficient care. We sought to understand the association between healthcare integration and quality of prostate cancer care.
Materials and Methods
We used SEER-Medicare data to perform a retrospective cohort study of men > 65 years old with prostate cancer diagnosed between 2007 and 2011. We defined integration within a healthcare market based on the number of discharges from a top 100 integrated delivery system and compared rates of adherence to well-accepted prostate cancer quality measures in markets with no integration versus full integration (>90% of discharges from an integrated system).
Results
The average man treated in a fully integrated market was more likely to receive pre-treatment counseling by a urologist and radiation oncologist (62.6% vs 60.3%, p =0.03), avoid inappropriate imaging (72.2% avoided vs 60.6%, p<0.001), avoid treatment when life expectancy was less than 10 years (23.7% vs 17.3%, p<0.001) and avoid multiple hospitalizations in the last 30 days of life (50.2% vs 43.6%, p=0.001) than when treated in markets with no integration. Additionally, patients treated in fully integrated markets were more likely to have complete adherence to all eligible quality measures (OR 1.38, 95% CI 1.27–1.50).
Conclusion(s)
Integrated systems are associated with improved adherence to several prostate cancer quality measures. Expansion of the integrated healthcare model may facilitate greater delivery of high quality prostate cancer care.
Keywords: prostate cancer, quality, integrated delivery systems, accountable care organizations, Medicare
Introduction
The quality of prostate cancer care in the U.S. varies widely.1 Uncertainty surrounding how best to treat men with localized prostate cancer is fueled by the considerable morbidity of treatments and the associated decrements in quality of life. Without question, public pressure to improve is mounting. The recent release of surgeon-specific complication rates, and the associated media attention, only further amplify calls for accountability and meaningful improvement.
While surgeons can hone their surgical skills through collaborative quality improvement and other self-learning methods to work toward better outcomes,2,3 the best means to facilitate improvements in prostate cancer quality more broadly remains uncertain. Some believe that recent health reforms, which heighten the focus on stewardship of population health and accountable care, will inevitably drive quality improvement.4 Enthusiasm for accountable care is based in part on successes to improve quality and efficiency by fully integrated delivery systems, such as Geisinger and Intermountain Healthcare.5,6 By definition, integrated delivery systems are organized, collaborative networks that link health care providers who are clinically and fiscally accountable for patient populations across the continuum of care.7 Integrated delivery systems are often typified by a focus on fully coordinated, evidence-based healthcare and have systems in place to manage and improve clinical outcomes. However, whether or not such integration is associated with higher quality prostate cancer care is uncertain.
For this reason, we performed a study to better understand the implications of healthcare integration for prostate cancer quality. We hypothesized that higher levels of integration would be associated with better quality. Our findings will help stakeholders anticipate the implications of Accountable Care Organizations (ACOs), the progeny of integrated delivery systems, for specialist-managed diseases such as prostate cancer.
Materials and Methods
Using SEER-Medicare data, we performed a retrospective cohort study of 72,411 men aged 66 and older with newly diagnosed prostate cancer between 2007 and 2011, with follow-up data through December 31, 2013. All men were followed for at least one year after diagnosis. Our study was limited to men continuously enrolled in Medicare Parts A and B throughout the study period and excluded men participating in Medicare managed care plans.
Measuring market-level healthcare integration
We examined quality at the market level rather than the hospital or facility level in an effort to understand patterns in quality of care for all prostate cancer patients. Only surgical patients routinely receive care in a hospital setting, where attribution to a hospital that is part of an integrated delivery system is straightforward. For the nearly 80% of newly diagnosed prostate cancer patients who receive care outside of a hospital setting, understanding the affiliation of the treating physician with an integrated system is a necessary step and these relationships are currently poorly defined. For these reasons, we elected to examine the effects of integration at the market level.
