Abstract
Background
The 21-gene recurrence score assay (RS) stratifies early-stage, estrogen receptor-positive breast cancer by recurrence risk. Few studies have examined how physicians use RS to recommend adjuvant systemic chemotherapy or patient experiences with testing and decision-making.
Methods
We surveyed 3,880 women treated for breast cancer in 2013-2014, identified by the Los Angeles County and Georgia SEER registries (response rate: 71%). Women reported chemotherapy recommendations, chemotherapy receipt, testing experiences, and decision satisfaction. Registries linked tumor data, RS, and surveys. Regression models examined factors associated with chemotherapy recommendations and receipt by RS and subgroups.
Results
Of 1,527 patients with Stage I-II, ER/PR(+), HER2-negative disease: 778 received RS (62.6% for node-negative favorable, 24.3% in node-negative, unfavorable, and 13.0% in node-positive disease, p<.001). Overall, 47.2% of patients received a recommendation against chemotherapy and 40.6% received a recommendation for it. RS results correlated with recommendations: nearly all patients with high scores (31-100) received a chemotherapy recommendation (86.9%-96.5% across clinical subgroups), while the majority of patients with low-risk results (0-18) received a recommendation against it (49.2%-78.2% across subgroups). Most patients with high RS received chemotherapy (87.0%, 91.1%, 100% across subgroups), while few patients with low scores received it (2.9%, 9.5%, 26.6% across subgroups). There were no substantial racial/ethnic differences in testing and treatment. Women were largely satisfied with RS and chemotherapy decisions.
Conclusions
Oncologists use RS to personalize treatment, even in node-positive disease. High satisfaction and absence of disparities in testing and treatment suggests that precision medicine advances have improved systemic breast cancer treatment.
Keywords: Breast neoplasms, Genomics, Chemotherapy, adjuvant, Surveys and questionnaires, Health services
Graphical abstract
Precis: In a population-based sample of women diagnosed with early-stage breast cancer, the majority of women received 21-gene recurrence score testing, including a third of women with lymph node-positive disease. Test results correlated with guideline-recommended treatment in most cases, and patient satisfaction was high.
INTRODUCTION
A key goal of precision medicine is to reduce treatment burdens in patients with a favorable cancer prognosis. Precision medicine advances have influenced decisions more strongly for breast cancer than for other conditions.1, 2 Until recently, results from cancer staging (in particular, lymph node status) and from tests performed on breast tumors (estrogen and progesterone receptors, human epidermal growth factor-2 (HER2) receptor, and grade) largely determined clinician recommendations regarding adjuvant chemotherapy use in patients with newly diagnosed, early stage, curable invasive breast cancer.3, 4 In recent years, however, the 21-gene recurrence score assay (RS) - which stratifies a woman’s risk for distant breast cancer recurrence into low, intermediate, and high categories and predicts the marginal benefit of adjuvant chemotherapy - has diffused rapidly into clinical practice, supported by guidelines based on strong evidence of its clinical validity and utility.5-7
Current guidelines recommend RS testing for all patients with favorable-prognosis (ER/PR-positive, HER2-negative, node-negative disease), but not for patients with ER/PR-positive, HER2-negative, node-positive disease, for which adjuvant treatment is recommended (independently of RS testing).3, 4 Several studies have shown that RS use for node-negative patients generally follows guideline recommendations with variable evidence of disparities in testing.8-11 Furthermore, RS results are strongly associated with treatment. RS may reduce overall use of chemotherapy 12, 13 because about half of tested patients have low scores that indicate minimal benefit from chemotherapy, while only about 10% have high scores that indicate a strong benefit of chemotherapy. About one-third of patients have an intermediate score, in which case chemotherapy’s benefit is less certain.14 A Canadian study showed that RS testing was followed by a marked increase in the percentage of patients who received a recommendation about chemotherapy, particularly against it.15 However, little is known about how RS results are used by medical oncologists to recommend chemotherapy, and whether patients follow these recommendations. Moreover, recommendations and decisions over testing and treatment is less understood in the United States, where RS use is more common and treatment occurs in more diverse settings. Published studies of RS and treatment decision-making have been limited by older diagnosis cohorts, lack of generalizability, incomplete ascertainment of RS testing and/or chemotherapy treatment, and by lack of granular measures of communication and decision-making linked to RS results and treatment.
