Summary
Astrocytes associate with synapses throughout the brain and express receptors for neurotransmitters that can elevate intracellular calcium (Ca2+) 1-3. Astrocyte Ca2+ signaling has been proposed to modulate neural circuit activity 4, but pathways regulating these events are poorly defined and in vivo evidence linking changes in astrocyte Ca2+ to alterations in neurotransmission or behaviors is limited. Here we show Drosophila astrocytes exhibit activity-regulated Ca2+ signaling events in vivo. Tyramine (Tyr) and octopamine (Oct) released from Tdc2+ neurons signal directly to astrocytes to stimulate Ca2+ increases through the octopamine-tyramine receptor (Oct-TyrR) and the TRP channel Waterwitch (Wtrw), and astrocytes in turn modulate downstream dopaminergic (DA) neurons. Tyr or Oct application to live preparations silenced dopaminergic (DA) neurons and this inhibition required astrocytic Oct-TyrR and Wtrw. Increasing astrocyte Ca2+ signaling was sufficient to silence DA neuron activity, which was mediated by astrocyte endocytic function and adenosine receptors. Selective disruption of Oct-TyrR or Wtrw expression in astrocytes blocked astrocyte Ca2+ signaling and profoundly altered olfactory-driven chemotaxis behavior and touch-induced startle responses. Our work identifies Oct-TyrR and Wtrw as key components of the astrocyte Ca2+ signaling machinery, provides direct evidence that Oct- and Tyr-based neuromodulation can be mediated by astrocytes, and demonstrates that astrocytes are essential for multiple sensory-driven behaviors.
Astrocytes are intimately associated with brain synapses and positioned to broadly regulate synaptic activity. It has been widely proposed that astrocytes modulate neural circuits and behavior 5-7, although in vivo data demonstrating astrocytes are directly activated by neurotransmission and signal back to neurons to modulate output remains elusive. Astrocytes exhibit dynamic fluctuations in intracellular Ca2+ in vitro 8,9 and in vivo 10,11 suggesting Ca2+ signaling might be a useful measure of astrocyte “activity”. Despite decades of studies on astrocytic Ca2+ transients, signaling pathways controlling these transients remain poorly defined, and their in vivo relevance remains controversial. If astrocytes indeed actively participate in information processing in circuits, it is imperative we characterize such mechanisms as they would represent a potentially widespread mechanism for controlling brain function.
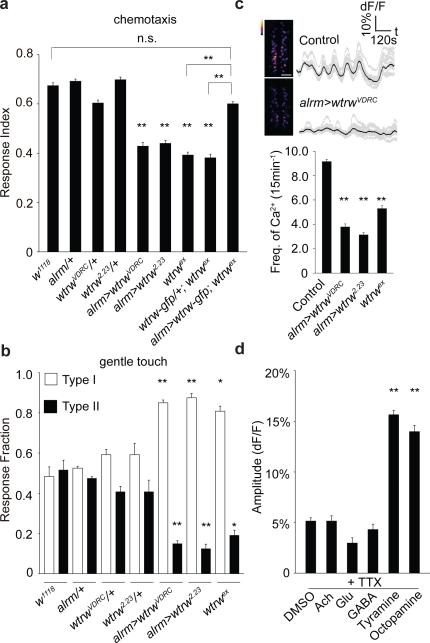
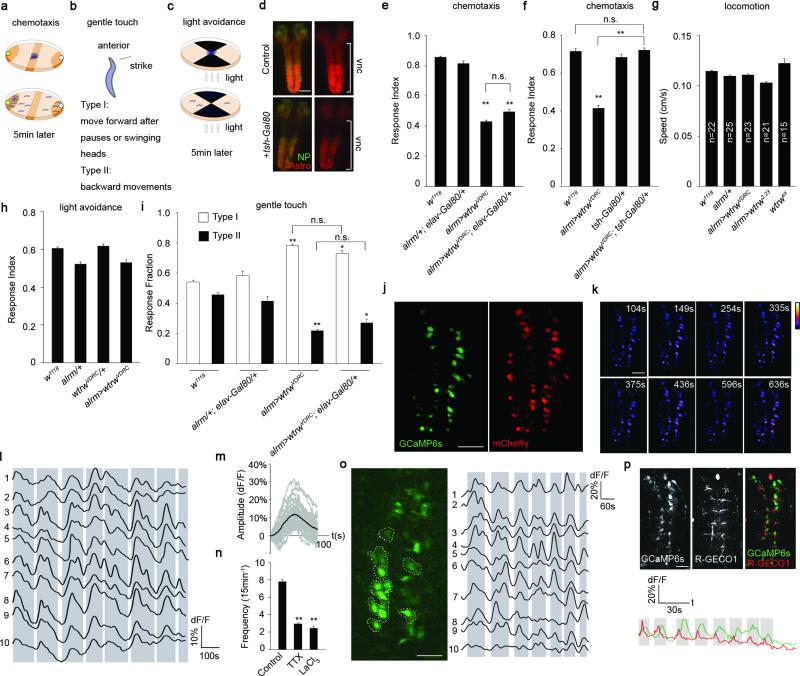
We performed an RNAi-based screen in Drosophila where we individually knocked-down ~500 Ca2+ signaling-related genes selectively in astrocytes using the astrocyte-specific Gal4 driver line alrm-Gal4 12, and assayed larval olfactory-driven chemotaxis behavior (Extended Data Fig. 1a). We found astrocyte knockdown of the TRP channel Water witch (Wtrw)13 led to a significant (~50%) decrease in larval chemotaxis toward iso-amyl acetate (IAA)(Fig. 1a). Similar results were found with a second, non-overlapping RNAi construct (Fig. 1a) and was specific to glia as shown by insensitivity to blockade of Gal4/UAS in neurons with elav-Gal80 (Extended Data Fig. 1e). The null allele wtrwex was equally defective in chemotaxis, which was rescued by re-expression of wtrw only in astrocytes (Fig. 1a). We also found that blockade of Gal4/UAS driven wtrwRNAi with tsh-Gal80 in the ventral nerve cord (VNC) resulted in normal chemotaxis responses (Extended Data Fig. 1f), revealing a critical role for VNC astrocytes in this behavior, although this does not exclude an additional role for brain astrocytes. Chemotaxis defects did not result from simple alterations in motility, as wtrwRNAi animals exhibited normal locomotion (Extended Data Fig. 1g) and light avoidance responses (Extended Data Fig. 1c,h). We conclude that Wtrw is required in astrocytes for normal larval olfactory-driven behavior.