We measured the level of integration within a healthcare market by determining the proportion of discharges coming from a top 100 integrated delivery system in each market.8 The designation of a top 100 integrated delivery system was determined by Becker’s Hospital Review based on rankings provided by a healthcare analytics firm, IMS Health™, as well as an overall assessment of each health systems’ financial, clinical and operations strength. Specifically, health systems are ranked on their ability to operate as a unified organization among key domains including integrated technology use, contractual capabilities, outpatient utilization, financial stability, services and access, hospital utilization and physicians. Markets were defined by the boundaries of hospital referral regions (HRRs) as described in the Dartmouth Atlas of Healthcare.9 Each of the 306 HRRs in the U.S. consists of a collection of zip codes within which residing patients receive the bulk of their healthcare. Of these HRRs, 105 are completely, or have a majority, located within SEER regions and are therefore included in this study to ensure capture of all healthcare services and clinical and pathologic data.10,11 The proportion of discharges from a top 100 integrated delivery system was measured at the level of the HRR. The corresponding proportions of market level integration were assigned to the patients of the cohort based on their HRR and served as the exposure. In an effort to overcome heterogeneity of integration at the hospital level within HRRs, we compared quality at the ends of the spectrum (i.e., no integration versus fully integrated) to ensure that the presence of integration is uniform for patients within the HRRs examined. Fully integrated markets were defined as those HRRs with ≥90% of discharges from a top 100 integrated delivery system.
Outcomes
Measures to assess prostate cancer quality have been developed by researchers at RAND Health, the American Urological Association and the Physician Consortium for Performance Improvement, as shown in Table 1.12 Subsequent studies have confirmed the feasibility of applying these indicators for evaluating quality,1,13–16 and the National Quality Forum has endorsed several of them for performance measurement. Each quality measure was assessed at the patient level. The numerator was the count of eligible men whose care adhered to the performance measure; the denominator was the number of men for whom the measure applies. For example, for the “avoidance of imaging in low risk patients” measure the numerator would be the count of patients with low D’Amico risk prostate cancer (i.e., Gleason score of 6 or less and clinical stage T2a or less, not accounting for PSA17) who did not undergo pretreatment imaging; the denominator would be the count of all men with low risk prostate cancer.
Table 1.
Measures of prostate cancer quality
| Timing relative to primary treatment | Measure | Endorsing Organization | Hypersensitive to health system integration |
|---|---|---|---|
| Pre-treatment | |||
| Received pretreatment counseling by a urologist and radiation oncologist | RAND | ✔ | |
| Avoided imaging in patients with low risk prostate cancer | PCPI, NQF | ||
| Obtained imaging in patients with high risk prostate cancer | PCPI, NQF | ||
| Peri-treatment | |||
| Treated by a high volume provider | |||
| Avoided treatment when life expectancy <10 years | AUA | ✔ | |
| Received ADT with radiation for high risk prostate cancer | PCPI, NQF | ||
| Received adjuvant radiation after surgery for high risk prostate cancer | RAND | ||
| Post-treatment | |||
| At least 2 follow-up visits with treating provider after treatment | RAND | ✔ | |
| Experienced GI toxicity related to treatment | RAND | ||
| Experienced GU toxicity related to treatment | RAND | ||
| Received chemotherapy in the last 14 days of life | NQF | ✔ | |
| Avoidance of multiple hospital admissions in last 30 days of life (>1) | NQF | ✔ | |
AUA: American Urological Association, PCPI Physician Consortium for Performance Improvement, NQF: National Quality Forum
Integrated delivery systems are more likely to adopt electronic health information systems with decision support and have access to patient-centered care management programs.18 Thus, the extent to which these quality measures are sensitive to healthcare integration almost certainly varies as some (e.g., adherence to clinical guidelines on imaging use) are more reliant on organizational infrastructure and systems in place to guide clinical practice while others (e.g., use of adjuvant radiation in men with high risk pathology) are more influenced by the preferences of the physician and/or patient. Additionally, to the degree that primary care physicians are most often the orchestrators of integrated, patient-centered care, we hypothesized that quality measures that involve primary care physicians are likely hypersensitive to health system integration. Based on the literature surrounding integrated delivery systems and quality, we a priori selected a set of measures that we believed to be “hypersensitive” to integration; also indicated in Table 1.