We used a large, contemporary, diverse, population-based sample of patients recently diagnosed with early-stage breast cancer to examine the relationship between RS results, clinician recommendations, chemotherapy receipt, and patient experiences with testing and treatment decision-making.
METHODS
Sampling and Data Collection
The iCanCare study16 selected 3,880 women aged 20-79 diagnosed with early-stage breast cancer and treated in 2013-14 as captured by rapid reporting systems from the Surveillance, Epidemiology, and End-Results (SEER) registries of Georgia and Los Angeles County (LA). African-American and Latinas were oversampled in LA to ensure diversity in the sample. We identified cases approximately two months after breast surgical treatment. Women with stage III or IV cancer, tumors >5 centimeters (cm) in size, or those with >3 positive lymph nodes were excluded. Non-Hispanic Whites and African Americans below the age of 50 in LA were not available for sampling due to an ongoing study in those populations. Modified Dillman techniques17-18 were used to solicit high patient response rates. Women were invited to participate by mail with an upfront $20 cash incentive. Extensive follow-up was conducted for non-responders. Materials were sent in English, except for women with Spanish surnames who received materials in both English and Spanish.19 From 3,880 identified women, 249 were ineligible due to a prior breast cancer diagnosis or stages III-IV; residing outside the SEER registry area; or being deceased, too ill, or unable to complete a survey in Spanish or English. Another 1,053 women did not return surveys or refused participation. SEER registries collect RS as part of routine surveillance operations but there are concerns for completeness. Through an agreement between Genomic Health, Inc. and the National Cancer Institute SEER Program, records from the two datasets were linked using probabilistic methods including manual review and adjudication of potential linked pairs to assure the highest specificity while simultaneously maximizing sensitivity. Results showed that 97.2% of patients with a SEER-confirmed RS test linked to the Genomic Health, Inc. test dataset. The SEER registries then provided limited SEER data and RS results for iCanCare participants to the University of Michigan, which were merged to survey data under IRB approvals from partnering Universities and State Departments of Public Health of Georgia and California. RS results were linked to the sample of 2,578 women (71% of eligible patients). Our analytic sample consisted of 1,527 patients with Stage I-II, ER/PR-positive, HER2-negative disease.
Measures
We classified patients into three mutually exclusive clinical categories: lymph node-negative, more-favorable (age at diagnosis ≥50 years and/or tumor grade 1-2); node-negative, less-favorable (age at diagnosis <50 years, and/or grade 3 disease), and node-positive. Age and tumor grade were used to derive subgroups as these variables are prognostic for distance recurrence. 3, 20 We examined three outcomes: receipt of RS testing (obtained from Genomic Health, Inc.), medical oncologists’ recommendation for adjuvant systemic chemotherapy, and receipt of chemotherapy (both reported from the patient survey). RS results indicated whether the test was done or not and the numeric score (0-100 for tested subjects, with higher values reflecting increased likelihood of distant metastatic breast cancer recurrence and greater benefit from chemotherapy). Scores were categorized in accordance with current guidelines and laboratory reporting (low 0-17, intermediate 18-30, high 31-100). Surveyed patients reported their medical oncologists’ recommendations for adjuvant systemic chemotherapy across five responses: strongly against, against, left it up to the patient, for, or strongly for chemotherapy. We categorized these into three responses: against, left it up to patient, or for chemotherapy. Women indicated whether they had begun or were planning to begin chemotherapy, or whether they had refused/had no plans to begin chemotherapy.
Covariates were obtained from the patient survey and SEER registries. Tumor stage (I or II), grade (1, 2, or 3), and lymph node status (all nodes negative, 1-3 nodes positive for disease) were obtained from registries. Patients provided the following variables from surveys: age at diagnosis; education (high school or less, some college, college graduate or higher); family income (< $20,000/year, $20-60,000/year, >$60,000/year); race/ethnicity (white, black, Latina, Asian), and diagnosis of comorbidities, including chronic lung disease, heart disease, diabetes, or stroke (no diagnosis, one condition, two or more conditions).
We also examined patient experiences with testing and chemotherapy decisions. We first asked tested women how helpful RS was in making a chemotherapy decision, on a 5-point Likert scale (1 = not at all helpful, 5 = extremely helpful). Next, women indicated whether RS made them “much less”, “less”, ”no change in their mind”, “more”, or “much more” interested in chemotherapy. We asked women about their satisfaction with decisions surrounding RS and chemotherapy, on a 5-point Likert scale (1 = not at all satisfied, 5 = totally satisfied).
