Abstract
Conjugate (or 1,4-) additions of carbanionic species to α,β-unsaturated carbonyl compounds are vital to research in organic and medicinal chemistry, and there are several known chiral catalysts that facilitate the catalytic enantioselective additions of nucleophiles to enoates1. However, catalytic enantioselective 1,6-conjugate additions are uncommon, and ones that are able to incorporate readily functionalizable moieties, such as propargyl or allyl groups, into acyclic α,β,γ,δ-doubly unsaturated acceptors are unknown2. Chemical transformations that could generate a new bond at the C6 position of a dienoate are particularly desirable, as the resulting products would be subjected to further modifications; such reactions, especially when dienoates contain two equally substituted olefins, are scarce3 and are confined to reactions promoted by a phosphine–copper (with alkyl Grignard4,5, dialkylzinc or trialkylaluminum compounds6,7), a diene–iridium (with arylboroxines)8,9, and a bisphosphine–cobalt catalyst (with monosilyl-acetylenes)10. 1,6-conjugate additions are otherwise limited to substrates where there is full substitution at C411. It is not clear why certain catalysts favor bond formation at C6, and – while there are a small number of catalytic enantioselective conjugate allyl additions12,13,14,15 – related 1,6-additions and processes involving a propargyl unit are non-existent. In this manuscript, we show that an easily accessible organocopper catalyst can promote 1,6-conjugate additions of propargyl and 2-boryl-substituted allyl groups to acyclic dienoates with high selectivity. A commercially available allenylboron compound or a monosubstituted allene may be used. Products can be obtained in up to 83 percent yield, >98 percent diastereo- (for allyl additions) and 99:1 enantiomeric ratio. Mechanistic details, including the origins of high site- (1,6- versus 1,4-) and enantioselectivity as a function of the catalyst structure and reaction type, have been elucidated by means of density functional theory (DFT) calculations.
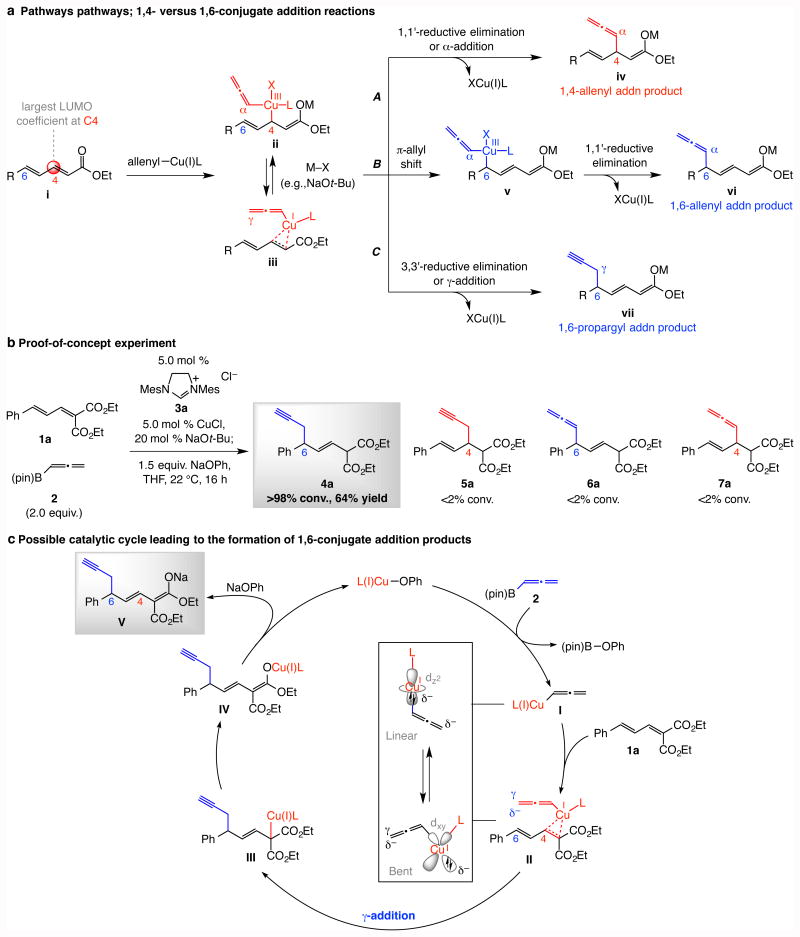
Designing an efficient 1,6-conjugate addition is difficult is that the largest coefficient of the lowest unoccupied molecular orbital (LUMO; see i, Fig. 1a) is at C4 and consequently this is where the bond is preferentially generated. We surmised that any conjugate addition should begin by interaction of the nucleophilic allenylcopper species with dienoate C4 by an oxidative addition to give ii or π-complexation16 to afford iii (Fig. 1a). 1,6-Addition could then be favorable if certain alternative processes were faster than the 1,1′-reductive elimination that affords 1,4-allenyl addition compound iv (Route A, Fig. 1a). We envisioned two possible scenarios in this regard: 1) Compound ii might undergo a 1,3-shift followed by 1,1′-reductive elimination to deliver 1,6-allenyl addition product vi via v by forming a bond between dienoate's C6 and the Cα of the allenylmetal system (Route B); this type of π-allyl isomerization has been previously suggested5 but experimental or computational support was not provided. 2) Organocopper complex iii might be directly transformed to the 1,6-propargyl-addition product vii by a creation of a bond between the dienoate C6 and the Cγ of the allenyl–copper moiety (Route C, Fig. 1a). That is, the allenylcopper complex in its bent form (see Fig. 1c) may interact with the C3–C4 π cloud, placing the nucleophilic Cγ near the dienoate C6. This pathway would be reminiscent of a 3,3′-reductive elimination proposed vis-à-vis enantioselective allyl–allyl coupling with Ni- and Pd complexes17,18. A similar reaction mode with an organocopper species has been mentioned in just one instance (again, without experimental or computational support)19.
