Abstract
Purpose
Stereotactic body radiation therapy (SBRT) has emerged as an effective treatment for early-stage lung cancer. Histologic subtyping in surgically resected lung adenocarcinomas is recognized as a prognostic factor, with the presence of solid or micropapillary patterns predicting poor outcomes. Herein, we describe outcomes following SBRT for early-stage lung adenocarcinoma by histologic subtype.
Materials and Methods
We identified 119 consecutive patients (124 lesions) with stage I-IIA lung adenocarcinoma who were treated with definitive SBRT at our institution between August 2008 and August 2015 and had undergone core biopsy. Histologic subtyping was performed according to the 2015 WHO Classification. Thirty-seven tumors (30%) were of high risk subtype, defined as containing a component of solid and/or micropapillary pattern. Cumulative incidences of local, nodal, regional and distant failure were compared between high risk vs. non-high risk adenocarcinoma subtypes with Gray’s test, and multivariable-adjusted hazard ratios were estimated from propensity score-weighted Cox regression models.
Results
Median follow-up for the entire cohort was 17 months and 21 months for surviving patients. The 1-year cumulative incidence and adjusted hazard ratio (HR) of local, nodal, regional and distant failure, respectively, in high risk versus non-high risk lesions were 7.3% vs 2.7% (HR 16.8; 95% CI 3.5–81.4), 14.8% vs 2.6% (HR 3.8; 95% CI 0.95–15.0), 4.0% vs 1.2% (HR 20.9; 95% CI 2.3–192.3) and 22.7% vs 3.6% (HR 6.9; 95% CI 2.2–21.1). No significant difference was seen with regard to overall survival.
Conclusions
Outcomes following SBRT for early-stage adenocarcinoma of the lung are highly correlated with histologic subtype, with micropapillary and solid tumors portending significantly higher rates of locoregional and metastatic progression. In this context, histologic subtype based on core biopsies is a prognostic factor and may have important implications for patient selection, adjuvant treatment, biopsy methods and clinical trial design.
Introduction
Stereotactic body radiotherapy (SBRT) has emerged in recent years as an effective treatment for inoperable as well as operable early-stage non small cell lung cancer (NSCLC). Adenocarcinomas are currently the most common type of lung cancer [1, 2], accounting for ~50% of cases [3, 4]. In 2011, the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) proposed a new classification for primary lung adenocarcinomas, subsequently adopted by the 2015 WHO Classification describing five distinct histologic subtypes: lepidic, papillary, acinar, micropapillary and solid[5, 6]. Micropapillary and solid subtypes portend significantly worse outcomes and survival following surgical resection for early-stage adenocarcinoma [7–15]. To our knowledge, the impact of adenocarcinoma subtype has not yet been investigated in the context of SBRT. Herein, we describe outcomes and patterns of failure for patients with early-stage lung adenocarcinoma treated with SBRT according to histologic subtype, which represents a potential novel prognostic factor.
Methods and Materials
Patient and study details
This study was approved by the Institutional Review Board at our institution. We identified 119 consecutive patients (124 lesions) who received SBRT for inoperable biopsy-proven stage I-IIA NSCLC (T1-2aN0M0) at our institution between August 2008 and August 2015 and had undergone core needle biopsy with pathological confirmation of invasive adenocarcinoma, and histologic subtyping by a pathologist specializing in thoracic malignancies. All patients were evaluated by a thoracic surgeon and either refused surgery or were determined to not be operative candidates. The last followup of the cohort was in January 2016.