Statistical Analysis
First, we described the association of patient characteristics and receipt of chemotherapy and RS tests. We then described medical oncologists’ recommendations for adjuvant systemic chemotherapy by clinical group and RS score. We then assessed chemotherapy use by clinical and RS groups. Next, we constructed a multivariable logistic model that examined receipt of RS testing as a function of clinical group, comorbidities, and various demographic characteristics including race, education, income, and geographical site. Further, we estimated the effect of RS on the likelihood of chemotherapy receipt, while controlling for clinical group and demographic characteristics listed above. Finally, we described patient recall of RS testing and their satisfaction with testing and treatment decision-making.
Survey design and non-response weights were created to compensate for differential probability of selection and to adjust for survey non-response to report results that resemble the target populations in LAC and Georgia.21 To reduce potential non-response bias due to missing data and changes in versions of the questionnaire we multiply imputed data using a sequential regression multiple imputation framework.22 We generated five independently-imputed data sets and then computed inferential statistics, combining estimates across the datasets.23 Unless noted, results reported used multiply imputed weighted data.(SAS version 9.4).
RESULTS
Table 1 shows distributions of key variables from observed, unweighted data and receipt of testing and chemotherapy by covariate group (unweighted %), with corresponding standard errors and p-values. One-fifth (19.8%) had node-positive disease; 19.4% had node-negative, less-favorable disease; and 60.1% had node-negative, more-favorable disease. Over one-quarter (27.3%) had one or more comorbidities. Patients were widely distributed across race/ethnicity, education, and income. Overall, 50.9% of patients in the analytic sample received RS: 62.6% of those with node-negative, more-favorable disease, 24.3% with node-negative, less-favorable disease, and 13.0% with node-positive disease. RS testing was more common in the Georgia versus LA cohort (65.8% vs 34.2%, p<.001). Overall, 30.9% of patients received chemotherapy. Few patients in the more-favorable group received chemotherapy (25.2%), compared to those with less-favorable disease (30.3% less-favorable, node-negative; 44.3% in node-positive disease). Chemotherapy use was less frequent in older women and those with more comorbidities. Among RS recipients (n=778), low scores (61.7%) were more common than intermediate (30.0%) or high scores (8.3%).
Table 1.
Patient Characteristics
|
Full Sample
N=1527 |
Received
Recurrence Score Assay N=778 |
Received
Systemic Chemo N=472 |
|||
|---|---|---|---|---|---|
| Characteristics a | |||||
| Age (in years) at diagnosis |
Mean (95% CI) | 61.0 (60.5,61.6) |
59.1 (58.4,59.8)* |
57.2 (56.2,58.2)* | |
| N | %(SE) | %(SE) | %(SE) | ||
| Clinical group | Lymph-node negative, more favorable: node-, age≥50 or grade=1-2 |
917 | 60.1(1.3) | 62.6(1.7)*** | 25.2(2.0)*** |
| Node-negative less favorable: node-, age<50 or grade=3 |
297 | 19.4(1.0) | 24.3(1.5) | 30.3(2.1) | |
| Node-positive disease | 303 | 19.8(1.0) | 13.0 (1.2) | 44.3(2.3) | |
| Missing | 10 | 0.7(0.2) | 0.1(0.1) | 0.2(0.2) | |
| Comorbiditiesb | No diagnosis | 1102 | 72.2(1.2) | 74.6(1.6)** | 76.1(2.0)** |
| One condition | 328 | 21.5(1.1) | 21.0(1.5) | 19.7(1.8) | |
| Two or more conditions | 88 | 5.8(0.6) | 3.9(0.7) | 3.8(0.9) | |