Figure 1. Possible conjugate addition pathways, the initial experiment and a plausible catalytic cycle.
a, In a conjugate reaction, addition to the C4 site is kinetically favored (→ii or iii); subsequent 1,1′-reductive elimination (or α-addition) could afford product iv (Route A), or a 1,3—π-allyl shift (→v) may precede reductive elimination, affording 1,6-allenyl addition product vi (Route B). Alternatively, ii/iii may be directly converted to vii by a γ-addition (3,3′-reductive elimination type) process (Route C). b, Proof-of-principle experiment indicates that with an allenyl–copper intermediate, Route C predominates. c, Plausible catalytic cycle for the preferential formation of the 1,6-propargyl addition product. Abbreviations: R, or G, various organic functional groups; LUMO, lowest unoccupied molecular orbital; M, metal; pin, pinacolato; Mes, 2,4,6-trimethylphenyl.
We first carried out a model transformation involving dienoate 1a and commercially available allenyl–B(pin) 2 with a Cu complex derived from imidazolinium salt 3a and CuCl. We opted for NaOPh versus an alkoxide as the stroichiometric base (e.g., NaOt-Bu) because the residual CuOPh is less Lewis basic and would not interfere with the function of a chiral catalyst; small amounts of either may however be used to deprotonate the imidazolinium salt to generate the NHC–Cu complex. In the event, at ambient temperature and after 16 hours 4a was isolated in 64% yield as a single alkene isomer (>98% β,γ-enoate); the 1,6-allenyl, 1,4-propargyl or 1,4-allenyl addition products were not detected (5a–7a). The exclusive formation of 4a implies that the pathway involving a π-allyl shift is not operative (Route B); otherwise, allenyl compound 6a would be formed. The catalytic cycle in Fig. 1c is probably the most relevant. Under the same conditions but with the corresponding α,β,γ,δ-unsaturated mono-ester there was minimal conversion (<5%).
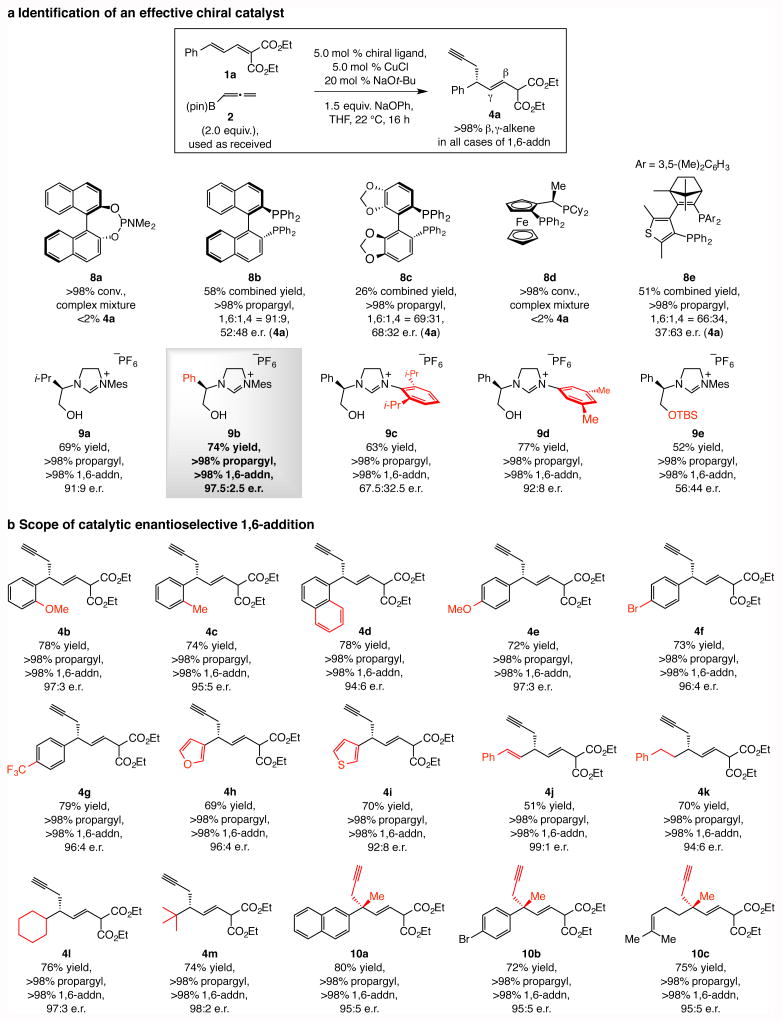
Next, we examined the effect of chiral phosphorous-based ligands. In certain cases either a complicated mixture of compounds was generated (8a and 8d, Fig. 2a) or, unlike the aforementioned N-heterocyclic carbene (NHC) copper species, appreciable amounts of the 1,4-addition product (5a) were formed (mechanistic analysis below). Enantioselectivity was uniformly low. Matters improved with NHC–Cu complexes (9a-e, Fig. 2a): 4a was generated exclusively (>98:2 1,6-:1,4-propargyl addition), and the complex derived from phenylglycine-derived 9b delivered it in 74% yield and 97.5:2.5 enantiomeric ratio (e.r.). Nevertheless, some of the screening data were unexpected. Whereas reaction with the larger 9c afforded substantially reduced selectivity (67.5:32.5 e.r.), with imidazolinium salt 9d, which contains a less imposing 3,5-dimethylphenyl moiety, 4a was formed in 92:8 e.r. Further, unlike the related catalytic allylic substitution processes20, protection of the NHC hydroxy group was detrimental to enantioselectivity: reaction with silyl protected 9e gave nearly racemic product.