Figure 1. Larval chemotaxis and startle-induced responses require the astrocyte-expressed TRP channel Water witch.
a, Chemotaxis assay (n=12). b, Gentle touch assay (n=30). c, Pseudocolored maximum intensity projections of 15min movies, averaged traces of 16 individual astrocytes and quantifications of the frequency of somatic Ca2+ transients (n=10, 160 cells total). Scale bar, 50μm. d, Responses of astrocytes to neurotransmitters/neuromodulators in the presence of tetrodotoxin (n=6, 96 cells total). *p<0.05, **p<0.01, n.s., not significant, Error bars, s.e.m. Wilcoxon and Mann-Whitney tests followed by Bonferroni-Holm post hoc test (a). One-way ANOVA followed by Tukey's post hoc test (b,c,d).
Ca2+ transients in mammalian astrocytes in awake behaving mice are induced during periods of elevated arousal 14-16. We therefore used a gentle anterior touch assay17 to examine the role of astrocyte Ca2+ signaling in larval startle-induced behaviors. Crawling larvae touched anteriorly with a hair responded by pausing and continuing forward (Type I response), or by moving backward and executing an escape response (Type II response) (Extended Data Fig. 1b). Control animals exhibited roughly equal frequencies of Type I and Type II responses, however, wtrwRNAi in astrocytes resulted in a dramatic alteration in behavior: ~80% of larvae exhibited Type I responses, a phenomenon mimicked by wtrwex mutants (Fig. 1b) and the phenotype is independent of neurons (Extended Data Fig. 1i). These data indicate astrocyte expressed Wtrw also modulates startle-induced behavioral changes in Drosophila larvae.
To explore in vivo Ca2+ signaling we developed a semi-dissected preparation to image the larval central nervous system (CNS). GCaMP6s was used to image astrocyte cytosolic Ca2+ changes (UAS-GCaMP6s), mCherry (UAS-mCherry) was used as a reference for astrocyte position, and dorsal astrocyte cell bodies were imaged in the VNC. We found astrocyte cell bodies exhibited coordinated, population-wide slow oscillations (termed somatic Ca2+ transients) (Extended Data Fig. 1j-I; Supplementary video 1). Somatic Ca2+ transients occurred approximately every 2 minutes and exhibited an average ~11% change in dF/F (Extended Data Fig. 1m). Interestingly, blocking neuronal activity with tetrodotoxin (TTX) suppressed transients by approximately 60%, as did application of the broad Ca2+ channel blocker lanthanum chloride (LaCl3)(Extended Data Fig. 1n). Similar astrocyte Ca2+ transients were observed when we imaged intact immobilized larvae (Extended Data Fig. 1o), indicating our dissected preparation preserves in vivo patterns of astrocyte activity.
TRP channels regulate Ca2+ levels in astrocytes 18, we therefore reasoned Wtrw might drive Ca2+ signaling in Drosophila astrocytes. Control larvae exhibited 8-9 rhythmic oscillations in somatic Ca2+ transients over 15 minutes. In contrast, astrocyte-specific wtrwRNAi led to a roughly 50% decrease in somatic astrocyte Ca2+ transients, which was also observed in the wtrwex mutant (Fig. 1c). Bath application of acetylcholine, glutamate, and γ-aminobutyric acid in the presence of TTX did not elicit a change in Ca2+ levels in astrocytes. In contrast, application of tyramine (Tyr) or octopamine (Oct), the invertebrate analogues of norepinephrine, which has been shown to induce Ca2+ transients in mammalian astrocytes 14,15,19,20, potently elevated somatic Ca2+ levels in astrocytes (Fig. 1d), indicating astrocyte somatic Ca2+ signaling is regulated by these neuromodulators.