Analysis
We compared patient characteristics and regional characteristics from the Area Resource File (urban/rural status, Medicare Advantage penetration, and number of urologists, radiation oncologists, and hospital beds per 100,000 population) for HRRs with no integration versus those with full integration using Chi-square tests.
Next, we constructed a series of multivariable logistic regression analyses to assess the association between the integration of healthcare markets and adherence to individual quality measures, adjusting for patient and regional characteristics. The models yielded predicted probabilities of compliance for HRRs with no integration versus full integration for the average patient (i.e., white, 70–74 years old, low risk (Gleason 6 and clinical stage ≤ T2a), no comorbidities, middle socioeconomic tercile, largest population tercile, intermediate number of urologists, radiation oncologists, hospital beds, and intermediate Medicare Advantage penetration). Using the same statistical approach, we then fit an “all-or-nothing” model in which compliance required that all quality measures for which the patient was eligible be adhered. As before, we compared the effect of no integration versus full integration.
To assess whether market integration had a particularly strong effect on what we hypothesized were the most sensitive of the quality measures (hypersensitive), we constructed a logistic model at the measure level (as opposed to the patient level), using generalized estimating equations to account for multiple measures per patient. Finally, an interaction term, formed by multiplying integration (continuous) and hypersensitivity (dichotomous), was added to the model.
All analyses were carried out in SAS version 9.4 (Cary, NC). The probability of a two-tailed Type 1 error was set at 0.05. The study protocol was judged to be exempt by the institutional review board of the University of Michigan.
Results
A total of 72,411 patients met inclusion criteria. Patient and market characteristics were contrasted according to the degree of market level integration, where 19,848 patients received care in 37 markets with no integration and 1,237 patients receive care in one of three fully integrated markets (Table 2). While similar in terms of age and D’Amico risk, patients treated in fully integrated markets were more often white (91.7% vs. 80.5%, p<0.001) and without comorbidities (65.4% vs. 60.9%, p=0.01). Fully integrated markets were more likely to be located in metropolitan areas with a population over 1 million (74.1% vs. 43.6%, p<0.001) and had the highest per capita concentration of urologists and hospital beds. During the study interval, our cohort experienced a 1.7% prostate cancer specific mortality rate and 15.7% all cause mortality rate.
Table 2.
Characteristics of patients treated in markets with no versus full integration