Figure 2. Catalytic enantioselective 1,6-propargyl conjugate additions.
a, Screening of a variety of chiral phosphine and NHC ligands indicated that the chiral copper catalysts derived from the latter series are significantly more effective, and that corresponding to imidazolinium salt 9b is optimal. b, The catalytic process is broadly applicable, affording products uniformly with >98% propargyl and 1,6-addition selectivity and in up to 80% yield and 98:2 e.r. Products containing a tertiary or an all-carbon quaternary carbon stereogenic center can be accessed. Abbreviations: pin, pinacolato; TBS, tert-butyldimethylsilyl, Mes, 2,4,6-trimethylphenyl; Mes, 2,4,6-trimethylphenyl.
Reactions were performed under N2 under the conditions shown in the box for 4a, except for 10a-c, where 10 mol % 9b and CuCl were used and mixture was allowed to stir for 24 h. Conversions, propargyl:allenyl and 1,6-:1,4-addition ratios were measured by analysis of 1H NMR spectra of unpurified mixtures; the variance of values estimated to be <±2%. Yields correspond to isolated and purified products and represent an average of at least three runs (±5%). See the Supplementary Information for experimental details and spectroscopic analyses.
The enantioselective protocol has considerable scope (Fig. 2b). Dienoates withan aryl unit (4b-g), whether it is electron donating (4e) or electron withdrawing (4g), react efficiently to give products in >98:2 propargyl:allenyl and 1,6-:1,4 selectivity and 94:6–97:3 e.r. A bromoaryl group is tolerated (4f), which is notable since an unhindered aryl–bromine bond can be prone to undergoing oxidative insertion with a copper(I) complex. High efficiency and selectivity was observed with heterocyclic substrates (4h,i) or those that bear an alkenyl (4j), a linear or a branched aliphatic group (4k,l). The sterically congested tert-butyl-substituted 4m was isolated in 74% yield and 98:2 e.r. (>98% propargyl and 1,6-addition); the ease with which this C–C bond is formed corroborates the initial addition occurring at C4, distally from the quaternary site at C6, followed by an intramolecular event that is less susceptible to steric pressure. All-carbon quaternary stereogenic centers21 were formed efficiently and with exceptional group-, site- and enantioselectivity (10a-c, Fig. 2b). Again, none of the allenyl- or 1,4-addition byproducts were generated and a single olefin isomer (>98% E) was detected (400 MHz 1H NMR analysis; see the Supplementary Information for details.)
