Abstract
Poxviruses comprise a large family of enveloped DNA viruses that infect vertebrates and invertebrates. Poxviruses, unlike most DNA viruses, replicate in the cytoplasm and encode enzymes and other proteins that enable entry, gene expression, genome replication, virion assembly and resistance to host defenses. Entry of vaccinia virus, the prototype member of the family, can occur at the plasma membrane or following endocytosis. Whereas many viruses encode one or two proteins for attachment and membrane fusion, vaccinia virus encodes four proteins for attachment and eleven more for membrane fusion and core entry. The entry-fusion proteins are conserved in all poxviruses and form a complex, known as the Entry Fusion Complex (EFC), which is embedded in the membrane of the mature virion. An additional membrane that encloses the mature virion and is discarded prior to entry is present on an extracellular form of the virus. The EFC is held together by multiple interactions that depend on nine of the eleven proteins. The entry process can be divided into attachment, hemifusion and core entry. All eleven EFC proteins are required for core entry and at least eight for hemifusion. To mediate fusion the virus particle is activated by low pH, which removes one or more fusion repressors that interact with EFC components. Additional EFC-interacting fusion repressors insert into cell membranes and prevent secondary infection. The absence of detailed structural information, except for two attachment proteins and one EFC protein, is delaying efforts to determine the fusion mechanism.
Keywords: Vaccinia virus, Membrane fusion, Virus entry, Hemifusion, Endocytosis
1. Introduction
Many viruses contain an outer lipoprotein envelope that fuses with a cellular membrane allowing entry of the nucleoprotein core into the cytoplasm [1]. The viral fusion pathway usually involves membrane apposition, hemifusion and pore formation, which is similar to that occurring during fusion of cellular membranes although the molecular mechanisms vary [2]. The study of viral fusion, in addition to being vital for elucidating the biology of viruses, may provide important clues that can increase understanding of the fusion of cellular vesicles and organelles. One or two proteins are required for entry of most viruses although three or four are needed for herpesviruses [1]. Viral fusion mechanisms have been divided into three types. Class I fusion proteins, as exemplified by the influenza virus hemagglutinin, form trimers of hairpins that contain a central α-helical coiled-coil domain and a N-terminal hydrophobic fusion peptide that inserts into the cell membrane. Class II fusion proteins, encoded by flaviviruses, have β-structures with an internally located hydrophobic fusion loop. Class III fusion proteins, including both the vesicular stomatitis G protein and the herpes simplex gB protein, have an internal bipartite fusion loop and some characteristics of both class I and class II fusion proteins. For all three classes, a conformational change in the fusion protein brings the viral and cellular membranes together [1].
This review provides current information related to the fusion of poxviruses with cell membranes during entry, which is distinct from that of other viruses and requires at least 11 viral proteins that form an entry fusion complex (EFC) in addition to four attachment proteins [3]. Other aspects of poxvirus cell entry will be covered only briefly as they have been subjects of other recent reviews [3–6].
2. Poxvirus biology
2.1. Poxvirus family
Poxviruses comprise a large family of enveloped double-stranded DNA viruses that replicate entirely within the cytoplasm of vertebrate and invertebrate cells [7]. Variola virus, the most notorious member of the family, was responsible for smallpox until eliminated from nature by vaccination with the closely related vaccinia virus (VACV). Members of the chordopoxvirus subfamily have genomes of 150,000 to 300,000 base pairs encoding approximately 200 proteins. About half of the proteins are highly conserved and dedicated to essential functions including cell entry, genome replication, transcription and virion assembly [8]; the remainder are more variable and many engage host defense mechanisms [9]. This review focuses on VACV, the prototype poxvirus.
2.2. Virion structure
The VACV mature virion (MV) has a barrel shape with dimensions of ~360 × 270 × 250 nm and consists of an internal nucleoprotein core flanked by protein structures called lateral bodies and an outer membrane envelope composed of a single lipoprotein bilayer [10–12]. Purified MVs contain at least 80 viral proteins of which 20 or more are associated with the membrane [13–15]. The MV membrane appears to be derived by modification of the endoplasmic reticulum with viral proteins [6]. During late steps in morphogenesis some MVs becomes surrounded by an additional double-membrane derived from trans-Golgi or endosomal membranes [16–18] and are propelled through the cytoplasm on microtubules [19,20]. Upon reaching the periphery, the outermost membrane fuses with the plasma membrane resulting in exocytosis. The MV has a single lipoprotein membrane whereas the extracellular virion (EV) has one additional membrane containing at least six viral proteins distinct from those of the MV. MVs, which are released by cell lysis, are stable and thought to be important for animal-to-animal spread of infection, whereas EVs provide efficient cell-to-cell spread and resistance to antibody neutralization.
Lipids, mostly phospholipids and cholesterol, constitute approximately 5% of the MV mass [21]. Phosphatidylcholine and lesser amounts of phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine and semilysobisphosphatidic acid (also known as acylbis(monoacylglycero)phosphate or acylphosphatidylglycerol) are present [22–25]. Extraction of virion-associated lipids with a non-ionic detergent renders MVs non-infectious, but incubation with either uninfected cell membrane preparations or phosphatidylserine-containing liposomes partially restores infectivity [26,27]. More recently, these findings were extended and an apoptotic mimicry model for poxvirus entry, in which virion-associated phosphatidylserine serves to flag virions as apoptotic debris for cell uptake by macropinocytosis, was proposed [28]. However, a specific phosphatidylserine receptor for VACV has not been identified and reconstitution of detergent-extracted MVs with some other anionic phospholipid species also facilitate virus entry [29]. Nonetheless, these studies indicated the importance of the MV membrane lipid composition, specifically the presence of anionic phospholipid species, in cell entry of VACV MVs.
3. Entry
3.1. Entry pathways
Entry of poxviruses is defined as the step in which the nucleoprotein core of the virion passes into the cytoplasm. MVs can enter cells either at the plasma membrane at neutral pH or through a low pH-dependent endocytic route [30,31] depending in part on the virus strain and cell type [32,33]. Modification of entry pathways could have occurred during extensive cell culture passaging of VACV strains. The endocytic route appears to be the primary one used by orthopoxviruses that have been recently isolated from nature [32] and may provide an advantage in facilitating transit through the dense cell cortex. Engulfment of MVs occurs by macropinocytosis or fluid phase endocytosis and is dependent on actin dynamics and cell signaling [28,34,35].