| Number (%) | No integration (n=19,848) | Full integration (≥90%) (n=1,237) | p-value |
|---|---|---|---|
| Age (years) | 0.16 | ||
| 66–69 | 5404 (27.2) | 358 (28.9) | |
| 70–74 | 6411 (32.3) | 417 (33.7) | |
| 75–79 | 4521 (22.8) | 272 (22.0) | |
| 80–84 | 2411 (12.1) | 125 (10.1) | |
| 85+ | 1101 (5.5) | 65 (5.3) | |
| Race (%) | <0.001 | ||
| White | 15983 (80.5) | 1134 (91.7) | |
| Black | 1849 (9.3) | 72 (5.8) | |
| Asian/Hispanic | 1170 (5.9) | 11 (0.9) | |
| Other | 846 (4.3) | 20 (1.6) | |
| Charlson comorbidity index | 0.01 | ||
| 0 | 12093 (60.9) | 809 (65.4) | |
| 1 | 4628 (23.3) | 265 (21.4) | |
| 2 | 1753 (8.8) | 87 (7.0) | |
| 3 | 1374 (6.9) | 76 (6.1) | |
| D’Amico Risk | 0.84 | ||
| Low | 7514 (37.9) | 462 (37.3) | |
| Intermediate | 7312 (36.8) | 466 (37.7) | |
| High | 5022 (25.3) | 309 (25.0) | |
| Socioeconomic Status Tercile | <0.001 | ||
| Low | 7885 (39.7) | 296 (23.9) | |
| Medium | 5966 (30.1) | 808 (65.3) | |
| High | 5997 (30.2) | 133 (10.8) | |
| Urban | <0.001 | ||
| ≥1 million metropolitan county | 8659 (43.6) | 916 (74.1) | |
| <1 million metropolitan county | 7707 (38.8) | 228 (18.4) | |
| Urban population of 2,500+ | 3270 (16.5) | 69 (5.6) | |
| Rural or <2,500 urban population | 204 (1.0) | 24 (1.9) | |
| Urologists per 100,000 population | <0.001 | ||
| Low (≤42.3) | 9220 (46.5) | >307* | |
| Intermediate | 4220 (21.3) | <11* | |
| High (≥71.5) | 6408 (32.3) | 919 (74.3) | |
| Radiation oncologists per 100,000 population | <0.001 | ||
| Low (≤19.2) | 8285 (41.7) | >137* | |
| Intermediate | 4731 (23.8) | 1097 (88.7) | |
| High (≥31.5) | 6832 (34.4) | <11* | |
| Hospital beds per 100,000 population | <0.001 | ||
| Low (≤4,028) | 6115 (30.8) | 85 (6.9) | |
| Intermediate | 6858 (34.6) | 28 (2.3) | |
| High (≥5,683) | 6875 (34.6) | 1124 (90.9) | |
| Medicare Advantage penetration | <0.001 | ||
| Low (≤15.8%) | 11590 (58.4) | >298* | |
| Intermediate | 3720 (18.7) | 929 (75.1) | |
| High (≥27.5%) | 4538 (22.9) | <11* |
Exact value redacted/altered due to small cell size
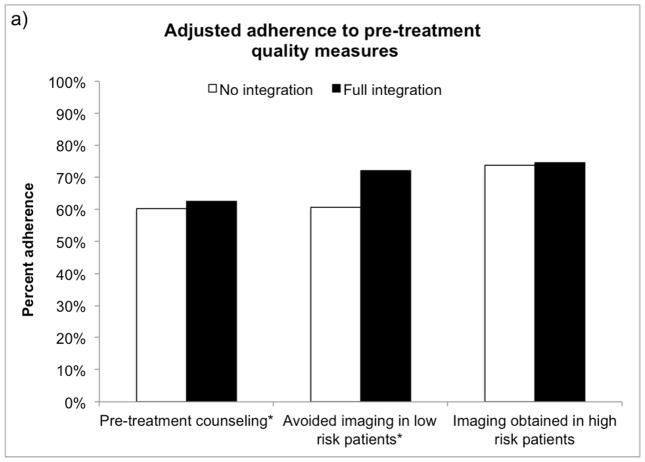
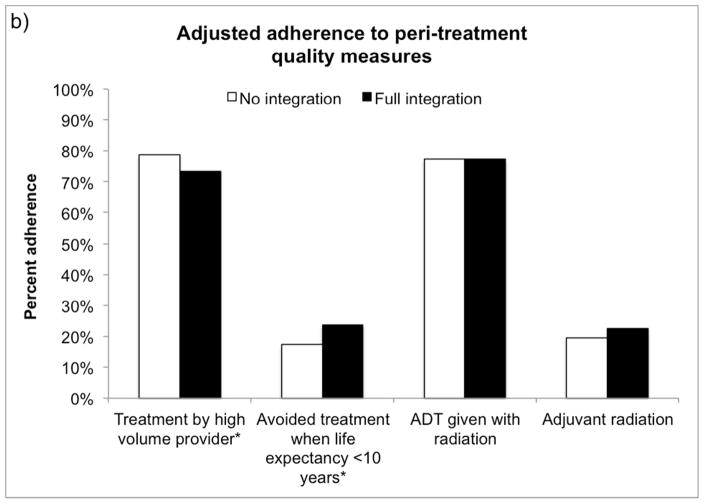
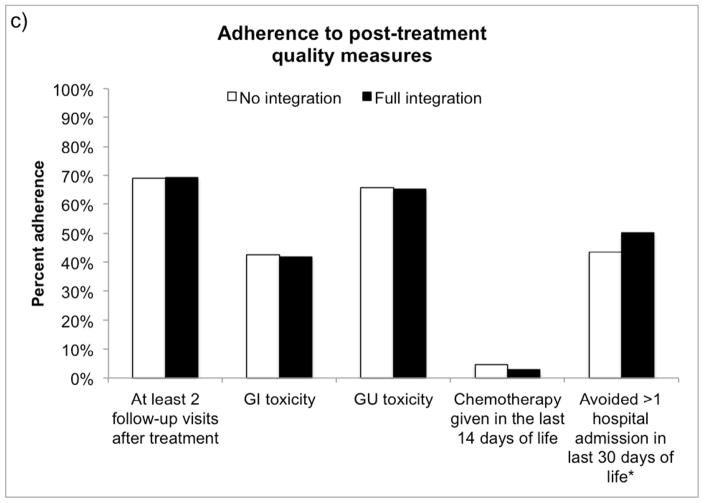
As illustrated in Figure 1, the average prostate cancer patient receiving treatment in a fully integrated market was more likely to receive pre-treatment counseling (62.6% vs. 60.3%, p=0.03) and avoid inappropriate imaging (72.2% avoided vs. 60.6%, p<0.001) during the pre-treatment period than if he were treated in a market with no integration. Men with prostate cancer treated in fully integrated markets were more likely to avoid treatment when non-cancer life expectancy was less than 10 years (23.7% avoided vs. 17.3%, p<0.001), however they were slightly less likely to be treated by a high volume provider (73.3% vs. 78.7%, p<0.001). In the post-treatment phase, fully integrated markets performed better at avoiding multiple hospitalizations (more than 1) in the last 30 days of life (50.2% avoided vs. 43.6%, p=0.001).