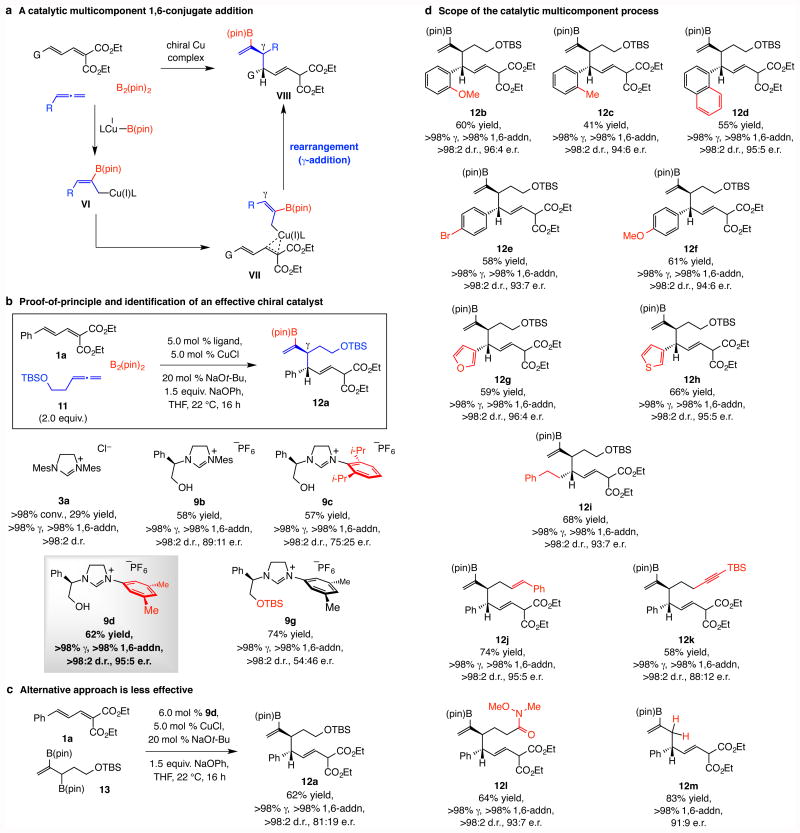
We then evaluated the possibility of a multicomponent enantioselective 1,6-addition by which a dienoate, a monosubstituted allene and B2(pin)2 may be combined (Fig. 3a). We envisioned association of allylcopper species VI with a substrate to yield VII, which could rearrange to give VIII. Electronic modification of one of the reacting alkenes by a B(pin) moiety could counter γ-addition. The other concern was that diastereomeric mixtures could form.
Figure 3. Catalytic diastereo- and enantioselective multicomponent 1,6-conjugate addition of 2-B(pin)-substituted allyl moieties.
a, The pathway through which 1,6-addition products may be generated by a multicomponent process involving a dienoate, an allene and B2(pin)2. b, Preliminary experiment with an achiral NHC–Cu complex demonstrates that, although inefficient, reactions are exceptionally γ-, group- and 1,6-selective. Screening studies to identify an effective chiral catalyst indicates that a different NHC ligand is optimal for these transformations (vs. propargyl additions). c, The alternative approach entailing initial synthesis of a diboryl reagent leads to lower enantioselectivity. d, The catalytic protocol has considerable scope. Abbreviations: pin, pinacolato; TBS, tert-butyldimethylsilyl.
Reactions were performed under N2 under the conditions shown for synthesis of rac-12a (Fig. 3b). Conversions, propargyl:allenyl, 1,6-:1,4-addition and diastereomeric ratios (d.r.) were measured by analysis of 1H NMR spectra of unpurified mixtures; the variance of values estimated to be <±2%. Yields correspond to isolated and purified products and represent an average of at least three runs (±5%). Ketone 12k was obtained after oxidative work-up. See the Supplementary Information for experimental details and spectroscopic analyses.
Reaction of dienoate 1a, allene 11 and B2(pin)2 with 5.0 mol % imidazolinium salt 3a and CuCl afforded 12a exclusively and with exceptionally high 1,6-:1,4- and diastereomeric ratios (d.r.; Fig. 3b). The low yield of 12a arises from a breakdown in chemoselectivity, namely by competitive boryl 1,4-addition. The optimal catalyst would therefore have to deliver high enantioselectivity and favor Cu–B addition to the allene over its reaction with a dienoate. Examination of different chiral imidazolinium salts led to encouraging results, as 12a was generated more efficiently (57–74% yield). The Cu complex derived from imidazolinium salt 9d proved optimal, affording 12a in 62% yield, >98:2 d.r. and 95:5 e.r. However, the trends in enantioselectivity were again puzzling. It was not a surprise that the transformations with catalysts derived from imidazolinium salts 9b,c gave 12a in 89:11 and 75:25 e.r., respectively, as there was a similar trend (albeit with a larger difference) with the propargyl additions (cf. Fig. 2a). What was perplexing was that enantioselectivity was higher with the less sterically congested 9d (95:5 e.r.).
Use of a diboryl compound such as 1322 (Fig. 3c) is a less attractive option. The need for the initial synthesis of an allylboron compound notwithstanding, the two-stage alternative, although highly γ-, site- and diastereoselective, proceeds with diminished enantioselectivity (Fig. 3c): with 13 as the reagent, 12a was obtained in 81:19 e.r. (vs. 95:5 through the multicomponent process). Control experiments indicate that lower e.r. originates from efficient addition of an achiral allylcopper species, generated from allyl(PhO)CuNa with 13, to the dienoate to give racemic product; the background reaction produces the 1,6-addition isomers exclusively (see below for further discussion the Supplementary Information for mechanistic/computational analysis). In the multicomponent processes, on the other hand, only an NHC–Cu(OPh) complex can efficiently activate the B–B bond in B2(pin)2.