EVs have been described as entering through the plasma membrane by fusion of a MV-like particle released upon shedding the outer membrane at the cell surface [36] and by macropinocytosis [37]. Since most entry studies have been carried out with purified MVs and the fusion proteins are located in the MV membrane, there will be little further discussion of EVs in this review.
3.2. Cell binding and internalization
The binding of MVs to the cell surface is mediated by at least four viral proteins: D8 binds chondroitin [38], A27 and H3 bind heparan [39–42] and A26 binds laminin [43]. The crystal structure of a trimeric form of A27 [44] and a dimeric form of H3 [45] have been solved. Inactivation of any one of the attachment proteins does not prevent entry, though infectivity is severely reduced by deletion of the genes encoding A27 or H3. The four proteins have additional roles unrelated to attachment. Another protein, L1, has been reported to bind an unidentified cell surface protein for entry [46] and its crystal structure has been solved [47]. As discussed below, L1 is a component of the EFC.
The binding of MVs to cells also involves association with integrin β1 and CD98 receptor molecules and activation of several serine/threonine kinases that induce the formation of actin-enriched membrane protrusions that engulf virus particles during macropinocytosis or fluid phase endocytosis [28,34,48–50]. Fluorescence microscopy studies indicate that endocytosed MVs traffic to early endosomes and recycling endosome compartments prior to membrane fusion [51].
Little is known regarding the mechanism or proteins required for binding of EVs to cells [52]. Electron micrographs reveal dissociation of the EV membrane at the cell surface indicating that it is not fusogenic [36]. Rupture of the outer EV membrane requires the EV membrane glycoproteins A34 and B5 and interactions with cell surface glycosoaminoglycans following virus attachment [36,53].
4. Membrane fusion
4.1. The EFC
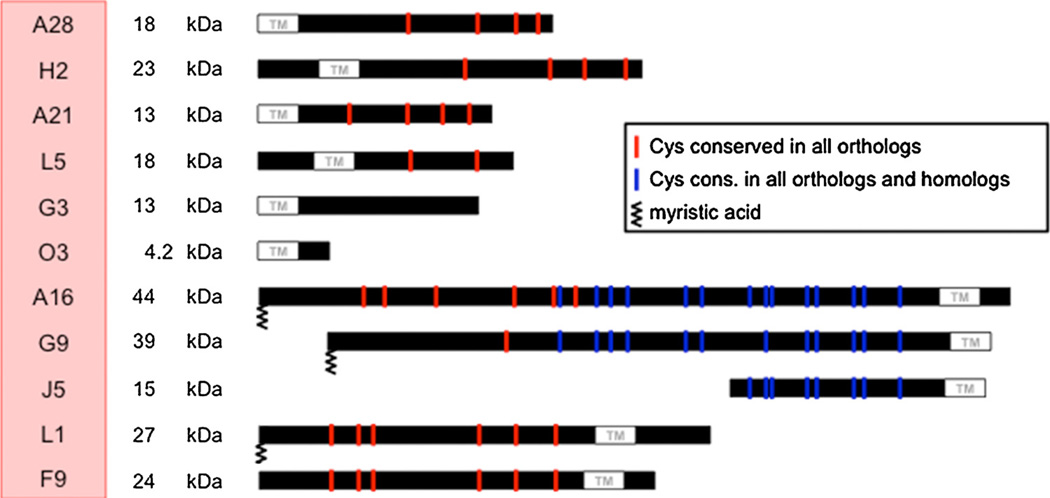
A complex of 11 proteins embedded in the MV membrane mediate a post attachment step of virus entry that is required for both MVs and EVs (Table 1, Fig. 1). The first components of the EFC were discovered by searching the VACV genome for putative transmembrane proteins conserved in all poxviruses and then by making conditional lethal mutants [54–57]. Additional components were found by their physical association with the first identified EFC proteins and mass spectrometry [58] and others by mutagenesis experiments [59–61]. The EFC proteins are difficult to extract from MVs with non-ionic detergents and reducing agent, suggesting that some are anchored to proteins beneath the membrane. The EFC proteins range in size from 4 to 43 kDa with a median of 16 kDa. Three of the proteins (A16, G9 and J5) are homologs arising by duplications but with low sequence identities. The transmembrane domains of five of the EFC proteins (A21, A28, G3, H2 and O3) are N-terminal and the others (A16, F9, G9, J5, L1 and L5) are C-terminal. Remarkably, none of the EFC proteins have a cleavable signal peptide and none are normally glycosylated, raising questions as to how they traffic to the MV membrane. Most of the EFC proteins have conserved cysteines that form intramolecular disulfide bonds (Fig. 1), which are catalyzed by three virus-encoded thiol oxidoreductases within the cytoplasm [62]. Interestingly, the three redox proteins as well as the eleven EFC proteins are conserved in all chordopoxviruses suggesting an evolutionarily important relationship.
Table 1.
VACV MV attachment and entry proteins.
| Protein | kDa | TMa | S-Sb | Conservedc | Properties |
|---|---|---|---|---|---|
| Attachment proteins | |||||
| A26 | 58 | − | − | − | Binds laminin; associates with A27; interacts with A16 and G9; fusion suppressor |
| A27 | 13 | − | − | − | Binds heparan; associates with A26 and A17 membrane protein; Ntd |
| D8 | 35 | N | − | − | Binds chondroitin; Nt |
| H3 | 38 | C | − | P | Binds heparan; Nt |
| Entry-fusion complex | |||||
| A16 | 43 | C | + | P | Homolog G9 and J5; interacts with G9 and A26; complex with G9 interacts with A56:K2 complex |
| A21 | 14 | N | + | P | |
| A28 | 16 | N | + | P | Interacts with H2; Nt |
| F9 | 24 | C | + | P | |
| G3 | 13 | N | P | Interacts with L5 | |
| G9 | 39 | C | + | P | Homolog A16 and J5; interacts with A16 and A26; complex with A16 interacts with A56:K2 complex; Myre |
| H2 | 22 | N | + | P | Interacts with A28 |
| J5 | 15 | C | + | P | Homolog A16 and G9; Myr |
| L1 | 27 | C | + | P | Myr; Nt |
| L5 | 15 | C | + | P | Interacts with G3 |
| O3 | 4 | N | C | Smallest VACV protein | |
TM, N- or C-terminal transmembrane domain.