| Missing | 9 | 0.6(0.2) | 0.6(0.3) | 0.4(0.3) | |
| Race/Ethnicity | White | 869 | 56.9(1.3) | 62.0(1.7)*** | 51.7(2.3)* |
| Black | 233 | 15.3(0.9) | 15.0(1.3) | 17.2(1.7) | |
| Latina | 268 | 17.6(1.0) | 13.2(1.2) | 20.3(1.9) | |
| Asian | 112 | 7.3(0.7) | 6.7(0.9) | 7.2(1.2) | |
| Missing | 45 | 2.9(0.4) | 3.1(0.6) | 3.6(0.9) | |
| Education | High School/GED or less | 449 | 29.4(1.2) | 26.0(1.6)*** | 28.8(2.1) |
| Some college or technical school | 491 | 32.2(1.2) | 31.9(1.7) | 32.2(2.2) | |
| College graduate or higher | 567 | 37.1(1.2) | 40.9(1.8) | 37.3(2.2) | |
| Missing | 20 | 1.3(0.3) | 1.3(0.4) | 1.7(0.6) | |
| Annual Family Income |
< $20,000 | 234 | 15.3(0.9) | 14.7(1.3)*** | 15.0(1.7) |
| $20,000-$60,000 | 417 | 27.3(1.1) | 24.8(1.6) | 26.1(2.0) | |
| > $60,000 | 583 | 38.2(1.2) | 43.3(1.8) | 42.2(2.3) | |
| Missing | 293 | 19.2(1.0) | 17.2(1.4) | 16.7(1.7) | |
| Site | Georgia | 839 | 54.9(1.3) | 65.8(1.7)*** | 51.7(2.3) |
| Los Angeles County | 688 | 45.1(1.3) | 34.2(1.7) | 48.3(2.3) | |
Except for age, data are expressed as No. (%) of patients, with corresponding standard errors (SEs). All %s are unweighted.
Patient reported a doctor in the past told them they had chronic lung disease, heart disease, diabetes, or stroke
p < .001
p < .01
p <.05
Factors associated with Recurrence Score testing
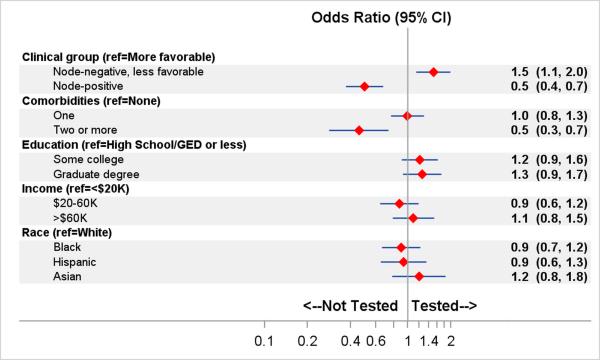
Figure 1 shows the results of a logistic regression model that estimates factors associated with RS receipt. Compared with women with node-negative, more-favorable disease, women with node-negative, less-favorable disease were more likely to receive RS (OR 1.5, 95% CI 1.1-2.0), while women with node-positive disease were less likely to receive RS (OR 0.5, 95% CI 0.4-0.7). Women with two or more comorbidities were less likely to receive RS than women without a comorbidity (OR 0.5, 95% CI 0.3-0.7). There were no significant differences in RS use across education, income, and race/ethnicity.
Figure 1. Factors associated with recurrence score (RS) testing.
Adjusted Odds ratios (95% confidence intervals) estimated using weighted logistic regression model on multiply imputed data. Adjusted for geographic site. Ref indicates reference group; K indicates thousand.
Factors associated with chemotherapy recommendations
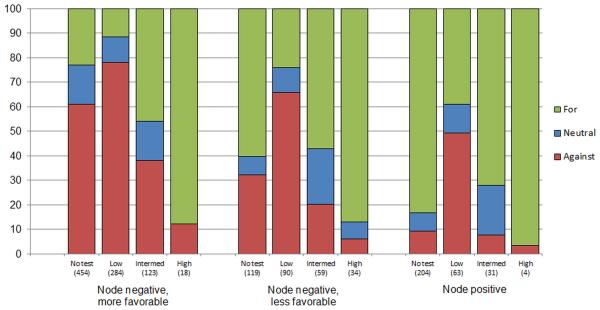
Overall, 47.2% of patients reported that their medical oncologist recommended against systemic chemotherapy, 12.3% reported their oncologist left the decision to them, and 40.5% reported their oncologist recommended for chemotherapy. Figure 2 shows the relationship between RS results and medical oncologist recommendations for the three clinical groups. RS results were highly associated with recommendations: virtually all patients with high scores (31-100) received a chemotherapy recommendation (86.9%-96.5% across subgroups). For women with node-negative disease, the majority with low-risk RS results (0-18) received a recommendation against chemotherapy (65.9%-78.2% across subgroups). Most women with favorable-risk, node-negative disease received a recommendation against chemotherapy (78.2%) and 11.7% received a recommendation for chemotherapy. Recommendations for chemotherapy varied in untested patients: 23.1% of those in the more-favorable group, 60.2% in the node-negative, less-favorable group and 83.2% in the node-positive group (p<.001). Women with less-favorable disease and intermediate RS results (19-30) reported the highest proportion (22.9% and 20.2% node-negative and node-positive disease, respectively) of a neutral oncologist’s recommendation.