The multicomponent process has ample range as well (Fig. 3e). Aryl- (12b-f), heteroaryl- (12g,h), or alkyl-substituted (12i) dienoates can be converted to the desired products with >98% γ-, site-, and diastereoselectivity and 93:7–99:1 e.r. Compounds containing an alkene (12j), an alkyne (12k) or a Weinreb amide (12l) were synthesized in 58–74% yield and 88:12–95:5 e.r., and unsubstituted allene may be used (12m). Unlike with allenylboronate 2 (cf. Fig. 2b), reactions with the more highly substituted enoates that would afford quaternary carbon centers were inefficient (<10% conv.); this is probably because of the increased steric repulsion caused by the sizeable B(pin) moiety.
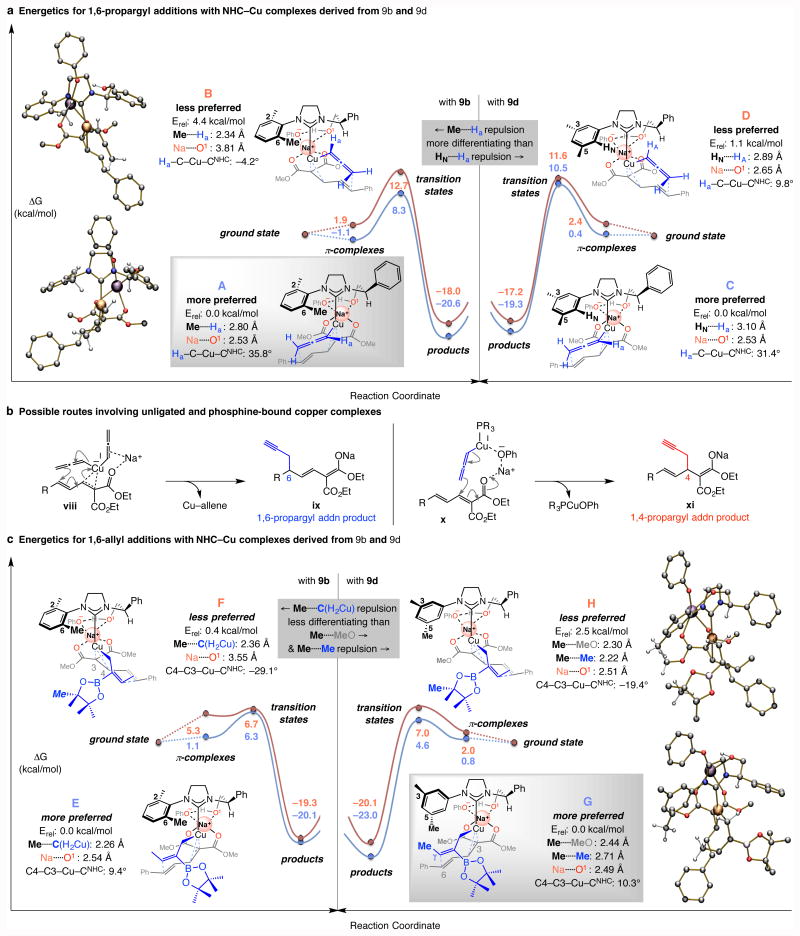
Several key mechanistic questions needed to be addressed at this point: Why does the identity of the optimal chiral Cu complex vary significantly, and why is it that, unlike multicomponent allylic substitutions20, the unprotected hydroxyl group within the catalyst structure is needed for high enantioselectivity? To shed light on these issues, DFT calculations were performed at ωB97XD/Def2TZVPP//ωB97XD/Def2SVPTHF(PCM) level of theory (see the Supplementary Information for details). Computational studies (Fig. 4) indicate that the linear Cu(I)–allenyl species derived from imidazolinium salts 9b and 9d associate with the C3–C4 bond, furnishing a square planar-type π-complex16 (A–D, Fig. 4a), precursors to 1,6-propargyl addition products (Fig. 2). The propensity of NHC–Cu complexes to afford an η2 complex at the C3–C4 site may be attributed to the stronger electron donating ability of the heterocyclic ligands (vs. phosphine), which raises the energy of copper's d-orbitals, causing stronger binding with the corresponding π* orbital16,23. 1,6-Addition products can be formed once an NHC–Cu-π complex is formed. A relevant experimental finding is that, similar to NHC–Cu but unlike phosphine Cu complexes, reactions with allenyl(t-BuO)CuNa (generated without a ligand) proceed with exceptional 1,6-:1,4-selectivity (>98:2). This might be because, as with an NHC–Cu system, the strongly π-basic in situ-formed (allenyl)2cuprate species [from allenyl(t-BuO)CuNa] can establish a complex with the C3–C4 alkene (see viii→ix, Fig. 4b), resulting in 1,6-propargyl addition. With the less Lewis basic phosphines, back-bonding is less favored. Additionally, DFT calculations indicate that with a weaker σ-donating phosphine unit, the highest occupied molecular orbital (HOMO; dz2, Fig. 1c) in a linear allenyl–Cu–phosphine complex is lower in energy (−7.43 eV for L = PPh3 vs. −7.14 eV for L = NHC). Consequently, aryloxide–Cu complexation can be stronger (less “filled–filled” electronic repulsion) during x→xi (Fig. 4b), leading to 1,4-propargyl addition. It follows that transformations proceeding via these conformationally more flexible transition structures are less enantioselective (vs. NHC–Cu systems; cf. Fig. 2a).