S-S, intramolecular disulfide bond(s).
Conserved in all poxviruses (P) or all chordopoxviruses (C).
Nt, target of neutralizing antibody.
Myr, myristoylated.
Fig. 1. Protein components of the EFC.
The locations of the transmembrane domains of the 11 proteins comprising the EFC are shown. Nine of the proteins have cysteines that are conserved in all poxirus orthologs. Three proteins (A16, G9 and J5) are homologs derived by duplications. Three proteins (A16, G9 and L1) are myristoylated.
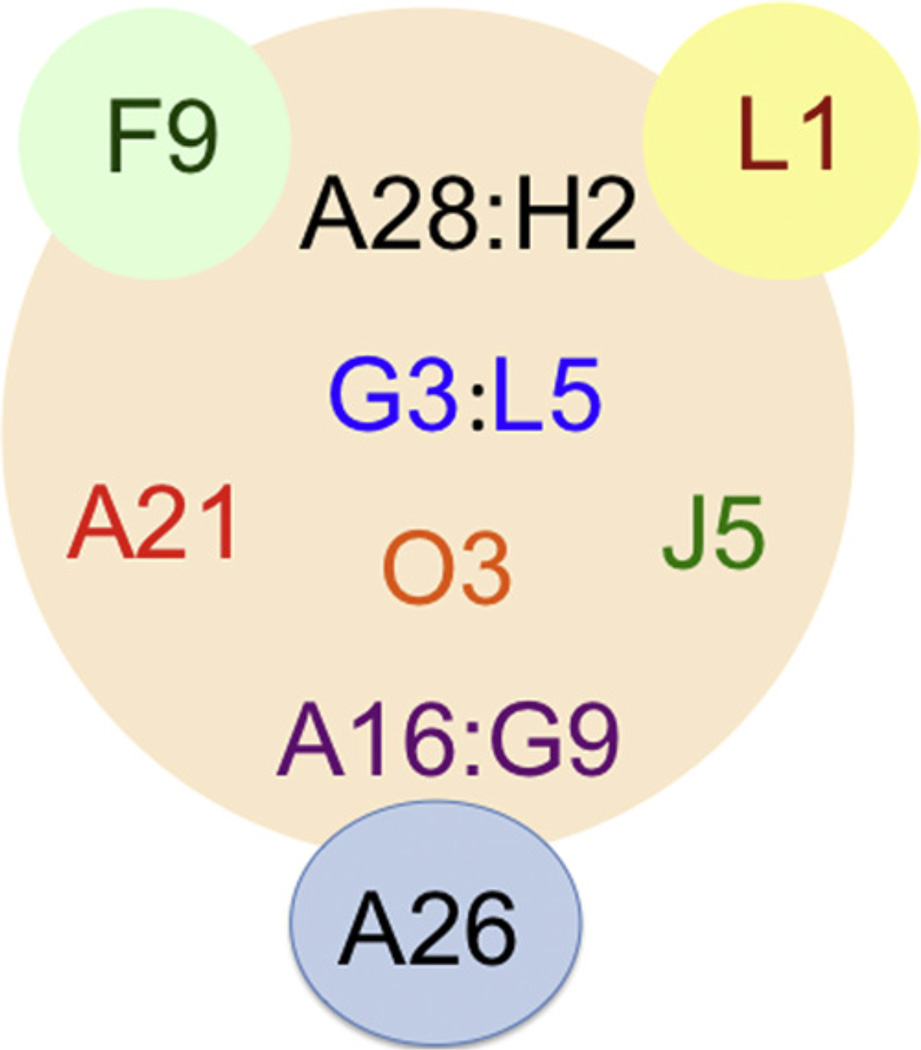
Ten of the eleven EFC proteins are essential for VACV replication and deletion of the 35 amino acid open reading frame encoding the O3 protein severely impairs replication. The role of the O3 protein can be fulfilled solely by its sequence flexible putative transmembrane domain [63]. Each EFC component can be immunoaffinity purified from infected cell membranes with antibody to any of the others indicating one or more complexes. Moreover, if expression of any EFC protein except for L1 and F9 is repressed, the network is disrupted and only subcomplexes remain. Nine proteins are thought to form the core of the EFC with L1 and F9 peripherally associated (Fig. 2). The subcomplexes that have been identified thus far include: A28-H2 [64], A16-G9 [65] and G3-L5 [66]. Mutation of a conserved LGYSG sequence between two cysteines in H2 prevent interaction with A28. The A16-G9 subcomplex also interacts with a complex of two other viral proteins, A56-K2, on the cell surface to prevent superinfection and cell–cell fusion at neutral pH [65] and also with the viral A26 protein on the MV surface to suppress neutral pH fusion after cell attachment [67] (Fig. 2). Two of the EFC proteins, L1 and A28, are targets of neutralizing antibodies [68,69]. The association of the A28 protein with the H2 protein enhances its immunogenicity [70].
Fig. 2. Interactions of components of EFC.
Of the eleven EFC components, nine (A16, A21, A28, G3, G9, H2, J5, L5 and O3) constitute the core and two (F9 and L1) are peripherally located. The EFC proteins that are known to interact directly (A28:H2, G3:L5, and A16:G9) are shown in the same color separated by a colon. The A26 fusion suppressor protein interacts with the A16:G9 subcomplex.