Radiation therapy
Our stereotactic body radiation therapy method has been described previously[16]. Briefly, patients were immobilized with their arms raised above the head. They then underwent a 4-dimensional CT simulation. Treatment planning was performed using in-house treatment planning software or with Eclipse (Varian Medical Systems, Inc., Palo Alto, CA). Target delineation was based on standard International Commission of Radiation Units and Measurements definitions. The gross tumor volume was defined on free-breathing computed tomography and modified based on the 4-dimensional CT scan to create an internal target volume (ITV) for all patients. The ITV was expanded with a 2- to 3-mm margin for microscopic disease extension to create a clinical target volume, which was then expanded 5 mm to the planning target volume (PTV). Dose was prescribed to the 100% isodose line surrounding the PTV using inhomogeneity corrections. The median prescribed dose was 48 Gy in 4 fractions, ranging from 40 to 60 Gy in 3 to 5 fractions. Tumors were typically treated with a risk-adapted approach using 8 to 10 Gy × 5 fractions for tumors within 2 cm of the proximal bronchial tree, 12 Gy × 4 fractions for tumors within 1 cm of the chest wall, and 18 Gy × 3 fractions for all other peripherally located tumors. All patients received treatment every other day. Treatment setup was verified using cone-beam computed tomography at each fraction, and adjustments and shifts were performed for optimal alignment.
Follow-up
Patients typically underwent a CT scan of the chest and upper abdomen, including the adrenal glands and liver, every 3 months during years 1 and 2, every 6 months during years 3 and 4, and annually thereafter. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) was performed at baseline and was only performed again if there was radiographic suspicion of recurrence on CT scan. Recurrences were documented by biopsy procedures whenever feasible unless there was clear evidence of metastatic disease on CT or PET/CT scan or the risks for biopsy were unacceptably high.
Histologic subtyping
Pre-SBRT core biopsy samples were obtained with either endobronchial or transthoracic CT-guided core biopsy.
All specimens were formalin fixed and stained with hematoxylin and eosin in the routine manner. In poorly differentiated tumors, immunohistochemistry was performed as needed using antibodies for TTF-1 and p40 to confirm the diagnosis of adenocarcinoma. Slides were reviewed by a board certified pathologist with subspecialty expertise in thoracic pathology and histologic subtyping performed according to the IASLC/ATS/ERS and 2015 WHO Classifications documenting the presence of lepidic, papillary, acinar, micropapillary and/or solid patterns[5, 6]. Lesions with components of more than one subtype were scored as “mixed” while lesions with a single histologic pattern were scored as “pure”. Tumors containing any component of micropapillary and/or solid pattern were labeled as high risk subtypes.
End points and statistics
Recurrences were defined as local if progression of the irradiated index tumor occurred in or adjacent to the PTV; nodal if progression occurred in mediastinal or supraclavicular lymph nodes; regional if progression occurred in the same lobe of the lung but distinct from the treated lesion; or distant if progression occurred via malignant pleural effusion or extrathoracic spread. Tumors that developed in a different lobe of the ipsilateral or contralateral lung in the absence of intervening lymph nodes and no evidence of metastases, as well as new tumors of separate histology, were considered new primary tumors rather than treatment failure.
Local, nodal, regional, and distant failure were estimated separately using cumulative incidence curves and compared using Gray’s test. Time to each event was measured from the end of SBRT until the event of interest or last follow-up. Death without the event of interest was considered a competing risk event. Overall survival was defined as the time from end of SBRT to death or last follow-up and estimated by the Kaplan-Meier method. Groups were compared by the log rank test. Lesions were assumed to be independent in the analyses.
A multivariable analysis of each individual failure was limited by the low numbers of each event. We used inverse probability propensity score weighting to first balance patient characteristics between the high risk and non-high risk histologic subtypes. Covariates included in the propensity score model of high risk subtype were patient age, KPS at diagnosis, tumor size (maximum dimension), and biologically effective dose (BED) using an alpha/beta ratio of 10. KPS, tumor size and BED were treated as continuous variables. Each endpoint was then compared between high risk and non-high risk subtypes using propensity score-weighted Cox regression models. High and non-high risk groups were compared on characteristics using the Chi-square test for categorical variables or the Wilcoxon rank sum test for continuous variables. Patient characteristics (gender, KPS and age) were compared on a patient level (n=119) while the tumor characteristics (tumor size, dose, BED) were compared on lesion level (n=124). The propensity score analysis was performed using the twang R library (R version 3.2, www.R-project.org). Statistical significance for all analyses was 2-sided and used a 5% significance level (P < .05).