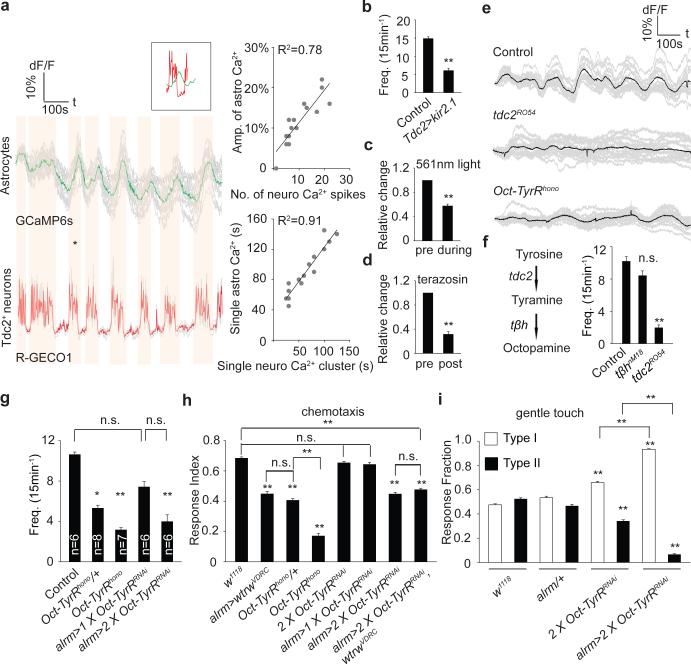
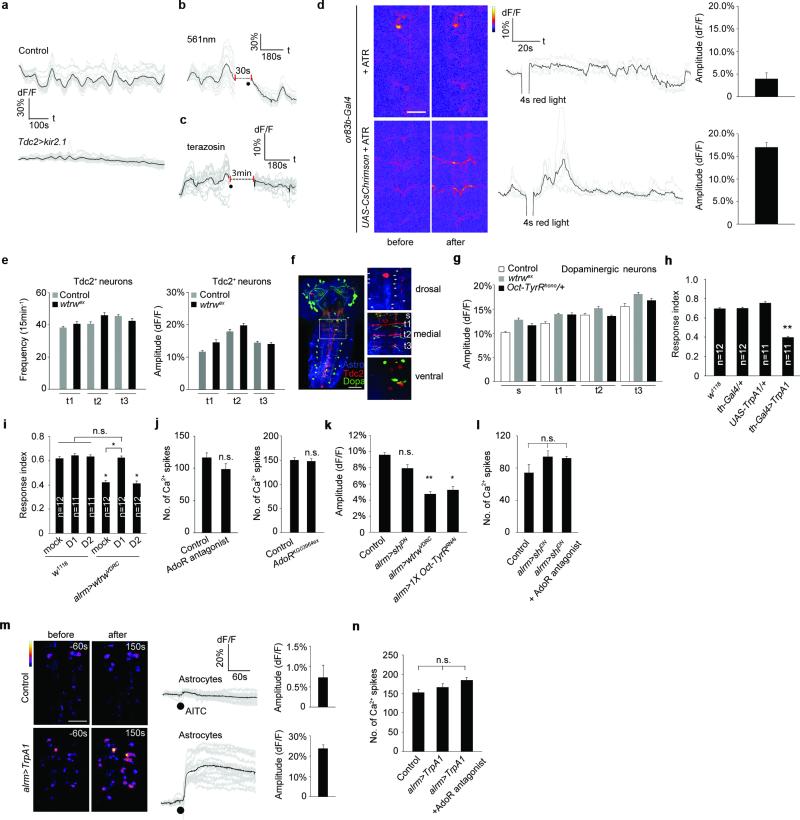
Tdc2+ neurons are the only known source of Tyr and Oct in the larval VNC 21. To explore their relationship with astrocytes we expressed the red-shifted Ca2+ indicator R-GECO1 in Tdc2+ neurons (using tdc2-LexA/LexAop-R-GECO1) and GCaMP6s in astrocytes (using alrm-Gal4/UAS-GCaMP6s) and examined in vivo activity. We observed a striking positive correlation between Tdc2+ neuron activity and somatic astrocyte Ca2+: when Tdc2+ neurons were active, astrocyte somatic Ca2+ levels increased, and when Tdc2+ neurons were silent, astrocyte somatic Ca2+ levels decreased (Fig. 2a; Supplementary video 2). A similar correlation was observed in intact larvae (Extended Data Fig. 1p). Moreover, the amplitude and duration of somatic astrocyte Ca2+ rise was tightly correlated with Ca2+ spikes in Tdc2+ neurons (Fig. 2a). When we chronically silenced Tdc2+ neurons by expressing the K+ leak channel Kir2.122, rhythmic oscillations in astrocyte Ca2+ were eliminated (Fig. 2b; Extended Data Fig. 2a); and acute optogenetic blockade of Tdc2+ neuron activity using halorhodopsin (tdc2-Gal4>UAS-NpHR) led to a decrease in astrocyte somatic Ca2+ (Fig. 2c; Extended Data Fig. 2b). Interestingly, astrocyte Ca2+ signaling was also blocked by the α1 adrenergic receptor antagonist terazosin (Fig. 2d; Extended Data Fig. 2c), which inhibits arousal-evoked Ca2+ responses in cortical astrocytes in mammals 14,15, further supporting the notion that astrocyte modulation is highly conserved between species. Consistent with astrocyte Ca2+ signaling being regulated by Oct and Tyr, we found somatic astrocyte Ca2+ transients were nearly absent (~80% reduced) in tdc2RO54 mutants, which lack both Tyr and Oct, although they persisted in tβhnM18 mutants, which lack Oct but retain Tyr signaling (Fig. 2e,f). Finally, Tdc2+ neurons were activated when olfactory neurons were optogenetically stimulated in intact larvae (using or83b-Gal4), suggesting sensory cues flow to Tdc2+ neurons, which are upstream of astrocyte Ca2+ signaling (Extended Data Fig. 2d).