Figure 1.
Adjusted adherence to a) pre-treatment, b) peri-treatment and c) post-treatment quality measures for an average patient according to market-level integration. *p<0.05
Patients treated in fully integrated markets were more likely to receive “ideal” care (i.e., adhere to all eligible quality measures) than patients treated in markets with no integration (16.5% vs. 12.5%, OR 1.38, 95% CI 1.27–1.50, Table 3).
Table 3.
Association between market level integration and adherence to all eligible quality measures
| Characteristic | Odds Ratio | p-value |
|---|---|---|
| Fully integrated market | 1.38 (1.27–1.50) | <0.001 |
| Age (years) | ||
| 66–69 | Ref | |
| 70–74 | 1.18 (1.12–1.24) | <0.001 |
| 75–79 | 1.56 (1.47–1.65) | <0.001 |
| ≥80 | 2.64 (2.49–2.80) | <0.001 |
| Race (%) | ||
| White | Ref | |
| Black | 1.19 (1.11–1.28) | <0.001 |
| Asian | 1.14 (1.02–1.28) | 0.03 |
| Hispanic | 1.12 (0.97–1.28) | 0.11 |
| Other | 1.05 (0.94–1.18) | 0.36 |
| Charlson comorbidity index | ||
| 0 | Ref | |
| 1 | 0.92 (0.88–0.97) | 0.001 |
| 2 | 0.93 (0.87–0.99) | 0.03 |
| 3 | 0.86 (0.80–0.93) | <0.001 |
| D’Amico Risk | ||
| Low | Ref | |
| Intermediate | 0.39 (0.37–0.40) | <0.001 |
| High | 0.28 (0.27–0.30) | <0.001 |
| Socioeconomic Status Tercile | ||
| Low | Ref | |
| Medium | 1.02 (0.97–1.07) | 0.47 |
| High | 1.09 (1.03–1.15) | 0.003 |
| Urban | ||
| ≥1 million metropolitan county | Ref | |
| <1 million metropolitan county | 1.12 (1.07–1.17) | <0.001 |
| Urban population of 2,500+ | 1.21 (1.13–1.30) | <0.001 |
| Rural or <2,500 urban population | 0.86 (0.74–1.01) | 0.07 |
| Urologists per 100,000 population | ||
| Low (≤42.3) | Ref | |
| Intermediate | 0.91 (0.85–0.96) | 0.001 |
| High (≥71.5) | 0.98 (0.91–1.05) | 0.55 |
| Radiation oncologists per 100,000 population | ||
| Low (≤19.2) | Ref | |
| Intermediate | 1.14 (1.08–1.21) | <0.001 |
| High (≥31.5) | 1.20 (1.12–1.29) | <0.001 |
| Hospital beds per 100,000 population | ||
| Low (≤4,028) | Ref | |
| Intermediate | 1.02 (0.97–1.07) | 0.52 |
| High (≥5,683) | 0.84 (0.79–0.90) | <0.001 |
| Medicare Advantage penetration | ||
| Low (≤15.8%) | Ref | |
| Intermediate | 1.19 (1.14–1.26) | <0.001 |
| High (≥27.5%) | 1.37 (1.30–1.44) | <0.001 |
Despite significantly improved odds of complying with measures that we deemed hypersensitive (OR 0.70 95% CI 0.69–0.71), we found no significant interaction between the degree of integration within the market and hypersensitive measures (interaction term p-value 0.47). In other words, while adherence to hypersensitive measures is better than non-hypersensitive measures, this is true across healthcare markets and not significantly better in more integrated markets.
Discussion