Results
The analyzed cohort included 119 patients with 124 treated lesions. Patient, tumor and treatment characteristics are presented in table 1. The median tumor size was 1.9 cm (range 0.7–5.3cm). The most common dose/fractionation scheme was 48 Gy in 4 fractions (BED=105, 46%) followed by 54 Gy in 3 fractions (BED=151, 24%), and 50 Gy in 5 fractions (BED=100, 19%). The median follow-up time for the whole cohort was 17 months and 21 for patients alive at time of analysis.
Table 1.
Baseline patient and tumor characteristics.
| Total | High Risk | Non-High Risk | p-value | |
|---|---|---|---|---|
| Number of patients | 119 | 35 (29%) | 84 (71%) | |
|
| ||||
| Gender | 0.35 | |||
| Male | 50 (42%) | 17 (49%) | 33 (39%) | |
| Female | 69 (58%) | 18 (51%) | 51 (61%) | |
|
| ||||
| KPS | 0.34 | |||
| median | 80 | 80 | 80 | |
| interquartile range | 80–90 | 70–90 | 80–90 | |
| range | 40–100 | 60–90 | 40–100 | |
|
| ||||
| Age (years) | 0.03 | |||
| median | 79 | 75 | 81 | |
| Interquartile range | 71–83 | 66–82 | 74–84 | |
| range | 51–95 | 51–93 | 55–95 | |
|
| ||||
| Number of lesions | 124 | 37 (30%) | 87 (70%) | |
|
| ||||
| Tumor size (cm) | 0.16 | |||
| median | 1.9 | 1.7 | 1.9 | |
| interquartile range | 1.4–2.5 | 1.2–2.4 | 1.4–2.5 | |
| range | 0.7–5.3 | 0.7–5.3 | 1.0–5.1 | |
|
| ||||
| Total dose (cGy) | 0.11 | |||
| median | 4800 | 4800 | 4800 | |
| Interquartile range | 4800–5000 | 4800–5000 | 4800–5400 | |
| range | 4000–5400 | 4500–5400 | 4000–5400 | |
|
| ||||
| BED (Gy, α/β=10) | 0.15 | |||
| median | 105 | 105 | 105 | |
| Interquartile range | 100–105 | 100–105 | 100–151 | |
| range | 81–151 | 86–151 | 81–151 | |
KPS= Karnofsky performance status, BED= Biologically effective dose.
Subtyping revealed acinar pattern to be the most common subtype (70%) and micropapillary to be the least common (9%, table 2). Forty-nine lesions (39.5%) were found to be pure (composed of a single histologic pattern) while 75 lesions (60.5%) were mixed.
Table 2.
Number of lesions analyzed according to histologic subtype.
| Number of lesions
| |||||
|---|---|---|---|---|---|
| Lepidic | Acinar | Papillary | Micropapillary | Solid | |
| Pure | 14 | 24 | 3 | 1 | 7 |
| Mixed | 41 | 63 | 22 | 10 | 22 |
| Total | 55 (44%) | 87 (70%) | 25 (20%) | 11 (9%) | 29 (23%) |
Five patients were treated for two separate primary tumors, three synchronously and two metachronously. Of these five patients, one patient experienced local failure that was clearly attributable to one of the treated lesions and not the other. Another patient was treated synchronous for right upper lobe and left upper lobe primaries of different subtypes (both non high-risk), and subsequently experienced bilateral mediastinal nodal and distant failure. As the responsible lesion could not be identified, nodal and distant failure was assigned to both lesions. The remaining three patients treated for two lesions did not experience treatment failure at last followup.