Figure 2. Tdc2+ neurons fire rhythmically to drive astrocyte somatic Ca2+ transients through the octopamine-tyramine receptor (Oct-TyrR).
a, Correlation analyses of neuronal activity and somatic Ca2+ transients in astrocytes. Averaged traces of 16 individual astrocytes (GCaMP6s) and 4 pairs of Tdc2+ neurites (R-GECO1) from the same sample. Vertical orange bars highlight concomitant activity in astrocytes and neurons (* marked events are shown in inset). Amplitude and duration of individual Ca2+ transients in astrocytes correlate highly with activity of Tdc2+ neurons. b, Frequency of Ca2+ transients in astrocytes (n=10,160 cells total). c,d, Relative changes in numbers of Ca2+ transients after acute blockade of octopamine/tyramine signaling by halorhodopsin (c) or terazosin (d) (n=6, 96 cells total). Black dots, when either 561nm light or terazosin was delivered. e, Representative traces of astrocytic Ca2+ transients in mutants defective in tyramine/octopamine signaling. f,g, Frequency of Ca2+ transients in tdc2RO54, tβhnM18 (f, n=6, 96 cells total) and Oct-TyrRhono mutants (g, n listed for each genotype, 16n cells total). h, Chemotaxis assay (n=12). i, Gentle touch assay (n=30). *p<0.05, **p<0.01, n.s., not significant. Error bar, s.e.m. Paired t-test (c,d), one-way ANOVA followed by Tukey's post hoc test (b,f,g,h,i).
To determine whether Tdc2+ neurons signal directly to astrocytes we knocked down the two Tyr receptors TyrR and TyrRII, and Oct-TyrR, a dual specificity receptor capable of binding both Oct and Tyr 23,24, in astrocytes and assayed Ca2+ signaling. Depletion of TyrR or TyrRII had no effect (Data not shown), however depletion of Oct-TyrR strongly suppressed astrocyte somatic Ca2+ transients. We observed strong inhibition of astrocyte somatic Ca2+ transients in the Oct-TyrRhono homozygous mutant, and in Oct-TyrRhono/+ heterozygous mutants, which were previously shown to have dominant effects 25 (Fig. 2g). Loss of one copy of Oct-TyrR (Oct-TyrRhono/+) also led to deficits in chemotaxis behavior similar to those observed with astrocyte-specific knockdown of wtrw; these behavioral changes were enhanced in the Oct-TyrRhono homozygous mutant, or by astrocyte-specific knockdown of Oct-TyrR with two copies of an Oct-TyrR RNAi construct. Co-expression of Oct-TyrRRNAi and wtrwRNAi, did not enhance chemotaxis defects, suggesting they act in a common genetic pathway (Fig. 2h). Finally, astrocyte-specific depletion of Oct-TyrR led to a strong inhibition of Type II escape responses in the gentle anterior touch assay (Fig. 2i). We conclude Wtrw and Oct-TyrR are required in astrocytes to trigger somatic Ca2+ transients in response to Oct and Tyr, and astrocyte Wtrw and Oct-TyrR are essential for normal olfactory-driven chemotaxis and startle-induced escape responses.
Astrocytes support neuronal function in many ways and it is plausible that alterations in astrocyte Ca2+ signaling might modify upstream Tdc2+ neuron firing to influence behaviors. We therefore examined the frequency and amplitude of firing events in Tdc2+ neurons in wtrwex null mutants and found no alterations compared to controls (Extended Data Fig. 2e). That Tdc2+ neurons fire normally in wtrw mutants indicates that blocking astrocyte Ca2+ signaling does not lead to a global disruption of neuronal activity in the larval CNS.
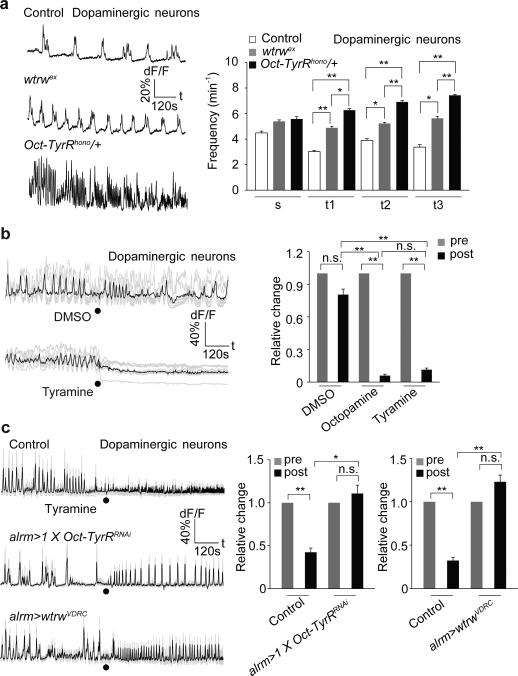
Biogenic amines often regulate dopaminergic (DA) neurons 26. Triple labeling revealed that astrocytes, Tdc2+ neurons and DA neurons are in close proximity in the larval CNS (Extended Data Fig. 2f). When we imaged DA neuron activity (using th-Gal4/UAS-GCaMP6s) we found wtrwex mutants exhibited a significant increase (~50-70%) in the frequency of DA Ca2+ transients in multiple segmental clusters of TH+ cells. A similar phenotype was observed in Oct-TyrRhono/+ mutants (Fig. 3a), although there were no significant changes in the average amplitude of DA neuron responses (Extended Data Fig. 2g). Thus Wtrw and Oct-TyrR negatively regulate DA neuron firing. Consistent with this observation, we found bath application of Oct or Tyr, but not vehicle (DMSO) to larval CNS suppressed DA neuron activity within seconds (Fig. 3b). Strikingly, depletion of the Oct-TyrR and Wtrw specifically from astrocytes using RNAi potently suppressed Tyr-induced Ca2+ elevation in astrocytes and silencing of DA neurons (Fig. 3c; Extended Data Fig. 2k). These data indicate that Tyr signals through astrocyte Oct-TyrR and Wtrw to inhibit downstream DA neuron activity. To explore how DA neuron modulation regulates larval chemotaxis behavior we performed a series of acute manipulations. We first expressed the temperature sensitive channel TrpA1 in DA neurons and found after 24-hour ectopic activation of DA neurons larvae exhibited profound chemotaxis defects similar to those observed when Wtrw or Oct-TyrR were depleted from astrocytes (Extended Data Fig. 2h). In addition, feeding larvae a dopamine D1-like receptor antagonist rescued deficits associated with wtrwRNAi in astrocytes in chemotaxis assays (Extended Data Fig. 2i). These data support the notion that the increased DA neuron activity observed after depletion of astrocyte Oct-TyrR and Wtrw is responsible for defects in chemotaxis behavior.