Thus far L1 is the only EFC protein with a determined structure, which comprises a fold composed of a bundle of α-helices packed against a pair of two-stranded β-sheets [47]. A potent neutralizing monoclonal antibody binds to a discontinuous epitope containing two loops that are held together by a disulfide bond [71]. There is a large hydrophobic cavity that could accommodate the N-terminal myristate moiety [47] although this has not been demonstrated. Mutations within the cavity inhibit infectivity without affecting myristoylation [72]. A “myristate switch” model in which the acyl chain is released from the cavity during entry has been proposed.
4.2. Steps in fusion
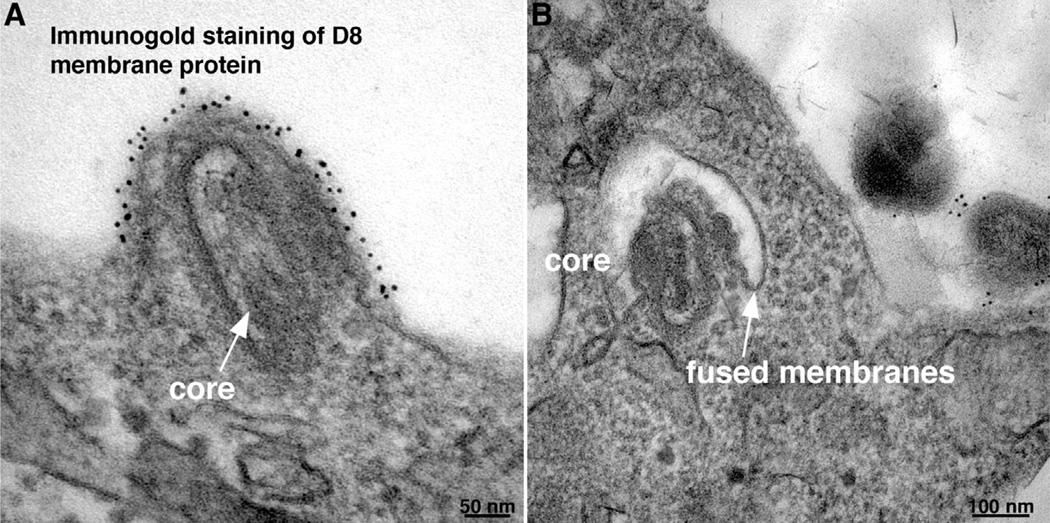
Fusion of the MV membrane with the plasma and endosomal membranes resulting in deposition of the core in the cytoplasm have been imaged directly by transmission electron microscopy [30,31,73] as shown in Fig. 3. The process of VACV entry, like that of other enveloped viruses [1], can be divided into several steps: close apposition of the viral and cellular membrane, lipid-mixing of the outer membrane leaflets leading to the formation of a hemifusion intermediate, and formation and expansion of a pore resulting in deposition of the core in the cytoplasm (Fig. 4).
Fig. 3. Fusion of vaccinia virus mature virions with cells visualized by transmission electron microscopy.
Immunogold staining with antibody to the D8 membrane protein was performed prior to embedding and cryosectioning. (A) Fusion at the plasma membrane 10 min after infection. Arrow points to virus core. (B) Fusion with endosomal membrane 20 min after infection. Arrow points to junction of viral and endosomal membrane.
Modified from: Townsley, A.C., et al. (2006). J. Virol. 80, 8899–8908.
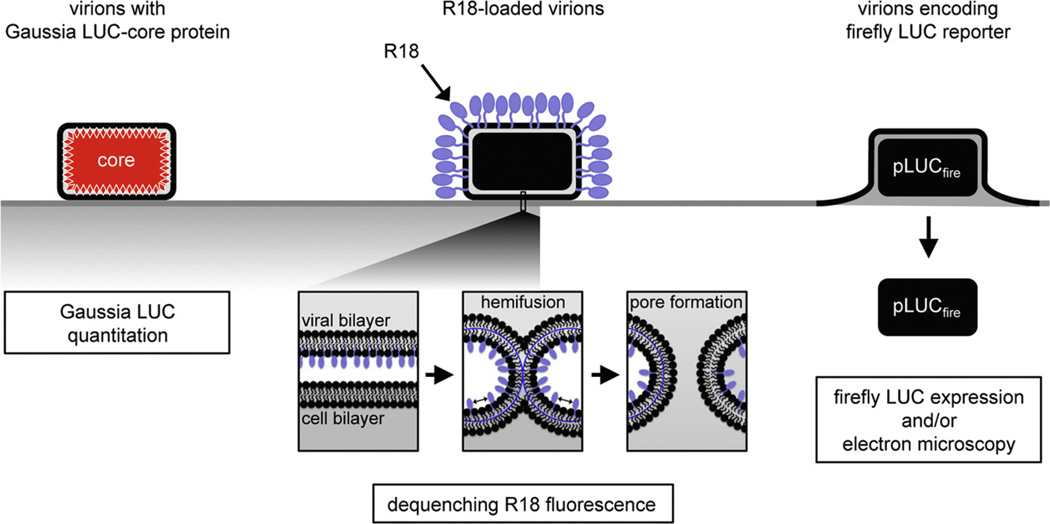
Fig. 4. Measurement of binding, hemifusion and core entry steps.
Attachment: a recombinant VACV with Gaussia luciferase (LUC) fused to a core protein is used to measure the binding of virions at 4 °C by assaying cell-associated LUC activity. Hemifusion: R18-loaded virions are bound to target cells at 4 °C, shifted to 37 °C, and the dequenching of R18 dimers due to lipid mixing is measured by increased fluorescence. Core entry: a recombinant VACV that expresses firefly LUC under an early promoter, is used to infect cells and newly synthesized LUC is measured.
Modified from: Laliberte, J.P., Weisberg, A.S., Moss, B., 2011. PLoS Pathog. 7, e1002446.
4.3. Hemifusion
Lipid mixing during hemifusion may be analyzed by loading the virion membrane with a self-quenching lipophilic dye such as R18 and measuring the increase in fluorescence [74,75]. After binding MVs to cells at 4 °C, fluorescence increases almost immediately upon raising the temperature suggesting an active transfer process. The rate of dequenching is not enhanced by low pH or reduced by bafilomycin, which prevents endosomal acidification. Treatment of cells with methyl-β-cyclodextrin resulting in a 74% reduction in total cellular cholesterol levels has only a mild effect on hemifusion. Although the site of hemifusion was not directly determined, the rapidity of lipid mixing and absence of a pH influence, imply that it begins at the plasma membrane.