The cumulative incidences of local, nodal, regional and distant failure were significantly higher for lesions with a high risk component compared to non-high risk lesions (Figure 1). Of the 87 non high-risk lesions, the absolute numbers of local, nodal, regional and distant failures were three, four, one and five; of the 37 high risk lesions, the absolute numbers of such failures were six, five, three and ten. The 1-year cumulative incidence of local, nodal, regional and distant failure, respectively, in high risk versus non-high risk lesions were 7.3% vs 2.7%, 14.8% vs 2.6%, 4.0% vs 1.2% and 22.7% vs 3.6%. Overall survival was not significantly different between the high risk and non-high risk subsets (Figure 2). Six patients from the high risk cohort experienced local failure, all of whom received SBRT with a BED of 86 to 105 Gy in 4 or 5 fraction regimens. None of the four patients with high risk lesions that were treated to a dose of 18 Gy × 3 fractions (BED=151 Gy) experienced local failure. Similarly, all three patients from the non-high risk cohort who experienced local failure were treated to a BED of 81–105.
Figure 1.
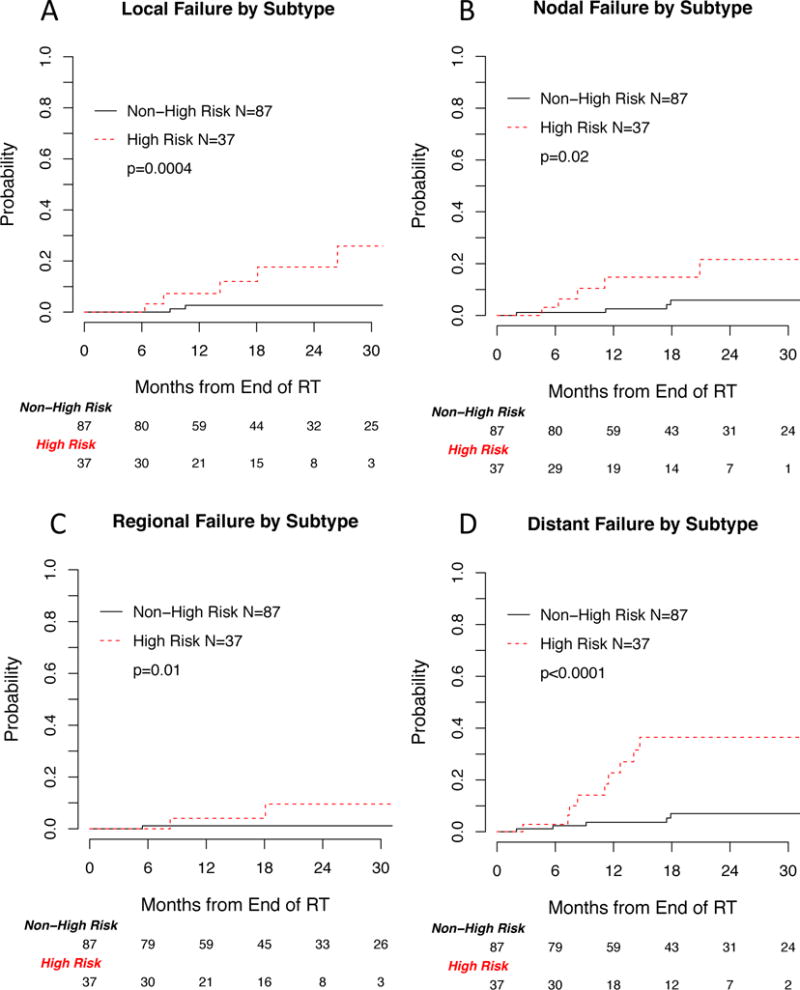
Cumulative incidence of (A) local, (B) nodal, (C) regional and (D) distant failure by high risk vs non-high risk adenocarcinoma subtype.
Figure 2.