Health system integration is associated with improved adherence to a number of prostate cancer quality measures. Specifically, patients treated in fully integrated markets are more likely to receive pre-treatment counseling by both a radiation oncologist and a urologist, avoid unnecessary imaging for low risk prostate cancer, avoid treatment when life expectancy is <10 years and avoid multiple hospital admissions at the end of life. In addition to individual quality measures, patients treated in fully integrated markets had a 38% higher odds of receiving “ideal” care or having complete adherence to all eligible quality measures.
The delivery of high quality prostate cancer care has become increasingly important for health systems, physicians and patients. As the number of quality measures grows and the financial risk associated with meeting them increases, integrated delivery systems, and by extension ACOs, may be better positioned to deliver high quality, efficient care. Improved quality of care has been noted in both integrated delivery systems and ACOs.19,20 Our findings add to this growing body of literature supporting increased health system integration as a means of improving quality of care.
Our study has several limitations. First, our study showed that integration is associated with improved adherence for certain prostate cancer quality measures, however not all measures show this relationship. Additionally, significant gaps in quality remain as evidenced by the overall low delivery of “ideal” care. Together, these data suggest that while integration facilitates the delivery of high quality care, integration alone is not sufficient to maximize quality of care. Improved patient and physician education, uniform guidelines across specialties, and multidisciplinary clinics may be other means of increasing quality care. Further financial incentives applied through recent policies focused on value and quality will likely act as additional levers to promote high quality care. Second, much of prostate cancer care occurs outside the hospital setting and providers administering outpatient prostate cancer care may have little or no relationship with the dominant integrated system in the market. However, we elected to assign integration at the market level and examine outcomes at the extremes of integration (none versus fully integrated) in an effort to minimize the impact of this limitation. Finally, it is conceivable that integration may be a surrogate measure for some other characteristic of these markets that facilitates higher quality care. For example, the improvements we observed for integrated systems may be mediated by factors such as the incorporation of multidisciplinary clinics, strong emphasis on shared decision making, the presence of patient care navigators or the robust use of electronic health records. While we hypothesized that integration represented a composite of these health system attributes, it is possible that one of these attributes or an unmeasured factor is driving the differences in quality that we observed.