Figure 3. Astrocytes mediate tyramine-induced inhibition of dopaminergic neurons through Oct-TyrR.
a, Enhanced activity of dopaminergic neurons in wtrwex mutant, Oct-TyrRhono /+ heterozygotes (n=10, 80 neurites). s, subesophageal segments. t, thoracic segments. b, Tyramine and octopamine (2.5mM) inhibit the activity of dopaminergic neurons (n=6, 48 neurites). c, Astrocyte-specific RNAi for Oct-TyrR or wtrw attenuates inhibition of tyramine on dopaminergic neurons (n=6, 48 neurites). Black dots, when tyramine was perfused. *p<0.05, **p<0.01, n.s., not significant. Error bar, s.e.m. Paired t-test (b, same treatment, c, same genotype), one-way ANOVA followed by Tukey's post hoc test (a,b,c).
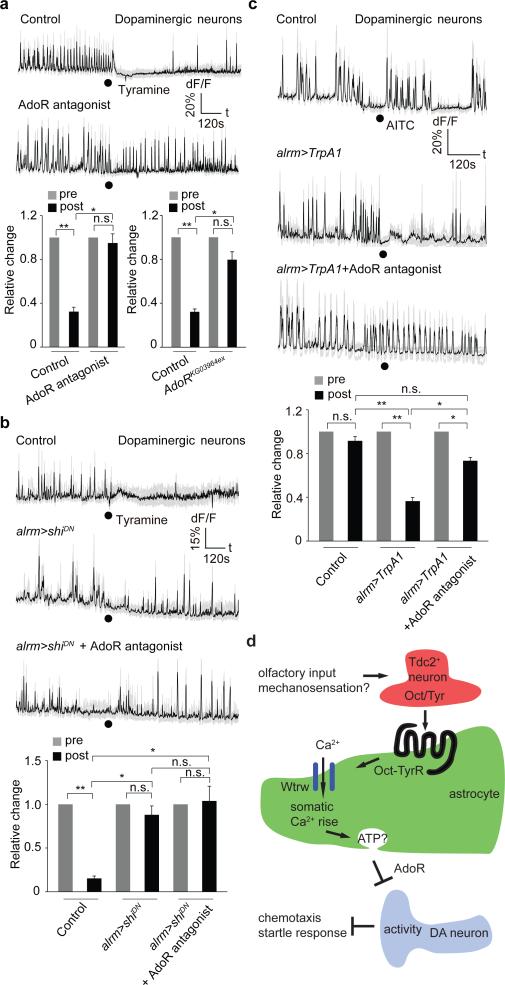
How do astrocytes regulate DA neuron firing? Astrocytic release of ATP and signaling through purinergic receptors is one mechanism by which astrocytes appear to modulate neuronal activity 27,28. The Drosophila genome contains a single seven transmembrane purinergic receptor, AdoR, that is most similar to mammalian adenosine receptors 29. Pretreatment of larval CNS preparations with the AdoR antagonist SCH-442416 blocked Tyr-induced DA neuron silencing without altering overall DA neuron activity before Tyr treatment. Similar results were observed in AdoR null mutants (Fig. 4a; Extended Data Fig. 2j). Purinergic signaling often results from vesicular release of ATP, which can be hydrolyzed to adenosine by ectoenzymes. We manipulated exocytosis selectively in astrocytes using a dominant negative version of the dynamin Shibire (ShiDN) and found this suppress the ability of Tyr to silence DA neurons. This blockade was not enhanced further by application of AdoR antagonists and had no effect on baseline DA neuron activity before Tyr treatment (Fig. 4b; Extended Data Fig. 2l). Finally, to determine whether astrocyte Ca2+ signaling through TRP channels is sufficient to suppress DA neuron firing we expressed the wasabi (AITC)-sensitive Drosophila TrpA1 channel in astrocytes and assayed the effects of AITC application on astrocyte Ca2+ signaling and DA neuron firing. Exposure of control animals to AITC did not alter astrocyte Ca2+ levels, however application of AITC to preparations expressing TrpA1 in astrocytes led to an increase in GCaMP6s signals of astrocytes of ~20% (Extended Data Fig. 2m), similar to that elicited by Tyr application (Fig. 1d). Moreover, AITC activation of astrocyte Ca2+ signaling resulted in a strong suppression of DA neuron activity. This effect was reversible by application of AdoR antagonists and did not occur when AITC was applied to control (i.e. non-TrpA1 expressing) animals (Fig. 4c; Extended Data Figure 2n). Thus TrpA1 channel mediated increases in astrocyte Ca2+ signaling are sufficient to silence DA neurons in vivo. DA neuron silencing is likely be mediated by ATP release from astrocytes and subsequent activation of AdoR on DA neurons.