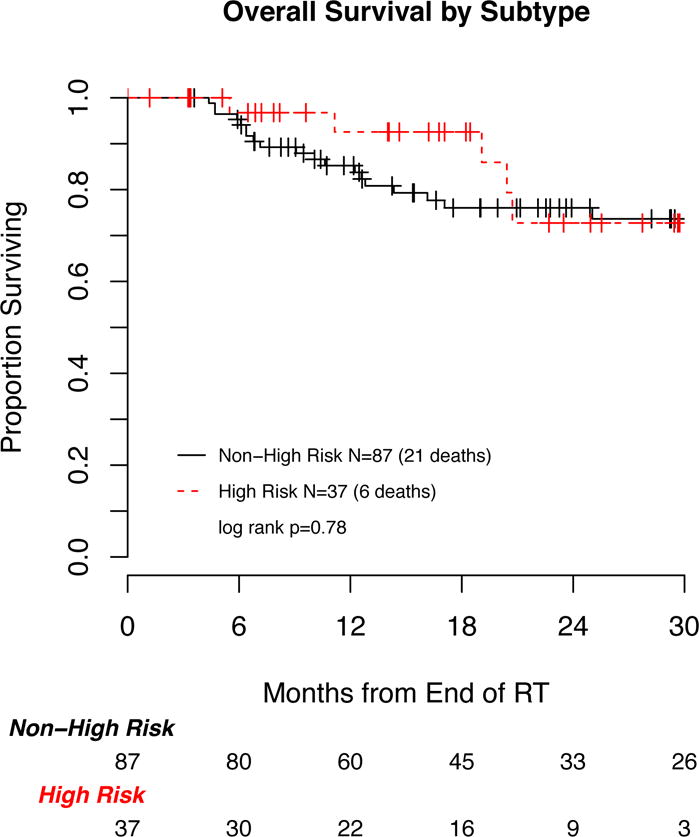
Overall survival according to high risk vs non-high risk adenocarcinoma subtype.
The multivariable adjusted hazard ratios from propensity score weighted Cox regression models revealed a significantly increased risk of local, regional and distant failures for the high risk compared to non-high risk subtypes (HR 16.8 95% CI 3.5–81.4, HR 20.9 95% CI 2.3–192.3 and HR 6.9 95% CI 2.2–21.1, respectively, table 3). The hazard ratios for nodal failure and overall survival were not statistically significant (HR 3.8 95% CI 0.95–15.0 and HR 0.8 95% CI 0.3–2.2, respectively).
Table 3.
Multivariable analysis using propensity score weighted Cox regression. Hazard ratios from Cox regression models were weighted by inverse propensity score. Twelve and 24 month cumulative incidences are shown. Covariates included in the propensity score model of high risk adenocarcinoma subtype included age, Karnofksy performance status, tumor size and radiation dose., CI = confidence interval.
| Endpoint | Non-High Risk | High Risk | |
|---|---|---|---|
| Local Failure | 1-year cumulative incidence (95%CI) | 2.7 (0, 6.4) | 7.3 (0, 17.3) |
| 2-year Cumulative incidence (95%CI) | 2.7 (0, 6.4) | 17.7 (1.0, 34.3) | |
| Hazard ratio (95%CI) | 1 | 16.8 (3.5, 81.4) | |
| p-value | 0.0005 | ||
|
| |||
| Nodal Failure | 1-year cumulative incidence (95%CI) | 2.6 (0, 6.1) | 14.8 (0.9, 28.7) |
| 2-year Cumulative incidence (95%CI) | 5.9 (0.2, 11.6) | 21.6 (3.0, 40.2) | |
| Hazard ratio (95%CI) | 1 | 3.8 (0.95, 15.0) | |
| p-value | 0.06 | ||
|
| |||
| Regional Failure | 1-year cumulative incidence (95%CI) | 1.2 (0, 3.5) | 4 (0, 11.9) |
| 2-year Cumulative incidence (95%CI) | 1.2 (0, 3.5) | 9.6 (0, 22.7) | |
| Hazard ratio (95%CI) | 1 | 20.9 (2.3, 192.3) | |
| p-value | 0.007 | ||
|
| |||
| Distant Failure | 1-year cumulative incidence (95%CI) | 3.6 (0, 7.7) | 22.7 (6.1, 39.4) |
| 2-year cumulative incidence (95%CI) | 7 (0.9, 13.1) | 36.5 (16.4, 56.5) | |
| Hazard ratio (95%CI) | 1 | 6.9 (2.2, 21.1) | |
| p-value | 0.0007 | ||
|
| |||
| Overall Survival | 1-year overall survival (95%CI) | 85% (75, 91) | 93% (73, 98) |
| 2-year overall survival (95%CI) | 76% (64, 84) | 73% (45, 88) | |
| Hazard Ratio (95%CI) | 1 | 0.80 (0.3, 2.2) | |
| p-value | 0.66 | ||
In all, 37 failures were found, of which 17 were biopsied. Among the 17 biopsied, 7 underwent fine needle aspiration and 10 core biopsies. Of the 10 core biopsies performed, all were found to be similar to the primary tumor in terms of morphology and subtype aside from one local failure in which a new component of micropapillary pattern was seen upon recurrence.