Studies with inhibitors suggest that actin dynamics is needed for hemifusion [75]. CK-636 and CK-548, which bind to the Arp2/3 complex and prevent actin nucleation, have little effect on virion attachment but are effective inhibitors of hemifusion. Dynasore, a small molecule inhibitor of the GTPase activities of dynamin 1, dynamin 2 and mitochondrial dynamin that interferes with dynamin-dependent endocytic pathways but also directly interacts with actin and regulates the actin cytoskeleton also severely inhibits hemifusion. Latrunculin A and cytochalasin D, which bind to G actin and inhibit actin polymerization, inhibit hemifusion by 50%. Extensive actin remodeling and mobilization occur during MV binding to cell surfaces [28,34,48] suggesting that actin-enriched projections enhance the intimacy of membrane contact allowing virus-cell membrane fusion.
Cell signaling is also important for hemifusion [75]. The small molecule tyrosine kinase inhibitors genestein, gefitinib (Iressa) and 324674 (PD153035) severely inhibit hemifusion. The latter could be related to the relative specificity of gefitinib and 324674 for epidermal growth factor receptor signaling, which causes rapid actin polymerization and rearrangement [76].
The importance of the EFC proteins for hemifusion was demonstrated using a panel of recombinant VACVs in which the expression of an individual EFC protein is regulated by the lac operator system [75]. When the infection is allowed to proceed in the absence of inducer, the progeny virus particles appear normal in all respects except for the absence or barely detectable amount of the regulated protein. These individual EFC protein-deficient MV particles are able to bind to cells but are unable to enter. Except for A28, L1 and L5, the particles are defective in hemifusion. Whether the latter three proteins are only required for a post-hemifusion step or the presence of minute amounts undetectable by Western blotting are sufficient for hemifusion but not pore expansion is difficult to assess. Nevertheless, the abilities of A28-, L1- and L5-deficient MVs to mediate hemifusion but not entry supports a two-step entry model with a hemifusion intermediate. The 2-step model is also supported by the ability of a potent neutralizing antibody to prevent entry but not hemifusion [75].
4.4. Core entry
Direct and indirect methods are used to measure core entry. The former include visualization of cytoplasmic cores by electron or fluorescence microscopy. When the medium is acidified and penetration occurs at the plasma membrane, numerous cores are detected in the cytoplasm at 10 min whereas 20–30 min are required for liberation from endosomes at neutral pH [31]. Indirect methods are based on the packaging within the virion of a complete transcription system including enzymes that cap and polyadenylate mRNA. Transcription is activated upon release of the core into the cytoplasm and followed by translation of the mRNA on the host ribosomes. Employment of a recombinant VACV that expresses firefly luciferase provides high sensitivity. Because of the additional steps, there is a delay of about 20 min between core entry as determined by electron microcopy and detection of appreciable luciferase activity. A panel of recombinant viruses encoding luciferase in which the lac operator system regulates expression of a single EFC protein demonstrated that at least 10 of the EFC proteins (an inducible J5 virus was not included in the panel) are required for core entry [75].
Although a partial reduction of cellular cholesterol with methyl-β-cyclodextrin has little effect on hemifusion, the drug has a major effect on core entry as measured by the luciferase expression assay. However, this drug also affects the actin cytoskeleton [77]. Another study has shown that MVs associate with cholesterol-rich regions of the plasma membrane and that cholesterol depletion reduces VACV entry as measured by visualizing cores in the cytoplasm [78]. Cytochalasin D and latrunculin have a greater inhibitory effect on VACV core entry than hemifusion, suggesting that actin dynamics may be required for hemifusion and pore formation as well as endocytosis [75].
5. Viral protein regulators of fusion
The fusion apparatus of viruses is generally maintained in an inactive state to prevent premature fusion during assembly or exit from the cell and may be activated by association with membranes, protein cleavage or low pH. For VACV strains that enter predominantly by endocytosis, exposing cells with attached virus to a pH of 4.5–5, which mimics the pH of late endosomes, triggers fusion at the plasma membrane [31]. Studies by the Chang laboratory [67,79] have shown that deletion of the MV membrane-associated A26 protein promotes fusion at the plasma membrane of human cells at neutral pH. Apparently the A26 protein prevents fusion by interacting with the A16 and G9 components of the EFC (Fig. 2). The finding that low pH causes partial dissociation of the A26 protein from MVs, which presumably occurs normally in endosomes, supports this model.
The process by which many viruses prevent secondary infection by the same or a related virus is known as superinfection exclusion. Exclusion can occur at various stages of the life cycle of a virus. In the case of VACV, exclusion has been shown to occur at the entry stage by two different mechanisms. Late in infection, a complex of two viral proteins A56 and K2 are associated with the plasma membrane [80]. A56 has a transmembrane domain whereas K2 binds to A56. The A56-K2 complex interacts with the A16-G9 EFC subcomplex but not with A16 or G9 alone [65,81]. Furthermore, cells that stably express A56-K2 are resistant to infection by VACV [82] and a block in entry occurs at a post-hemifusion step [83]. Thus, the A16-G9 subcomplex is a target for regulation of fusion by the A26 protein during entry and the A56-K2 proteins to prevent superinfection.
A second method of exclusion at the hemifusion step occurs early after VACV infection [83]. Although the mechanism involves the synthesis of viral early proteins, the interactions involved have not been defined. Exclusion of EVs resulting in enhancement of spread occurs by a repulsion mechanism dependent on the early expression of the viral A33 and A36 proteins [84].