Figure 4. Tyramine mediated inhibition of dopaminergic neurons depends on adenosine receptor and glial endocytic function.
a, AdoR is required for tyramine mediated inhibition of dopaminergic neurons (n=6, 48 neurites). b, Blockade of endocytic function by dominant negative shibire (shiDN) attenuates inhibition of tyramine on dopaminergic neurons (n=6, 48 neurites). Black dots, when tyramine was perfused. c, Ca2+ influx to astrocytes through TrpA1 is sufficient to inhibit dopaminergic neurons (n=6, 48 neurites). Black dots, when AITC was perfused. d, Model. Olfactory and likely mechanosensory information flow towards Tdc2+ neurons that release Oct and Tyr to activate the Oct-TyrR on astrocytes and in turn astrocyte Ca2+ entry through the TRP channel Wtrw. Increase in astrocyte Ca2+ is sufficient to silence DA neurons through a mechanism requiring the adenosine receptor AdoR, potentially through astrocyte ATP release and its breakdown to adenosine. Inhibition of DA neuron activity by astrocytes is essential for normal chemotaxis and startle-induced reversal behaviors. *p<0.05, **p<0.01, n.s., not significant. Error bar, s.e.m. Paired t test (a,b,c, same genotype), Wilcoxon and Mann-Whitney tests followed by Bonferroni-Holm post hoc test (a,b), one-way ANOVA followed by Tukey's post hoc test (c).
It has been assumed that neuromodulators alter neural circuit activity and behaviors by directly acting on neurons; however, astrocytes (and other glia) express a diverse array of neuromodulatory receptors suggesting they may contribute to the global effects of these neurotransmitters. In this study we show neuromodulatory signaling in at least some cases flows through astrocytes. We demonstrate Drosophila astrocytes exhibit robust Ca2+ signaling events remarkably similar to those observed in awake behaving mice 14-16. Similar to the global activation of astrocyte Ca2+ signaling by norepinephrine in mammals, release of tyramine (and likely octopamine) in vivo triggers synchronous activation of astrocytes. We identify Wtrw and Oct-TyrR as key components of the astrocyte Ca2+ signaling axis and their loss of function phenotypes provide direct evidence that astrocyte Ca2+ signaling modulates animal behaviors. Furthermore, we show that during suppression of DA neuron activity, Oct/Tyr signal directly to astrocytes, and astrocyte Ca2+ transients execute neuromodulatory events downstream (Fig. 4d). Octopamine and tyramine have been linked to arousal and aggression in insects and are considered to be functionally equivalent to norepinephrine in mammals. The profound modulation of astrocyte Ca2+ levels by norepinephrine in mammals 14-16 suggests that the Tyr/Oct/norepinephrine-mediated activation of astrocyte Ca2+ signaling, and in turn astrocyte-based neuromodulation, are ancient features of the metazoan nervous system. A reassessment of the cellular basis of neuromodulation is therefore warranted, with further reflection on direct roles for astrocytes in transducing neuromodulatory signals in neural circuits.
Material and Methods
Fly stocks and husbandry
Flies were cultured in standard cornmeal food at 25°C in 12h/12h light/dark cycles. Fly stocks include: w1118, alrm-Gal4 26, alrm-LexA::GAD, UAS-wtrw-gfp, LexAop-R-GECO1 (Bloomington, 52224), tdc2-Gal4 (Bloomington, 9313), 20XUAS-IVS-GCaMP6s (Bloomington, 42746), 13XLexAop2-IVS-GCaMP6s-p10 (Bloomington, 44274), UAS-Kir2.1-EGFP (Bloomington, 6595), UAS-Oct-TyrRRNAi (Bloomington, 28332), th-Gal4 (Bloomington, 8848), UAS-wtrwVDRC (VDRC, 107423), Oct-TyrRhono (Kyoto, 109038), UAS-shiDN (Bloomington, 5822), UAS-TrpA1 (Bloomington, 26263), UAS-NpHR (Kyoto, 117008), UAS-CsChrimson (Bloomington, 55135), AdoRKG03964ex (Bloomington, 30868), tdc2-LexA::p65 27, UAS-wtrw2.23 28, wtrwex 29, tβhnM18 30, tdc2RO54 31, th-LexA::p65 32, tsh-Gal80 33.
Behavioral tests
Chemotaxis and phototaxis
These two assays were performed using protocols described previously with minor modifications 34. Briefly, pools of ~100 3rd instar larvae (108-120h after egg lay) were allowed to freely move for 5 minutes on petri dishes with according settings for chemotaxis assay or phototaxis assay, then the numbers of larvae in odorant/vehicle circles, or in light/dark quadrants were scored respectively. Response indices were calculated as: RIchemotaxis=(Nodor-Nvehicle)/(Nodor+Nvehicle), RIphototaxis=(Ndark-Nlight)/(Ndark+Nlight). Isoamyl acetate (IAA) was diluted to 1:5000 in mineral oil, and pure mineral oil was used as the control. Light intensity in phototaxis assays was 2400 lux white light (5400K). Thermogenetic manipulation of dopaminergic neurons was accomplished by transferring 3rd instar larvae from 18°C to 25°C to allow activation of TrpA1 for 24h before testing. For chemotaxis assays related to D1-like or D2-like dopamine receptor inhibitors 3rd instar larvae were fed on cornmeal food mixed with inhibitors for 24h before testing.
Gentle touch assay
Adapted from 35, early 3rd instar larvae (84-96h after egg lay) were struck in the thoracic segments with a hair while moving straight. No response, a stop, head retraction and turn were grouped into type I response, initiation of at least one single full body retraction or multiple full body retractions were categorized as type II reversal responses.
Larval motility
3rd instar larvae (108-120h after egg lay) were videotaped and the maximal velocity was calculated by dividing the travel distance measured when larvae move straight without pause by time.