Figure 2. Medical oncologists’ recommendations for adjuvant systemic chemotherapy by clinical and recurrence score (RS) testing subgroups.
Distribution (%) of medical oncologists’ chemotherapy recommendations for chemotherapy (for, neutral, against) estimated from multiply-imputed data. Sample sizes reported are weighted and averaged across multiple imputation iterations. Intermed indicates an intermediate RS.
Factors associated with chemotherapy receipt
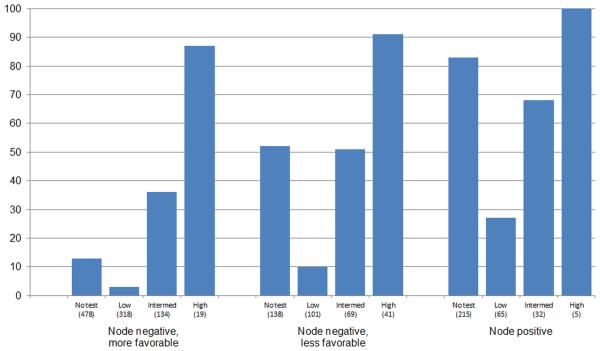
Figure 3 shows the distribution of chemotherapy receipt by clinical and RS groups. The relationship between receipt of chemotherapy and RS was consistent across the 3 clinical subgroups. Most patients with high RS received chemotherapy (87.0%, 91.1%, 100% for node-negative more-favorable, node-negative less-favorable, and node-positive groups). Low scores were associated with low rates of chemotherapy in all clinical subgroups (2.9%, 9.5%, and 26.6%, respectively). Intermediate scores yielded rates between the low and high score groups. Absolute differences in chemotherapy receipt were particularly marked for patients with low RS versus no testing. In node-positive disease, 83.2% of untested women received chemotherapy, versus 27.2% with low RS. In node-negative favorable disease, 13.0% of untested women received chemotherapy versus 3% in women with low RS.
Figure 3. Receipt of adjuvant systemic chemotherapy by clinical and recurrence score (RS) testing subgroups.
Sample sizes below each bar are weighted and averaged across multiple imputed datasets by subgroup. Intermed indicates an intermediate RS.
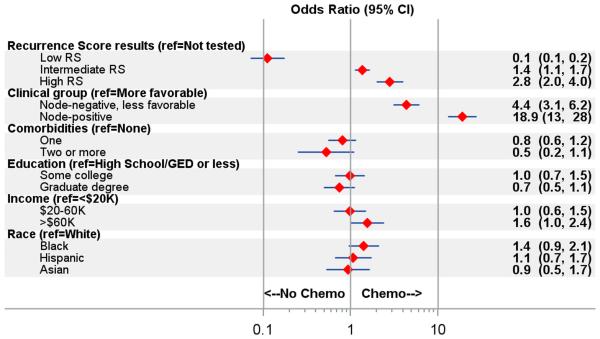
Figure 4 shows results of a multivariable logistic regression model to examine the association between chemotherapy receipt and selected covariates. Receipt of chemotherapy was associated with clinical subgroups and RS scores. Compared with women who did not have RS, women with low-risk RS results were less likely to receive chemotherapy (OR 0.1, 95% CI 0.1-0.2), while women with medium-risk and high-risk RS results were more likely to receive chemotherapy: ORs 1.4 (1.1-1.7) and 2.8 (2.0-4.0), respectively. Compared with women with more-favorable node-negative disease, women with node-negative disease but one unfavorable risk factor were more likely to receive chemotherapy (OR 4.3, 95% CI 3.0-6.1) while women with node-positive disease were considerably more likely to receive chemotherapy (OR 19.08, 95% CI 13.1-27.7). Higher-income patients were more likely to receive chemotherapy than lower-income patients (OR 1.6, 95% 1.0-2.5) but there were no differences in receipt by education or race/ethnicity. To investigate differences by race/ethnicity, we examined chemotherapy receipt by clinical subgroup, RS status, and race/ethnicity (full results not shown). The only subgroup where white women had notably higher rates of chemotherapy receipt than other racial/ethnic groups was for node-positive disease with intermediate RS (79% in whites, 50% in Asian women and Latinas, 20% in black women).