Discussion
In 2011, the IASLC/ATS/ERS proposed a new classification for primary lung adenocarcinomas[5] that was adopted by the 2015 WHO Classification[6], describing five separate histologic growth patterns: lepidic, acinar, papillary, micropapillary and solid. Multiple reports from the surgical literature have demonstrated histologic subtype to be a powerful predictor of clinical behavior and oncologic outcome[17, 18], however these predictors have not yet been confirmed in the context of SBRT. Specifically, the lepidic growth pattern is associated favorable recurrence-free survival and overall survival[17, 19] solid-predominant tumors are more prone to unfavorable recurrence-free and overall survival as well as earlier extrathoracic and multisite recurrences and inferior post-recurrence survival[14]. The presence of a micropapillary component also predicts an increased risk of recurrence and worse overall survival [7–10, 13, 17], particularly in the setting of close surgical margins[11], suggesting that such patients who undergo sublobar resection may need completion lobectomy or adjuvant therapy. Higher degree of micropapillary pattern has also been associated with occult pathologic mediastinal lymph node metastasis in cN0-N1 patients after surgical resection[15]. In a retrospective study of four large lung cancer cohorts, micropapillary and solid patterns were associated with significantly worse, disease free survival and lung cancer-specific disease free survival[12]. Studies of FDG-PET avidity identified micropapillary and solid-predominant subtype tumors to have the highest SUVmax [20, 21].
In the current study, rates of local failure following SBRT were significantly higher in the high risk adenocarcinoma histologic subtype cohort compared to the non-high risk cohort (1-year cumulative incidence of 7.3% vs 2.7%). Multivariable analysis accounting for tumor size and BED, among other factors, revealed a significantly increased risk of local failure in high risk lesions (HR 16.8, 95% CI 3.5–81.4). Interestingly, high risk subtypes, particularly micropapillary tumors, have also been associated with the pathologic phenomenon of “spread through alveolar air spaces” with extension of tumor cells into lung parenchyma adjacent to the main tumor, an entity that is associated with increased risk of recurrence [17, 22]. In the future, consideration could be given to dose or volume escalation for high risk lesions, given the propensity for local failure which may in part be due to microscopic extension beyond radiographically apparent gross disease[23–27]. In addition, all six of the patients in the current study with high risk lesions who experienced local failure received a BED of 86–105 and were not treated to the highest dose level of 151 BED (18 Gy × 3) due to proximity to the central airway or chest wall. While a cutoff of BED of 100 has been reported as a minimum dose necessary for optimal tumor control,[28] higher doses may be needed to ablate lesions with high risk histology.
In addition to local failure, high risk subtype was associated with a trend towards increased risk of nodal failure following SBRT which reached borderline significance (p=0.06). This finding will need to be validated in a larger cohort but may raises the possibility that elective nodal coverage in early-stage adenocarcinoma may warrant investigation for patients with high risk lesions. In addition, if this finding is validated, mediastinal nodal sampling, which was not routinely performed in our cohort, may be particularly important in patients with high risk subtypes to rule out occult nodal disease prior to SBRT.