6. Cell-cell fusion
Cells infected with VACV form syncytia under certain conditions. This occurs when cells infected with wild type virus are briefly exposed to low pH [74,85] or at neutral pH when cells are infected with virus containing mutations in the A56 [86] or K2 [87–89] gene. These conditions are similar to those promoting MV fusion with the plasma membrane since cell–cell fusion is mediated by the EFC and dependent on the externalization of virions on the cell surface [90] [54,56,57,81]. Low pH can also induce syncytium formation immediately after infection with a high multiplicity of MVs and this has been called fusion from without [85]. As discussed in section 5, the A56-K2 complex interacts with the A16 and G9 components of the EFC to prevent spontaneous activation of the fusion apparatus by progeny virions [65]. Uninfected cells expressing A56 and K2 are resistant to forming syncytia when mixed with cells infected with an A56 deletion mutant [82]. Therefore, the A56-K2 complex acts as a fusion suppressor for both virus entry and syncytia formation.
Ectopic expression of A17, a component of the MV membrane, causes fusion of transfected cells suggesting a role during entry [91]. However, it is possible that the fusion activity of A17 functions during morphogenesis rather than entry as conditional lethal A17 mutants are blocked in viral membrane formation [92,93]. Recent studies indicate that A17 is a reticulon-like protein that promotes curvature of the endoplasmic reticulum [94].
7. Summary and future directions
Poxviruses exemplified by VACV enter cells by a multistep process consisting of attachment, hemifusion and core entry that can occur at the plasma membrane or after endocytosis. Four proteins are involved in attachment to glycosaminoglycans and laminin and a complex of eleven proteins that are conserved in all poxviruses mediates the hemifusion and entry steps. Fusion is activated by low pH, which dissociates a fusion suppressor protein from the EFC. A specific cell receptor that activates fusion has not been identified. None of the components of the EFC resemble fusion proteins of other viruses and structural studies of the individual proteins as well as the complex will be crucial to further understanding of the entry mechanism.
Acknowledgments
I thank Jason Laliberte and Andrea Weisberg for help with illustrations. This work was supported by the Division of Intramural Research, NIAID, NIH.
References
- 1.White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008;43(3):189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123(3):375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4(5):688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laliberte JP, Moss B. Lipid membranes in poxvirus replication. Viruses Basel. 2010;2(4):972–986. doi: 10.3390/v2040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt FI, Bleck CK, Mercer J. Poxvirus host cell entry. Curr. Opin. Virol. 2012;2(1):20–27. doi: 10.1016/j.coviro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Moss B. Poxvirus membrane biogenesis. Virology. 2015;479–480:619–626. doi: 10.1016/j.virol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss B. Poxviridae. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; 2013. p. 2130. [Google Scholar]
- 8.Upton C, Slack S, Hunter AL, Ehlers A, Roper RL. Poxvirus orthologousclusters: toward defining the minimum essential poxvirus genome. J. Virol. 2003;77(13):7590–7600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GL, Benfield CTO, de Motes CM, Mazzon M, Ember SWJ, Ferguson BJ, Sumner RP. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013;94:2367–2392. doi: 10.1099/vir.0.055921-0. [DOI] [PubMed] [Google Scholar]
- 10.Dales S, Siminovitch L. The development of vaccinia virus in Earle’s L strain cells as examined by electron microscopy. J. Biophys. Biochem. Cytol. 1961;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollinshead M, Vanderplasschen A, Smith GL, Vaux DJ. Vaccinia virus intracellular mature virions contain only one lipid membrane. J. Virol. 1999;73(2):1503–1517. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cyrklaff M, Risco C, Fernandez JJ, Jimenez MV, Esteban M, Baumeister W, Carrascosa JL. Cryo-electron tomography of vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 2005;102(8):2772–2777. doi: 10.1073/pnas.0409825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CS, Chen CH, Ho MY, Huang CY, Liao CL, Chang W. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 2006;80(5):2127–2140. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. Protein composition of the vaccinia virus mature virion. Virology. 2007;358(1):233–247. doi: 10.1016/j.virol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Yoder JD, Chen TS, Gagnier CR, Vemulapalli S, Maier CS, Hruby DE. Poxproteomics: mass spectrometry analysis and identification of vaccinia virion proteins. Virol. J. 2006;3(1):10. doi: 10.1186/1743-422X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiller G, Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J. Virol. 1985;55:651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 1993;60(1):163–178. [PubMed] [Google Scholar]
- 19.Ward BM, Moss B. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J. Virol. 2001;75:11651–11663. doi: 10.1128/JVI.75.23.11651-11663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002;83(Pt. 12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 21.Zwartouw HT. The chemical composition of vaccinia virus. J. Gen. Microbiol. 1964;34:115–123. doi: 10.1099/00221287-34-1-115. [DOI] [PubMed] [Google Scholar]
- 22.Stern W, Dales S. Biogenesis of vaccinia: concerning the origin of the envelope phospholipids. Virology. 1974;62:293–306. doi: 10.1016/0042-6822(74)90393-6. [DOI] [PubMed] [Google Scholar]
- 23.Hiller G, Eibl H, Weber K. Characterization of intracellular and extracellular vaccinia virus variants:N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J. Virol. 1981;39:903–913. doi: 10.1128/jvi.39.3.903-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodeik B, Doms RW, Ericsson M, Hiller G, Machamer CE, van’t Hof W, van Meer G, Moss B, Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J. Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cluett EB, Machamer CE. The envelope of vaccinia virus reveals an unusual phospholipid in Golgi complex membranes. J. Cell Sci. 1996;109(Pt. 8):2121–2131. doi: 10.1242/jcs.109.8.2121. [DOI] [PubMed] [Google Scholar]
- 26.Ichihashi Y, Oie M. The activation of vaccinia virus infectivity by the transfer of phosphatidylserine from the plasma membrane. Virology. 1983;130:306–317. doi: 10.1016/0042-6822(83)90085-5. [DOI] [PubMed] [Google Scholar]