Figure 4. Factors associated with receipt of adjuvant systemic chemotherapy.
Adjusted Odds ratios (95% confidence intervals) estimated using weighted logistic regression model on multiply imputed data. Adjusted for geographic site. Chemo indicates receipt of adjuvant systemic chemotherapy; HS, high school education. Ref indicates reference group; K indicates thousand.
Patient experiences with testing and chemotherapy decisions
We compared observed self-reported RS results to RS test results from Genomic Health, Inc. About three quarters (76.5%) of patients accurately reported RS receipt, and among those who did receive RS, 61.7% correctly reported results by category of low, intermediate, or high risk. Among those who received RS, 63.9% of patients reported that it was “very” or “extremely” helpful. Among 420 women who reported low-risk RS results, 65.0% indicated that RS shifted their opinion against chemotherapy, whereas 73.1% of those who reported high scores reported that their RS result shifted their opinion toward receipt of chemotherapy. Satisfaction with decision-making about RS testing and receipt of chemotherapy was very high (4.4 out of 5.0 for both decisions) and these scores did not differ substantively by whether patients did or did not receive testing or chemotherapy.
DISCUSSION
We examined patient experiences with RS and chemotherapy use in a diverse, contemporary, population-based sample of breast cancer patients. RS use closely followed practice guidelines. A majority of patients with node-negative disease received RS, but fewer node-negative patients with less favorable characteristics (younger age or higher grade) received RS; this may reflect clinicians’ planned chemotherapy use for these higher-risk patients, thus negating the need for RS testing. Substantial RS use for node-positive patients underscores clinicians’ growing support of wider RS use to tailor treatment recommendations, despite guidelines that recommend chemotherapy (and no RS testing) for these patients. These results suggest that clinicians find RS useful when chemotherapy is less clearly indicated. Results from the RxPONDER trial will clarify the clinical utility of testing in patients with node-positive disease.24 The utility of RS in women with tumors < 0.5 cm without adverse features remains unclear.1
RS results correlated strongly with clinician recommendations and receipt of chemotherapy; chemotherapy was recommended in virtually all patients with high scores but discouraged in most patients with low scores. The RS effect appeared greatest in less-favorable disease. Importantly, we observed no marked educational or racial/ethnic gradient in RS testing or treatment. Patient recall of RS results was moderate (60% accuracy) suggesting that many patients deferred integration of RS results to the physician. This suggests an opportunity for targeted educational interventions to improve patient understanding of RS results and their role in patient decision making. Finally, patients were highly satisfied with the RS testing and treatment decision-making process.
Our findings add to prior studies that have examined RS use and treatment in breast cancer. In an Ontario study conducted between 2012-2013, patients and physicians completed surveys before and after RS testing.15 After RS results were shared, oncologists changed their initial recommendation 51% of the time, resulting in lower chemotherapy use. Patients’ decisional uncertainty was reduced after RS testing. Our study findings support low decision uncertainty in a diverse patient population with access to RS testing in the US. In a North Carolina study of women diagnosed with breast cancer between 2008-2013, approximately 40% of patients received RS, with similar rates between node-positive and node-negative patients9; however, RS testing was ascertained by pathology reports alone which may be prone to missing information.25 Our study suggests substantial RS testing and clinical impact in patients with node-positive disease. While investigators have documented high patient and clinician satisfaction with RS testing,26 others have noted substantial variations in the chemotherapy decision-making process.27 Potosky et al. showed that RS results were highly associated with chemotherapy use in a cohort that was treated prior to 2012 and found no socioeconomic disparities; however, few non-white patients were studied.10 Our study confirms the absence of socioeconomic testing differences in a large, diverse, population-based sample. A recent study suggested less than optimal adherence to guidelines with regard to testing and treatment.28 Our study suggests robust uptake of RS testing in guideline-concordant clinical subgroups and provides insight into reasons for testing patterns.