These limitations notwithstanding, our findings have important implications for policymakers, health system leadership and patients. For policymakers, evidence that health system integration is associated with improved adherence to quality measures supports the development and dissemination of initiatives, such as ACOs, that aim to further encourage deeply integrated networks. ACOs have expanded rapidly with an estimated 23.5 million patients covered in a commercial, Medicaid or Medicare ACO in 2015.21,22 As increasing financial bonuses or penalties are tied to quality, health system leadership and physicians may find that increased integration helps support the delivery of high quality care in general as well as for specific conditions such as prostate cancer. Patients treated in integrated delivery systems will inevitably benefit from the improved adherence to quality measures as well as the more coordinated, efficient care provided in these settings. Despite these benefits, policy makers will also be cognizant of the unintended consequences of consolidation, which may drive higher spending by increasing an organization’s bargaining power and reducing competition.23
For practicing urologists, our findings suggest participation in an integrated delivery system may help facilitate the delivery of high quality care. Importantly, the Medicare Access and Chip Reauthorization Act of 2015 (MACRA) changes how physicians are reimbursed for services provided to Medicare beneficiaries by tying payments to quality.24 Integrated delivery systems and ACOs will likely continue to grow under MACRA and urologists may have an opportunity to formally participate in these programs as an alternative to individual physician performance measurement. A greater understanding of factors that influence the delivery of quality care will become increasingly relevant for urologists in this new payment environment.
Conclusions
Moving forward, policies that encourage health system integration may have downstream effects of facilitating the delivery of high quality care. Ultimately, studies that explore the financial resources needed to build integrated networks and the costs associated with delivering high quality care will inform how wide dissemination of this model can realistically occur.
Acknowledgments
This work was supported by a Research Scholar Grant RSGI-13-323-01-CPHPS to BKH from the American Cancer Society. VBS is supported by funding from the NCI (R01 CA168691). DCM is supported by National Cancer Institute (1-R01-CA-174768-01-A1). FRS is supported by a Department of Veterans Affairs VISN1 Career Development Award. TAS is supported by a VA HSR&D Career Development Award - 2 (CDA 12-171)
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
This work was supported by a Research Scholar Grant RSGI-13-323-01-CPHPS to BKH from the American Cancer Society. VBS is supported by funding from the NCI (R01 CA168691). DCM is supported by National Cancer Institute (1-R01-CA-174768-01-A1). FRS is supported by a Department of Veterans Affairs VISN1 Career Development Award. TAS is supported by a VA HSR&D Career Development Award - 2 (CDA 12-171)
No funding organization or sponsor had a role in the design and conduct of the study; management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Key of Definitions for Abbreviations
- ACO
Accountable Care Organization
- HRR
Hospital referral region
- PSA
Prostate specific antigen
- MACRA
Medicare Access and CHIP Reauthorization Act
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schroeck FR, Kaufman SR, Jacobs BL, et al. Regional variation in quality of prostate cancer care. J Urol. 2014;191:957–62. doi: 10.1016/j.juro.2013.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg CC, Ghousseini HN, Pavuluri Quamme SR, et al. Surgical coaching for individual performance improvement. Ann Surg. 2015;261:32–4. doi: 10.1097/SLA.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical Skill and Complication Rates after Bariatric Surgery. N Engl J Med. 2013;369:1434–1442. doi: 10.1056/NEJMsa1300625. [DOI] [PubMed] [Google Scholar]