Our results also showed increased risk of distant metastasis in the high risk subtype cohort. While adjuvant systemic therapy is not routinely recommended following resection or SBRT for early-stage NSCLC[29], high risk histologic subtype may have prognostic value for selecting patients who would benefit from adjuvant chemotherapy or targeted therapy. Indeed, in one study, patients with micropapillary and solid tumor subtypes in surgically resected lung adenocarcinomas were shown to benefit most from adjuvant chemotherapy, though benefit from adjuvant chemotherapy was greater in stage II or III patients[12]. Interestingly, one study identified improved response to chemotherapy and longer survival in high risk subtypes (solid, papillary and micropapillary) in core biopsies when looking at patients with advanced disease (stage IIIB-IV).[30] The benefit of systemic therapy following surgery or SBRT in this high risk cohort remains to be investigated prospectively. However, the feasibility of administering adjuvant chemotherapy to patients following SBRT may be limited by the comorbidites of many SBRT patients who are not surgical candidates at baseline.
Subtyping of lung adenocarcinoma requires pathologic review of surgical or core biopsy specimens to assess histologic pattern of growth. While the majority of studies assessing subtype have analyzed surgical resection specimens, some studies have assessed biopsy samples and correlated subtype with outcomes and response to chemotherapy.[12, 30, 31] In one study, however, the predominant subtype identified on biopsy matched the predominant subtype identified on resection pathology only 66% of the time with concordance rates particularly low in smaller biopsy samples[32]. Biopsy-resection concordance rates were highest for solid and papillary-predominant tumors. While examination of surgical resected specimens allows for more accurate quantification of subtype components within a tumor, core biopsy may not sample all of the components of a tumor and thus provide less complete subtyping information. Nevertheless, it is intriguing that both for chemotherapy and radiation it appears that early data indicates adenocarcinoma histologic subtyping on core biopsy specimens appears to provide useful information that predicts response to key therapeutic approaches. Specimens from fine needle aspiration, which is commonly practiced for establishing the diagnosis of NSCLC, do not allow subtyping due to lack of histologic architecture information. As histologic subtype is emerging as a powerful predictor of response to treatment, core biopsy should be increasingly considered for the diagnosis of early-stage NSCLC, though further work is needed to establish the validity and accuracy of subtyping on biopsy samples.
Our study is limited by its retrospective nature and associated biases. Furthermore, the small number of events limited our analysis. Further validation of these findings is needed in larger cohorts of patients. No difference was seen in overall survival in high risk vs non-high risk adenocarcinoma histologic subtype despite significantly increased rates of recurrence and metastasis. This is likely due to limited follow up (median 17 months) in our cohort, as assessment and reporting of adenocarcinoma subtype has only recently become routine on core biopsy samples at our institution. Ultimately, a study of treatment outcomes performed across individual adenocarcinoma subtypes, as opposed to high risk vs. non-high risk groupings, will be important but the limited number of events in this study did not allow this type of analysis.
Conclusions
We have assessed the association of adenocarcinoma histologic pattern with outcome following SBRT for early-stage lung adenocarcinoma. While the impact of subtype has been well established in the surgical literature, further studies are needed to validate these findings in the setting of SBRT and to better establish the validity of histological pattern assessment on core biopsy samples. Adenocarcinoma histologic subtype is a prognostic factor that may ultimately prove to be valuable for selection of patients for SBRT dose or volume escalation, elective nodal treatment or adjuvant systemic or targeted therapy. Ultimately, prospective trials are needed in this regard.
Summary.
Histologic subtyping surgically resected lung adenocarcinoma has become established as a predictor of prognosis and recurrence. Herein, we demonstrate that high risk histologic subtypes (micropapillary or solid) in core biopsies are associated with significantly increased risk of local, nodal and distant failure after SBRT for early-stage lung adenocarcinoma. This may have important implications for patient selection, adjuvant treatment and clinical trial design.
Acknowledgments
Funding: This work was supported in part by NIH/NCI P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Rimner receives research funding from Boehringer Ingelheim and Varian Medical Systems.