Aspects of our study merit comment. Strengths include a large, contemporary, diverse, population-based sample; a high response rate; valid measures of recurrence score testing (including actual results obtained from the laboratory); clinical and treatment variables; and granular measures of patient experiences and appraisal of testing and treatment. Our analytic techniques reduced potential non-response bias and account for missing data. However, our results are limited to two large geographic regions of the United States. Measures of communication and decision-making were ascertained through patients and do not necessarily represent physician perspectives.
Implications
Our results suggest that a major advance in oncology precision medicine, tumor genomic profiling, may improve treatment decision-making and communication. In the context of early-stage breast cancer, the combination of genomic test results and clinical data now offers more precise targeting of patients for chemotherapy, especially among those with node-negative disease. Additional clarity about the prediction of the marginal benefit of adjuvant chemotherapy in patients with intermediate score range in patients with node negative disease is forthcoming.29
The majority of patients studied reported that their medical oncologist made a recommendation for or against chemotherapy, rather than leaving the decision up to the patient. Personalized recommendations appear to reduce potential overtreatment with chemotherapy and nearly eliminated socioeconomic disparities in treatment, after controlling for clinical factors. This is a notable benefit of incorporating RS into breast cancer treatment algorithms. Oncologists’ commitment to addressing overtreatment may be most evident by the substantial proportion of patients with node-positive disease who received RS, despite current guidelines that advise chemotherapy without RS testing. The impact of RS testing appeared greatest in node-positive patients because their baseline use of chemotherapy was high, such that RS results largely served to identify node-positive patients with low scores for whom chemotherapy might logically be omitted. However, definitive evidence for the benefit of RS testing among node-positive patients awaits the results of clinical trials.24 Finally, our results suggest that many patients rely on their oncologist to incorporate RS results into chemotherapy recommendations, and that patient satisfaction with RS testing and treatment decisions is very high. This underscores another potential impact of precision medicine: to reduce lingering uncertainty and improve the patient experience of treatment decision-making and communication.
ACKNOWLEDGEMENTS
Cancer incidence data collection was supported by the California Department of Public Health (California Health and Safety Code Section 103885); Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, (cooperative agreement 5NU58DP003862-04/DP003862); the NCI’s Surveillance, Epidemiology and End Results Program (contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California (USC), and contract HHSN261201000034C awarded to the Public Health Institute). Cancer incidence data collection in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the NCI, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred.
We thank Steve Shak, MD and Genomic Health, Inc. for collaboration on RS assay test linkage to iCanCare data. We acknowledge our project staff (Mackenzie Crawford and Kiyana Perrino from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, and Renee Bickerstaff-Magee from USC; Rebecca Morrison, Rachel Tocco, Alexandra Jeanpierre, Stefanie Goodell, and Rose Juhasz from the University of Michigan). We acknowledge with gratitude our survey respondents.
Funding: Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health under award number P01CA163233 to the University of Michigan.
Footnotes
Conflict of Interest: Allison W. Kurian has received research funding for work performed outside of the current study from Myriad Genetics, Invitae, Ambry Genetics, GenDx, and Genomic Health.