Figure 4. Mechanistic considerations.
a, Based on DFT calculations [ωB97XD/Def2TZVPP//ωB97XD/Def2SVP level of theory (THF)] stereochemical models were developed for NHC–Cu-catalyzed 1,6-propargyl additions with catalysts bearing an N-mesityl moiety (from 9b). The issue is the larger energetic differentiation arising from steric repulsion between an o-methyl unit of the N-aryl group and the allenylcopper moiety (i.e., Me⋯.Ha, 9b) versus one involving an aryl proton (i.e., HN⋯.Ha, 9d). b, Routes by which a phosphine–based and non-ligated Cu complex might generate products, respectively. c, Transition state energies for enantioselective allyl additions are consistent with the observation that the catalyst derived from 9d is optimal (vs. 9b). Steric repulsion involving a meta-methyl group of the NHC ligand with the carboxylic ester and the allylcopper substituents are the distinguishing elements. See the Supplementary Information for details of calculations. Abbreviations: NHC, N-heterocyclic carbene; Erel, relative energy.
DFT calculations reveal that high enantioselectivity originates from the structural organization caused by a cationic sodium interacting with the catalyst's hydroxyl unit, in turn H-bonded with the phenoxy counterion and the dienoate carbonyl groups. In the more favorable pathway with 9b (via A; Fig. 4a, left panel) there is less steric strain between the N-aryl's methyl group and the allenyl hydrogen Ha than in B (Me.....Ha distance of 2.80 vs. 2.34 Å, respectively). Steric pressure in B can be alleviated by rotation of the NHC ligand around the Cu–CNHC bond but this weakens the interaction between the hydroxy and the sodium cation (i.e., Na.....O1, 3.81 vs. 2.53 Å in B and A, respectively). With the complex derived from 3,5-dimethyl-substituted 9d (Fig. 4a, right panel) a smaller energy gap separates the two modes of reaction (C vs. D; 1.1 vs. 4.4 kcal/mol for A and B); this might be because there is less difference in steric repulsion between the allenyl hydrogen and the ortho hydrogen of the N-aryl group in D (HN.....Ha, 3.10 and 2.89 Å in C and D, respectively).
With the more sizeable pinacolatoboron-substituted allylcopper species (E-H, Fig. 4c) a similar complexation involving the catalyst's hydoxyl unit and a sodium cation takes hold. The smaller NHC–Cu system (from 3,5-dimethyl-phenyl- substituted 9d) can differentiate better between the two orientations of the allylic nucleophile compared to when 9b is involved (Fig. 4b, right panel). Specifically, in the transition state leading to the major enantiomer G the allylcopper moiety's Cγ is oriented such that there is optimal overlap with C6 of the dienoate. In complex H, alignment of the latter two components engenders distortion of the C4–C3–Cu–CNHC dihedral angle (−19.4° vs. +10.3° for H and G, respectively). The N-aryl methyl unit in H sits closer to the bulky pinacolato moiety (2.22 Å) and a methoxy group (2.30 Å) than in G when it is at a more favorable distance from the allylic methyl and ester substituents (2.71 and 2.44 Å, respectively). With the less selective catalyst derived from 9b, which contains an N-2,6-dimethylphenyl group (Fig. 4b, left panel), the steric repulsion involving the N-aryl methyl and the methylene unit of the allylcopper moiety is the dominant interaction in F as well as E (Me.....CH2, 2.26 and 2.36 Å in E and F, respectively). Hence, overall, the calculated energy difference between E and F amounts to just 0.4 kcal/mol (vs. 2.5 kcal/mol for H vs. G; Fig. 4c).