Molecular biology and transgenic flies
pUAST-wtrw::gfp was made by cloning full length wtrw tagged with gfp at C terminal into pUAST. Transgenic flies were obtained by standard germline injection (BestGene Inc, Chino Hills, CA).
Chemicals, buffers and antibodies
For live preparation studies all the chemicals were diluted in live imaging buffer (110mM NaCl, 5.4mM KCl, 1.2mM CaCl2, 0.8mM MgCl2, 10mM D-glucose, 10 mM HEPES, pH adjusted to 7.2), including 1μM tetrodotoxin, 0.1mM LaCl3, 2.5mM octopamine, 2.5mM glutamate, 2.5mM acetylcholine, 2.5mM GABA, 150μM AITC, 10μM SCH-442416, 1mM terazosin, 0.5mM tyramine was used unless otherwise stated. 0.2mg/ml SCH-23390 (dopamine D1 like receptor antagonist), 0.2mg/ml Eticlopride (dopamine D2 like receptor antagonist), 10mM all trans retinal. Rabbit anti-GAT was used at 1:5000.
Live imaging and data analysis
1st instar larvae were immersed in halocarbon oil 27 and immobilized between coverslips and slides to image the activity of neurons and astrocytes through the cuticle in intact larvae. 3rd instar wandering stage larvae were used to dissect the CNS. Briefly, intact CNS were transferred to a silicon pad after dissection and incubated with 100μl live imaging buffer. Samples were allowed to sit at room temperature for 15 minutes before imaging. To examine the effects of lanthanum chloride (LaCl3) or tetrodotoxin (TTX) on calcium transients, samples were pre-incubated in buffer containing chemicals for 15 minutes before imaging. Movies were acquired by using Volocity software on a spinning disc microscope equipped with a 20X (dissected CNS) or 40X (intact larvae) water immersion objective at 2 frames/second. Changes of GCaMP intensity of ROIs were calculated and expressed as (Ft1...n-F)/F (first 10 frames were averaged as F), which then were plotted over time. Plotted traces were semi-automatically analyzed by the multiple peak fitting function of Igor Pro (WaveMetrics Inc., OR) to obtain the numbers and amplitudes of peaks.
Bath application of drugs
Samples were prepared as described above, incubated with imaging buffer (with/without 1μM TTX). At ~240s (astrocytes) or ~300s (neurons) after imaging, 100μl live imaging buffer containing 2X final concentrations of chemicals (with/without 1μM TTX) were directly added to the medium, and imaging continued. GCaMP6s intensity of ROIs were plotted and analyzed as mentioned above. For terazosin experiments we first examined preparations for 6 min (control, pre-exposure window), terazosin was perfused for 3 min, and we then imaged for an additional 6min (exposed window). Data points of 6-9min that represented the perfusion window were omitted in graph.
Optogenetic activation/inactivation of neurons
For CsChrimson activation of olfactory neurons larvae were grown on cornmeal food daily topped with 100 μl all trans retinal for 3 days. Early 3rd instar larvae were immobilized between coverslips and slides in halocarbon oil 27, activity of Tdc2+ neurons reflected by GCaMP6s were continuously monitored except when red light (640nm, 3.41mW/ mm2) was delivered through an epifluorescence light source. Halorhodopsin mediated inhibition of Tdc2 neurons was accomplished by growing larvae on cornmeal food topped daily with 100 μl all trans retinal for 4-5 days. Larval brains were dissected from wandering 3rd instar larvae and prepared as described above for imaging somatic calcium transients in astrocytes. GCamP6s signals were recorded for 6 minutes, then halorhodopsin was activated (within ~30 seconds) by alternating delivering 561nm (0.26mW/mm2, 3s, for photoinhibition) and blue light 488nm (500msec, for imaging GCaMP6s labeled astrocytes) for another 6min. Data points from two consecutive movies were then assembled together to show changes of somatic calcium transients.
Statistical analysis
No statistical methods were used to predetermine sample size. All n numbers represent biological replicates. The experiments were not randomized or blinded. The Shapiro–Wilk test (normal distribution if P>0.05) was used to determine the normality of data. Statistical comparisons were performed using one-way ANOVA followed by Tukey's post hoc test or paired Student's t-test (two-tailed). Non-normally distributed data were compared using Wilcoxon and Mann-Whitney tests followed by Bonferroni-Holm post hoc test. P values less than 0.05 were considered significant. All the data in bar graphs are expressed as mean ± s.e.m.
Extended Data
Extended Data Figure 1. Synchronous somatic Ca2+ transients in Drosophila astrocytes.
a-c, Brief diagrams of behavioral tests. d, tsh-Gal80 suppression of alrm-Gal4 activity. Nc82, neuropil (NP). Astrocytes (Astro), alrm>myr::mtdTomato. Vnc, ventral nerve cord. Scale bar, 50μm. e,f, Chemotaxis assay (n=12). g, Locomotion assay (n listed). h, Light avoidance assay (n=12). i, Gentle touch assay (n=24). j, GCaMP6s and mCherry expression in astrocytes. Scale bar, 50μm. k, Representative pseudocolored images of 4 continuous Ca2+ transients. Scale bar, 50μm. l, Traces of normalized GCaMP6s intensity over mCherry of 10 individual astrocytes in 15min live imaging window. m, Averaged traces of individual somatic Ca2+ transients. n, somatic Ca2+ transients in astrocytes with treatments of TTX and LaCl3 (n=10, 160 cells). o, GCaMP6s expression in astrocytes and traces of 10 individual astrocytes from an intact larva. Scale bar, 20μm. Grey bars (l,o) represent population rise/fall in GCaMP6s signals. p, GCaMP6s labeled astrocytes and R-GECO1 labeled Tdc2+ neurites and their averaged traces in an intact larva. Scale bar, 20μm. *p<0.05, **p<0.01, n.s., not significant. Error bar, s.e.m. Wilcoxon and Mann-Whitney tests followed by Bonferroni-Holm post hoc test (e,n), one-way ANOVA followed by Tukey's post hoc test (f,g,h,i).