Author Contributions
Christopher R. Friese: Conceptualization, methodology, investigation, writing-original draft, writing-review and editing, and visualization
Yun Li: Methodology, investigation, software, formal analysis, writing-original draft, and writing-review and editing
Irina Bondarenko: Methodology, investigation, software, formal analysis, writing-original draft, writing-review and editing, and visualization
Tim Hofer: Methodology, investigation, formal analysis, writing-original draft, writing-review and editing
Dennis Deapen: Conceptualization, methodology, investigation, writing-original draft and writing-review and editing
Ann S. Hamilton: Conceptualization, methodology, investigation, writing-original draft and writing-review and editing
Kevin Ward: Conceptualization, methodology, investigation, writing-original draft and writing-review and editing
Allison W. Kurian: Conceptualization, methodology, investigation, writing-original draft, and writing-review and editing
Steven J. Katz: Conceptualization, methodology, investigation, writing-original draft, writing-review and editing, and visualization
REFERENCES
- 1.Pusztai L. Chemotherapy and the recurrence score--results as expected? Nat Rev Clin Oncol. 2015;12:690–692. doi: 10.1038/nrclinonc.2015.191. [DOI] [PubMed] [Google Scholar]
- 2.Hudis CA. Biology before Anatomy in Early Breast Cancer--Precisely the Point. N Engl J Med. 2015;373:2079–2080. doi: 10.1056/NEJMe1512092. [DOI] [PubMed] [Google Scholar]
- 3.Network NCC NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [accessed May 4, 2016]
- 4.Harris LN, Ismaila N, McShane LM, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH. Initial Trends in the Use of the 21-Gene Recurrence Score Assay for Patients With Breast Cancer in the Medicare Population, 2005-2009. JAMA Oncol. 2015;1:158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 8.Roberts MC, Weinberger M, Dusetzina SB, et al. Racial variation in adjuvant chemotherapy initiation among breast cancer patients receiving oncotype DX testing. Breast Cancer Res Treat. 2015;153:191–200. doi: 10.1007/s10549-015-3518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts MC, Weinberger M, Dusetzina SB, et al. Racial Variation in the Uptake of Oncotype DX Testing for Early-Stage Breast Cancer. J Clin Oncol. 2016;34:130–138. doi: 10.1200/JCO.2015.63.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potosky AL, O'Neill SC, Isaacs C, et al. Population-based study of the effect of gene expression profiling on adjuvant chemotherapy use in breast cancer patients under the age of 65 years. Cancer. 2015;121:4062–4070. doi: 10.1002/cncr.29621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan MA, Mi X, Reed SD, Lyman GH, Curtis LH. Association Between Use of the 21-Gene Recurrence Score Assay and Receipt of Chemotherapy Among Medicare Beneficiaries With Early-Stage Breast Cancer, 2005-2009. JAMA Oncol. 2015;1:1098–1109. doi: 10.1001/jamaoncol.2015.2722. [DOI] [PubMed] [Google Scholar]
- 12.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18:3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 13.Markopoulos C. Overview of the use of Oncotype DX((R)) as an additional treatment decision tool in early breast cancer. Expert Rev Anticancer Ther. 2013;13:179–194. doi: 10.1586/era.12.174. [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine MN, Julian JA, Bedard PL, et al. Prospective Evaluation of the 21-Gene Recurrence Score Assay for Breast Cancer Decision-Making in Ontario. J Clin Oncol. 2016;34:1065–1071. doi: 10.1200/JCO.2015.62.8503. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Surveillance and Outcomes Research Team CanSORT: Cancer Surveillance and Outcomes Research Team. Available from URL: http:\cansort.med.umich.edu [accessed Jun 2, 2015]
- 17.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302:1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. 2014 [Google Scholar]
- 19.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18:2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. Journal of Clinical Oncology. 2016 doi: 10.1200/JCO.2015.65.8013. [Epub ahead of print] DOI: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 21.Groves RM, Fowler FJ, Jr, Couper MP, Lepkowski JM, Singer E, Torangeau R. Survey Methodology. John Wiley & Sons; 2011. [Google Scholar]
- 22.Raghunathan TE, Lepkowski JM, VanHoewyk J, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; Hoboken, NJ: 1987. [Google Scholar]
- 24.Sun Z, Prat A, Cheang MC, Gelber RD, Perou CM. Chemotherapy benefit for 'ER-positive' breast cancer and contamination of nonluminal subtypes-waiting for TAILORx and RxPONDER. Ann Oncol. 2015;26:70–74. doi: 10.1093/annonc/mdu493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shak S, Petkov V, Miller DP, et al. Breast cancer specific mortality in patients with early-stage hormone receptor–positive invasive breast cancer and oncotype DX recurrence score results in the SEER database; American Society of Clinical Oncology Qualitiy Care Symposium; Phoenix, AZ. 2016. [Google Scholar]
- 26.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 27.Hamelinck VC, Bastiaannet E, Pieterse AH, et al. Patients' preferences for surgical and adjuvant systemic treatment in early breast cancer: a systematic review. Cancer Treat Rev. 2014;40:1005–1018. doi: 10.1016/j.ctrv.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Ray GT, Mandelblatt J, Habel LA, et al. Breast cancer multigene testing trends and impact on chemotherapy use. Am J Manag Care. 2016;22:e153–e160. [PMC free article] [PubMed] [Google Scholar]
- 29.National Cancer Institute The TAILORx Breast Cancer Trial. Available from URL: http://www.cancer.gov/types/breast/research/tailorx [accessed June 20, 2016]