References
- 1.Devesa SS, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. International journal of cancer. 2005;117(2):294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008;3(8):819–31. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 3.Asamura H, et al. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008;3(1):46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, et al. Non-small-cell lung cancer. Lancet. 2011;378(9804):1727–40. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6(2):244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis WD, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10(9):1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(8):992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami T, et al. Micropapillary pattern and grade of stromal invasion in pT1 adenocarcinoma of the lung: usefulness as prognostic factors. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(5):514–21. doi: 10.1038/modpathol.3800765. [DOI] [PubMed] [Google Scholar]
- 9.Makimoto Y, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi’s type C tumours) Histopathology. 2005;46(6):677–84. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi T, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. The American journal of surgical pathology. 2003;27(1):101–9. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Nitadori J, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. Journal of the National Cancer Institute. 2013;105(16):1212–20. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao MS, et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(30):3439–46. doi: 10.1200/JCO.2014.58.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsumida H, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(6):638–47. doi: 10.1038/modpathol.3800780. [DOI] [PubMed] [Google Scholar]
- 14.Ujiie H, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(26):2877–84. doi: 10.1200/JCO.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh YC, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification predicts occult lymph node metastasis in clinically mediastinal node-negative lung adenocarcinoma. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2016;49(1):e9–e15. doi: 10.1093/ejcts/ezv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spratt DE, et al. Recurrence Patterns and Second Primary Lung Cancers After Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance. Clinical lung cancer. 2016;17(3):177–183 e2. doi: 10.1016/j.cllc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warth A, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. The American journal of surgical pathology. 2015;39(6):793–801. doi: 10.1097/PAS.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 18.Motoi N, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. The American journal of surgical pathology. 2008;32(6):810–27. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, et al. Distinctive histopathological features of lepidic growth predominant node-negative adenocarcinomas 3–5 cm in size. Lung cancer. 2013;79(2):118–24. doi: 10.1016/j.lungcan.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Kadota K, et al. FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Annals of surgical oncology. 2012;19(11):3598–605. doi: 10.1245/s10434-012-2414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura H, et al. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake in positron emission tomography. Lung cancer. 2015;87(1):28–33. doi: 10.1016/j.lungcan.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Kadota K, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10(5):806–14. doi: 10.1097/JTO.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraud P, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. International journal of radiation oncology, biology, physics. 2000;48(4):1015–24. doi: 10.1016/s0360-3016(00)00750-1. [DOI] [PubMed] [Google Scholar]
- 24.Grills IS, et al. Clinicopathologic analysis of microscopic extension in lung adenocarcinoma: defining clinical target volume for radiotherapy. International journal of radiation oncology, biology, physics. 2007;69(2):334–41. doi: 10.1016/j.ijrobp.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Meng X, et al. Noninvasive evaluation of microscopic tumor extensions using standardized uptake value and metabolic tumor volume in non-small-cell lung cancer. International journal of radiation oncology, biology, physics. 2012;82(2):960–6. doi: 10.1016/j.ijrobp.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 26.Siedschlag C, et al. The impact of microscopic disease on the tumor control probability in non-small-cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011;100(3):344–50. doi: 10.1016/j.radonc.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 27.van Loon J, et al. Microscopic disease extension in three dimensions for non-small-cell lung cancer: development of a prediction model using pathology-validated positron emission tomography and computed tomography features. International journal of radiation oncology, biology, physics. 2012;82(1):448–56. doi: 10.1016/j.ijrobp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Onishi H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101(7):1623–31. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger DS, et al. Non-Small Cell Lung Cancer, Version 6.2015. Journal of the National Comprehensive Cancer Network: JNCCN. 2015;13(5):515–24. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 30.Campos-Parra AD, et al. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. The European respiratory journal. 2014;43(5):1439–47. doi: 10.1183/09031936.00138813. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, et al. Solid predominant histology predicts EGFR tyrosine kinase inhibitor response in patients with EGFR mutation-positive lung adenocarcinoma. Journal of cancer research and clinical oncology. 2013;139(10):1691–700. doi: 10.1007/s00432-013-1495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzawa R, et al. Factors influencing the concordance of histological subtype diagnosis from biopsy and resected specimens of lung adenocarcinoma. Lung cancer. 2016;94:1–6. doi: 10.1016/j.lungcan.2016.01.009. [DOI] [PubMed] [Google Scholar]