Extended Data Figure 2. Somatic Ca2+ transients of astrocytes inhibit the activity of dopaminergic neurons.
a-c, Representative traces of astrocyte Ca2+ transients with blockade of tyramine/octopamine signaling. d, Stimulation of olfactory neurons activates Tdc2+ neurons (n=3-4). Scale bar, 25μm. e, Activity of Tdc2+ neurons are not altered in wtrw mutants (n=8, 48 neurites). f, Astrocytes, Tdc2+ neurons and dopaminergic neurons in larval CNS. Dorsal (arrows point to astrocyte somas), medial (neurites intermingled with ramified processes of astrocytes for monitoring activity are labeled) and ventral (cell bodies of Tdc2+ and dopaminergic neurons) images from the boxed region are shown right. s, subesophageal. t, thoracic. Scale bar, 50μm. g, Amplitude of Ca2+ spikes of dopaminergic neurons (n=10, 80 neurites). h,i, Chemotaxis assay (n listed). j, Number of Ca2+ spikes of dopaminergic neurons (n=6, 48 neurites). k, Responses of astrocytes to tyramine (0.5mM) in the presence of TTX (n=6, 96 cells total). l, Number of Ca2+ spikes of dopaminergic neurons (n=6, 48 neurites). m, AITC induces Ca2+ influx to astrocytes expressing TrpA1 (n=5, 80 cells). Scale bar, 50μm. n, Number of Ca2+ spikes of dopaminergic neurons (n=6, 48 neurites). *p<0.05, **p<0.01, n.s., not significant. Error bar, s.e.m. One-way ANOVA followed by Tukey's post hoc test.
Supplementary Material
Acknowledgements
We are grateful to colleagues, the Vienna Drosophila RNAi Center and the Bloomington Stock Center for generously providing fly stocks. We thank members of the Freeman lab for comments on the manuscript. This work was supported by NINDS grant R01 NS053538 (to MRF). During the period of this study MRF was an Investigator with the Howard Hughes Medical Institute.
Footnotes
Author Information
The authors state that they have no competing financial interests.
Statement of Individual Contributions
ZM and MRF designed most experiments. ZM performed all experiments. TS provided alrm-LexA::GAD transgenic flies. AS generated pUAST-wtrw::gfp construct. ZM and MRF wrote the manuscript with editing by TS.
References
- 1.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 2.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 3.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 4.Smith SJ. Do astrocytes process neural information? Prog. Brain Res. 1992;94:119–136. doi: 10.1016/s0079-6123(08)61744-6. [DOI] [PubMed] [Google Scholar]
- 5.Khakh BS, McCarthy KD. Astrocyte Calcium Signaling: From Observations to Functions and the Challenges Therein. Cold Spring Harb Perspect Biol. 2015;7:a020404. doi: 10.1101/cshperspect.a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araque A, et al. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatatis A, Russell JT. Spontaneous changes in intracellular calcium concentration in type I astrocytes from rat cerebral cortex in primary culture. Glia. 1992;5:95–104. doi: 10.1002/glia.440050203. [DOI] [PubMed] [Google Scholar]
- 9.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 10.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty J, Logan MA, Taşdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 14.Ding F, et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–394. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paukert M, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan R, et al. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Cameron S, Chang W-T, Rao Y. Control of directional change after mechanical stimulation in Drosophila. Mol Brain. 2012;5:39. doi: 10.1186/1756-6606-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salm AK, McCarthy KD. Norepinephrine-evoked calcium transients in cultured cerebral type 1 astroglia. Glia. 1990;3:529–538. doi: 10.1002/glia.440030612. [DOI] [PubMed] [Google Scholar]
- 21.Vömel M, Wegener C. Neuroarchitecture of aminergic systems in the larval ventral ganglion of Drosophila melanogaster. PLoS ONE. 2008;3:e1848. doi: 10.1371/journal.pone.0001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J. Neurobiol. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 23.Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robb S, et al. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- 26.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman EA. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalo U, Rasooli-Nejad S, Pankratov Y. Exocytosis of gliotransmitters from cortical astrocytes: implications for synaptic plasticity and aging. Biochem. Soc. Trans. 2014;42:1275–1281. doi: 10.1042/BST20140163. [DOI] [PubMed] [Google Scholar]
- 29.Brody T, Cravchik A. Drosophila melanogaster G protein-coupled receptors. J. Cell Biol. 2000;150:F83–8. doi: 10.1083/jcb.150.2.f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Materials and methods references
- 30.Doherty J, Logan MA, Taşdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monastirioti M, Linn CE, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole SH, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 36.Galili DS, et al. Converging circuits mediate temperature and shock aversive olfactory conditioning in Drosophila. Curr. Biol. 2014;24:1712–1722. doi: 10.1016/j.cub.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 37.Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 38.Lilly M, Carlson J. smellblind: a gene required for Drosophila olfaction. Genetics. 1990;124:293–302. doi: 10.1093/genetics/124.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Cameron S, Chang W-T, Rao Y. Control of directional change after mechanical stimulation in Drosophila. Mol Brain. 2012;5:39. doi: 10.1186/1756-6606-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.