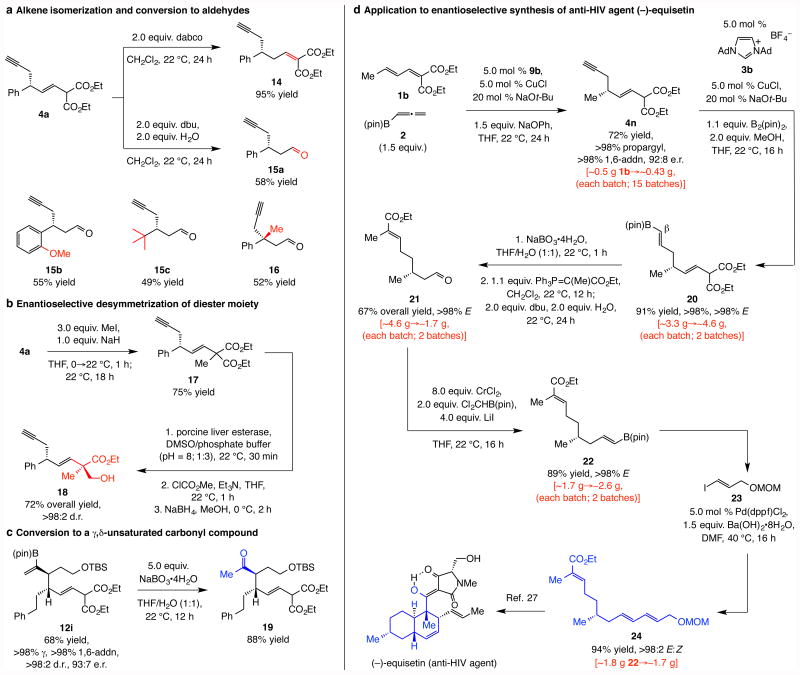
The enantiomerically enriched products can be converted to otherwise difficult-to-access molecules (Fig. 5). Synthesis of enyne 14 (95% yield, Fig. 5a) shows that the kinetically generated β,γ-alkene can be equilibrated to the lower energy, conjugated isomer. In contrast, with an amine base and water, the corresponding β-substituted aldehydes were formed exclusively: 15a-c and 16, containing a quaternary carbon stereogenic center, were obtained in 49–58% yield. These transformations offer an attractive entry for synthesis of β-substituted enantiomerically enriched aldehydes that cannot be prepared easily by alternative protocols; as already mentioned, there are no catalytic enantioselective 1,4-propargyl additions1. Synthesis of this type of enantiomerically enriched aldehydes by conjugate addition of an aryl- or alkyl-metal reagent (e.g., PhMgCl or Me2Zn) to an unsaturated ester followed by oxidation state adjustment would be problematic due to sensitivity of the methylene unit in the α,β,δ,ω- dienoate (or the corresponding enyne). The diester moiety offers additional opportunities; for example, through an alkylation/enzymatic desymmetrization24 sequence, 4a was converted to alcohol-ester 18 (Fig. 5b), which bears a quaternary carbon stereogenic center. As represented by 19 (Fig. 5c), oxidation of the alkenyl–B(pin) moiety affords γ,δ-unsaturated ketones with vicinal stereogenic centers; these fragments have been utilized in total synthesis of biologically active compounds and might be prepared by Claisen rearrangement25 (see the Supplementary Information for additional bibliography) but are difficult to access directly, especially in a catalytic and enantioselective manner26.
Figure 5. Functionalizations and demonstration of utility.
a, The kinetically favored alkene can be readily isomerized to the thermodynamically preferred isomer under one set of basic conditions, while with water present, cleavage of the diester moiety leads to the formation of β-substituted aldehydes. b, Alkylation followed by enzymatic desymmetrization of the diester unit proceeds with excellent stereochemical control. c, Oxidation of the alkenyl–B(pin) moiety affords otherwise difficult-to-access γ,δ-unsaturated ketones with vicinal stereogenic centers at the α- and β-carbon sites. d, Application to synthesis of gram quantities of enantiomerically enriched triene 24, previously used in the total synthesis of anti-HIV agent (–)-equisetin showcases utility of the catalytic approach. Abbreviations: dabco, 1,4-diazabicyclo[2.2.2]octane; dbu, 1,8-diazabicyclo[5.4.0]undec-7-ene ; DMSO, dimethylsulfoxide; Ad, adamantyl; dppf, 1,1′-bis(diphenylphosphino)ferrocene; MOM, methoxymethyl; pin, pinacolato.
Preparation of triene 24, employed in an intramolecular Diels-Alder reaction en route to anti-HIV agent (–)-equisetin27, highlights utility (Fig. 5c). Enyne 4n was secured with >98% propargyl and 1,6-selectivity in 72% yield and 92:8 e.r. This transformation was performed on ∼0.5 gram scale (15 times) and with unpurified commercially available organoboron reagent 2, underscoring the ease with which significant quantities of the ligand precursor can be synthesized, the scalability of the catalytic processes and the their high degree of reliability/reproducibility. NHC–Cu-catalyzed site- and stereoselective proto-boryl addition28 to the alkyne [(<2% addition to alkene, <2% Z alkenyl–B(pin)] afforded 20 in 91% yield (∼4.6 g). Oxidation of the C–B bond, Wittig reaction (>98% E), followed by cleavage of the β,γ-unsaturated diester moiety to the derived aldehyde (cf. Fig. 5a), afforded 21 in 67% overall yield (∼1.7 g). E-Alkenyl–B(pin) 22 was then prepared in 89% yield (∼2.6 g) by a Cr(II)-based reagent (oxidation state with low toxicity)29. Phosphine–Pd-catalyzed cross-coupling with alkenyl iodide 2330 delivered ∼1.7 grams of the desired triene 24 (94% yield; >98% E). Although a pathway of similar length may be envisioned starting from readily available enantiomerically pure starting materials (e.g., citronellol), this strategy has the advantage of being more easily amenable to preparation of various other analogues of the biologically active agent.