- 27.Oie M. Reversible inactivation and reactivation of vaccinia virus by manipulation of viral lipid composition. Virology. 1985;142(2):299–306. doi: 10.1016/0042-6822(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 28.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320(5875):531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 29.Laliberte JP, Moss B. Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc. Natl. Acad. Sci. U. S. A. 2009;106(41):17517–17521. doi: 10.1073/pnas.0909376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter GC, Law M, Hollinshead M, Smith GL. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 2005;86:1279–1290. doi: 10.1099/vir.0.80831-0. [DOI] [PubMed] [Google Scholar]
- 31.Townsley AC, Weisberg AS, Wagenaar TR, Moss B. Vaccinia virus entry into cells via a low pH-dependent-endosomal pathway. J. Virol. 2006;80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bengali Z, Satheshkumar PS, Moss B. Orthopoxvirus species and strain differences in cell entry. Virology. 2012;433(2):506–512. doi: 10.1016/j.virol.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bengali Z, Satheshkumar PS, Yang Z, Weisberg AS, Paran N, Moss B. Drosophila S2 cells are non-permissive for vaccinia virus DNA replication following entry via low pH-dependent endocytosis and early transcription. PLoS One. 2011;6(2):e17248. doi: 10.1371/journal.pone.0017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CY, Lu TY, Bair CH, Chang YS, Jwo JK, Chang W. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J. Virol. 2008;82(16):7988–7999. doi: 10.1128/JVI.00894-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercer J, Knebel S, Schmidt FI, Crouse J, Burkard C, Helenius A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc. Natl. Acad. Sci. U. S. A. 2010;107(20):9346–9351. doi: 10.1073/pnas.1004618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law M, Carter GC, Roberts KL, Hollinshead M, Smith GL. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt FI, Bleck CK, Helenius A, Mercer J. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. EMBO J. 2011;30:3647–3661. doi: 10.1038/emboj.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 1999;73(10):8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung C-S, Hsiao J-C, Chang Y-S, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparin sulfate. J. Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiao JC, Chung CS, Chang W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region ofA27L protein as the glycosaminoglycan-binding domain. J. Virol. 1998;72(10):8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin CL, Chung CS, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 2000;74(7):3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez MI, Esteban M. Identification of functional domains in the 14-kilodalton envelope protein (A27L) of vaccinia virus. J. Virol. 1999;73(11):9098–9109. doi: 10.1128/jvi.73.11.9098-9109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiu WL, Lin CL, Yang MH, Tzou DLM, Chang W. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J. Virol. 2007;81(5):2149–2157. doi: 10.1128/JVI.02302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang TH, Chang SJ, Hsieh FL, Ko TP, Lin CT, Ho MR, Wang I, Hsu STD, Guo RT, Chang W, Wang AHJ. Crystal structure of vaccinia viral A27 protein reveals a structure critical for its function and complex formation with protein. PLoS Pathog. 2013;9(8) doi: 10.1371/journal.ppat.1003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su HP, Singh K, Gittis AG, Garboczi DN. The structure of the poxvirus A33 protein reveals a dimer of unique C-type lectin-like domains. J. Virol. 2010;84(5):2502–2510. doi: 10.1128/JVI.02247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foo CH, Lou H, Whitbeck JC, Ponce-de-Leon M, Atanasiu D, Eisenberg RJ, Cohen GH. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology. 2009;385(2):368–382. doi: 10.1016/j.virol.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su HP, Garman SC, Allison TJ, Fogg C, Moss B, Garboczi DN. The 1.51-A structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 2005;102(12):4240–4245. doi: 10.1073/pnas.0501103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locker JK, Kuehn A, Schleich S, Rutter G, Hohenberg H, Wepf R, Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell. 2000;11(7):2497–2511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izmailyan R, Hsao JC, Chung CS, Chen CH, Hsu PWC, Liao CL, Chang W. Integrin beta 1 mediates vaccinia virus entry through activation of PI3K/Akt signaling. J. Virol. 2012;86(12):6677–6687. doi: 10.1128/JVI.06860-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroeder N, Chung CS, Chen CH, Liao CL, Chang W. The lipid raft-associated protein CD98 is required for vaccinia virus endocytosis. J. Virol. 2012;86:4868–4882. doi: 10.1128/JVI.06610-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsiao JC, Chu LW, Lo YT, Lee SP, Chen TJ, Huang CY, Ping YH, Chang W. Intracellular transport of vaccinia virus in HeLa cells requires WASH-VPEF/FAM21-retromer complexes and recycling molecules Rab11 and Rab22. J. Virol. 2015;89(16):8365–8382. doi: 10.1128/JVI.00209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderplasschen A, Smith GL. A novel virus binding assay using confocal microscopy: demonstration that intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts KL, Breiman A, Carter GC, Ewles HA, Hollinshead M, Law M, Smith GL. Acidic residues in the membrane-proximal stalk region of vaccinia virus protein B5 are required for glycosaminoglycan-mediated disruption of the extracellular enveloped virus outer membrane. J. Gen. Virol. 2009;90:1582–1591. doi: 10.1099/vir.0.009092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senkevich TG, Ward BM, Moss B. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 2004;78(5):2357–2366. doi: 10.1128/JVI.78.5.2357-2366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senkevich TG, Moss B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell–cell fusion. J. Virol. 2005;79:4744–4754. doi: 10.1128/JVI.79.8.4744-4754.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Townsley A, Senkevich TG, Moss B. The product of the vaccinia virus L5Rgene is a fourth membrane protein encoded by all poxviruses that is requried for cell entry and cell–cell fusion. J. Virol. 2005;79:10988–10998. doi: 10.1128/JVI.79.17.10988-10998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsley A, Senkevich TG, Moss B. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 2005;79:9458–9469. doi: 10.1128/JVI.79.15.9458-9469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. U. S. A. 2005;102(51):18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisht H, Weisberg AS, Moss B. Vaccinia virus L1 protein is required for cell entry and membrane fusion. J. Virol. 2008;82:8687–8694. doi: 10.1128/JVI.00852-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown E, Senkevich TG, Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related l1 protein. J. Virol. 2006;80(19):9455–9464. doi: 10.1128/JVI.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satheshkumar PS, Moss B. Characterization of a newly Identified 35 amino acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses. J. Virol. 2009;83:12822–12832. doi: 10.1128/JVI.01744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senkevich TG, White CL, Koonin EV, Moss B. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6667–6672. doi: 10.1073/pnas.062163799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satheshkumar PS, Chavre J, Moss B. Role of the vaccinia virus O3 protein in cell entry can be fulfilled by its sequence flexible transmembrane domain. Virology. 2013;444(1–2):148–157. doi: 10.1016/j.virol.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson GE, Wagenaar TR, Moss B. A conserved sequence within the H2 subunit of the vaccinia virus entry/fusion complex is important for interaction with the A28 subunit and infectivity. J. Virol. 2008;82(13):6244–6250. doi: 10.1128/JVI.00434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagenaar TR, Ojeda S, Moss B. Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex. J. Virol. 2008;82(11):5153–5160. doi: 10.1128/JVI.00162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolfe CL, Moss B. Interaction between the G3 and L5 proteins of the vaccinia virus entry-fusion complex. Virology. 2011;412:278–283. doi: 10.1016/j.virol.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang SJ, Shih AC, Tang YL, Chang W. Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH. J. Virol. 2012;86(7):3809–3818. doi: 10.1128/JVI.06081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolffe EJ, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211:53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]
- 69.Nelson GE, Sisler JR, Chandran D, Moss B. Vaccinia virus entry/fusion complex subunit A28 is a target of neutralizing and protective antibodies. Virology. 2008;380(2):394–401. doi: 10.1016/j.virol.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinoda K, Wyatt LS, Moss B. The neutralizing antibody response to the vaccinia virus A28 protein is specifically enhanced by its association with the H2 protein. Virology. 2010;405(1):41–49. doi: 10.1016/j.virol.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su HP, Golden JW, Gittis AG, Hooper JW, Garboczi DN. Structural basis for the binding of the neutralizing antibody 7D11, to the poxvirus L1 protein. Virology. 2007;368(2):331–341. doi: 10.1016/j.virol.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foo CH, Whitbeck JC, Ponce-de-Leon M, Saw WT, Cohen GH, Eisenberg RJ. The myristate moiety and amino-terminus of the vaccinia virus L1 constitute a bipartite functional region needed for entry. J. Virol. 2012;86:5437–5451. doi: 10.1128/JVI.06703-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armstrong JA, Metz DH, Young MR. The mode of entry of vaccinia virus into L cells. J. Gen. irol. 1973;21:533–537. doi: 10.1099/0022-1317-21-3-533. [DOI] [PubMed] [Google Scholar]
- 74.Doms RW, Blumenthal R, Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J. Virol. 1990;64:4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laliberte JP, Weisberg AS, Moss B. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 2011;7(12):e1002446. doi: 10.1371/journal.ppat.1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rijken PJ, Hage WJ, van Bergenen Henegouwen PM, Verkleij AJ, Boonstra J. Epidermal growth factor induces rapid reorganization of the actin microfilament system in human A431 cells. J. Cell Sci. 1991;100(Pt. 3):491–499. doi: 10.1242/jcs.100.3.491. [DOI] [PubMed] [Google Scholar]
- 77.Chubinskiy-Nadezhdin VI, Efremova TN, Khaitlina SY, Morachevskaya EA. Functional impact of cholesterol sequestration on actin cytoskeleton in normal and transformed fibroblasts. Cell Biol. Int. 2013;37(6):617–623. doi: 10.1002/cbin.10079. [DOI] [PubMed] [Google Scholar]
- 78.Chung CS, Huang CY, Chang W. Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts. J. Virol. 2005;79(3):1623–1634. doi: 10.1128/JVI.79.3.1623-1634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang SJ, Chang YX, Izmailyan R, Tang YL, Chang W. Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells. J. Virol. 2010;84(17):8422–8432. doi: 10.1128/JVI.00599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner PC, Moyer RW. The cowpox virus fusion regulator proteins SPI-3 and hemagglutinin interact in infected and uninfected cells. Virology. 2006;347(1):88–99. doi: 10.1016/j.virol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Wagenaar TR, Moss B. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 2007;81:6286–6293. doi: 10.1128/JVI.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagenaar TR, Moss B. Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J. Virol. 2009;83(4):1546–1554. doi: 10.1128/JVI.01684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laliberte JP, Moss B. A novel mode of poxvirus superinfection exclusion that prevents fusion of the lipid bilayers of viral and cellular membranes. J. Virol. 2014;88(17):9751–9768. doi: 10.1128/JVI.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doceul V, Hollinshead M, van der Linden L, Smith GL. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science. 2010;327(5967):873–876. doi: 10.1126/science.1183173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong SC, Lai CF, Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178(1):81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- 86.Ichihashi Y, Dales S. Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology. 1971;46:533–543. doi: 10.1016/0042-6822(71)90057-2. [DOI] [PubMed] [Google Scholar]
- 87.Turner PC, Moyer RW. An orthopoxvirus serpin-like gene controls the ability of infected cells to fuse. J. Virol. 1992;66:2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Law KM, Smith GL. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J. Gen. Virol. 1992;73:549–557. doi: 10.1099/0022-1317-73-3-549. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J, Sun XY, Fernando GJP, Frazer IH. The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell–cell fusion. Virology. 1992;189(2):678–686. doi: 10.1016/0042-6822(92)90591-c. [DOI] [PubMed] [Google Scholar]
- 90.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000 Dalton outer envelope protein. J. Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kochan G, Escors D, Gonzalez JM, Casasnovas JM, Esteban M. Membrane cell fusion activity of the vaccinia virus A17–A27 protein complex. Cell. Microbiol. 2008;10:1149–1164. doi: 10.1111/j.1462-5822.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 92.Rodríguez D, Esteban M, Rodríguez JR. Vaccinia virus A17L gene product is essential for an early step in virion morphogenesis. J. Virol. 1995;69:4640–4648. doi: 10.1128/jvi.69.8.4640-4648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolffe EJ, Moore DM, Peters PJ, Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J. Virol. 1996;70:2797–2808. doi: 10.1128/jvi.70.5.2797-2808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Erlandson KJ, Bisht H, Weisberg AS, Hyun SI, Hansen BT, Fischer ER, Hinshaw JE, Moss B. Poxviruses encode a reticulon-like protein that promotes membrane curvature. Cell Rep. 2016;14(9):2084–2091. doi: 10.1016/j.celrep.2016.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]