- 4.Pham HH, Cohen M, Conway PH. The Pioneer Accountable Care Organization Model Improving Quality and Lowering Costs. JAMA. 2014;312:1635–36. doi: 10.1001/jama.2014.13109. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Bothe A, Steele GD. INNOVATION PROFILE: How Geisinger Structures Its Physicians’ Compensation To Support Improvements In Quality, Efficiency, And Volume. Health Aff. 2012;31:2068–2073. doi: 10.1377/hlthaff.2011.0940. [DOI] [PubMed] [Google Scholar]
- 6.James BC, Savitz LA. How Intermountain Trimmed Health Care Costs Through Robust Quality Improvement Efforts. Health Aff. 2011;30:1185–1191. doi: 10.1377/hlthaff.2011.0358. [DOI] [PubMed] [Google Scholar]
- 7.Enthoven AC. Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15:S284–90. [PubMed] [Google Scholar]
- 8.Rodak S. 100 Integrated Health Systems to Know. Becker’s Hosp Rev. 2013 [Google Scholar]
- 9.The Dartmouth Atlas of Health Care. 2006 [Google Scholar]
- 10.Brooks GA, Li L, Sharma DB, et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst. 2013;105:634–42. doi: 10.1093/jnci/djt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollenbeck BK, Ji Hong, Ye Zaojun, et al. Misclassification of hospital volume with Surveillance, Epidemiology, and End Results Medicare data. Surg Innov. 2007;14:192–8. doi: 10.1177/1553350607307274. [DOI] [PubMed] [Google Scholar]
- 12.Spencer BA, Steinberg M, Malin J, et al. Quality-of-care indicators for early-stage prostate cancer. J Clin Oncol. 2003;21:1928–36. doi: 10.1200/JCO.2003.05.157. [DOI] [PubMed] [Google Scholar]
- 13.Miller DC, Litwin MS, Sanda MG, et al. Use of quality indicators to evaluate the care of patients with localized prostate carcinoma. Cancer. 2003;97:1428–35. doi: 10.1002/cncr.11216. [DOI] [PubMed] [Google Scholar]
- 14.Spencer BA, Miller DC, Litwin MS, et al. Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol. 2008;26:3735–42. doi: 10.1200/JCO.2007.13.2555. [DOI] [PubMed] [Google Scholar]
- 15.Miller DC, Spencer BA, Shah RB, et al. The quality of surgical pathology care for men undergoing radical prostatectomy in the U. S Cancer. 2007;109:2445–53. doi: 10.1002/cncr.22698. [DOI] [PubMed] [Google Scholar]
- 16.Miller DC, Spencer BA, Ritchey J, et al. Treatment choice and quality of care for men with localized prostate cancer. Med Care. 2007;45:401–9. doi: 10.1097/01.mlr.0000255261.81220.29. [DOI] [PubMed] [Google Scholar]
- 17.http://seer.cancer.gov/data/psa-values.html
- 18.Solberg LI, Asche SE, Shortell SM, et al. Is integration in large medical groups associated with quality? Am. J Manag Care. 2009;15:e34–41. [PubMed] [Google Scholar]
- 19.Rhoads KF, Patel MI, Ma Y, et al. How Do Integrated Health Care Systems Address Racial and Ethnic Disparities in Colon Cancer? J Clin Oncol. 2015;33:854–860. doi: 10.1200/JCO.2014.56.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McWilliams JM, Chernew ME, Zaslavsky AM, et al. Delivery System Integration and Health Care Spending and Quality for Medicare Beneficiaries. JAMA Intern Med. 2013;173:1447–1456. doi: 10.1001/jamainternmed.2013.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department of Health and Human Services. New hospitals and health care providers join successful, cutting-edge federal initiative that cuts costs and puts patients at the center of their care. 2016 http://www.hhs.gov/about/news/2016/01/11/new-hospitals-and-health-care-providers-join-successful-cutting-edge-federal-initiative.html.
- 22.Muhlestein D. Growth and Dispersion of Accountable Care Organizations in 2015. http://healthaffairs.org/blog/2015/03/31/growth-and-dispersion-of-accountable-care-organizations-in-2015-2/2015.
- 23.Neprash H, Chernew M, Hicks A, et al. Financial Integration between Physicians and Hospitals and Commercial Health Care Prices. JAMA Intern Med. 2015;02115:1–15. doi: 10.1001/jamainternmed.2015.4610. [DOI] [PubMed] [Google Scholar]
- 24.The Medicare Access and CHIP Reauthorization Act (P.L. 114-10).; 2015.