We thus introduce an approach for efficient catalytic enantioselective 1,6-conjugate addition of two types of valuable unsaturated organic moieties to dienoates. The mechanistic details regarding various features of an effective catalyst should help pave the way for achieving such important objectives. Considering that other readily available unsaturated organoboron and/or organocopper systems may be used, the low cost and ease with which the catalysts can be accessed, the reliably high selectivity values, and the versatility of the resulting products, the strategies delineated above adumbrate considerable impact on future advances in stereoselective catalysis and chemical synthesis.
Supplementary Material
Acknowledgments
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (GM-47480) and the National Science Foundation (CHE-1362763). We are grateful to Atsuhiro Iimuro for experimental assistance.
Footnotes
Author information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions F. M., and X. L. were involved in the discovery and development of the optimal catalysts, the corresponding methods and their various applications; S. T. and Y. S. performed the computational investigations, developed the models for the observed levels and patterns in selectivity; X. S. developed efficient routes for preparation of the dienoates. A. H. H. directed the investigations and wrote the manuscript with revisions provided by the other authors.
References
- 1.Alexakis A, Krause N, Woodward S. In: Copper-Catalyzed Asymmetric Synthesis. Alexakis A, Krause N, Woodward S, editors. VCH–Wiley; 2014. pp. 33–68. [Google Scholar]
- 2.Silva EMP, Silva AMS. 1,6-Conjugate addition of nucleophiles to α,β,γ,δ-diunsaturated systems. Synthesis. 2012;44:3109–3128. [Google Scholar]
- 3.Tissot M, Li H, Alexakis A. In: Copper-Catalyzed Asymmetric Synthesis. Alexakis A, Krause N, Woodward S, editors. VCH–Wiley; 2014. pp. 69–84. [Google Scholar]
- 4.den Hartog T, Harutyunyan SR, Font D, Minnaard AJ, Feringa BL. Catalytic enantioselective 1,6-conjugate addition of Grignard reagents to linear dienoates. Angew Chem Int Edn. 2008;47:398–401. doi: 10.1002/anie.200703702. [DOI] [PubMed] [Google Scholar]
- 5.den Hartog T, van Dijken DJ, Minnaard AJ, Feringa BL. An enantioselective approach to syn deoxypropionate units combining Cu-catalyzed 1,6- and 1,4-conjugate addition. Tetrahedron:Asymmetry. 2010;21:1574–1584. [Google Scholar]
- 6.Tissot M, Alexakis A. Enantio- and regioselective conjugate addition of organometallic reagents to linear polyconjugated nitroolefins. Chem Eur J. 2013;19:11352–11363. doi: 10.1002/chem.201300538. [DOI] [PubMed] [Google Scholar]
- 7.Magrez-Chiquet M, et al. Enantioselective 1,-6-conjugate addition of dialkylzinc reagents to acyclic dienones catalyzed by Cu-DiPPAM complex – extension to asymmetric sequential 1,6/1,4-conjugate addition. Chem Eur J. 2013;19:13663–13667. doi: 10.1002/chem.201302649. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura T, Yasuhara Y, Sawano T, Hayashi T. Iridium/chiral diene-catalyzed asymmetric 1,6-addition of arylboroxines to α,β,γ,δ-unsaturated carbonyl compounds. J Am Chem Soc. 2010;132:7872–7873. doi: 10.1021/ja1034842. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Noishiki A, Hayashi T. Electronic tuning of chiral diene ligands in iridium-catalyzed asymmetric 1,6-addition of arylboroxines to γ-aryl-α,β,γ,δ-unsaturated ketones. Chem Commun. 2012;48:973–975. doi: 10.1039/c2cc16973h. [DOI] [PubMed] [Google Scholar]
- 10.Sawano T, Ashouri A, Nishimura T, Hayashi T. Cobalt-catalyzed asymmetric 1,6-addition of (triisopropylsilyl)-acetylene to α,β,γ,δ-unsaturated carbonyl compounds. J Am Chem Soc. 2012;134:18936–18939. doi: 10.1021/ja309756k. [DOI] [PubMed] [Google Scholar]
- 11.Hénon H, Mauduit M, Alexakis A. Regiodivergent 1,4 versus 1,6 asymmetric copper-catalyzed conjugate addition. Angew Chem Int Edn. 2008;47:9122–9124. doi: 10.1002/anie.200803735. [DOI] [PubMed] [Google Scholar]
- 12.Sieber JD, Morken JP. Asymmetric Ni-catalyzed allylation of activated enones. J Am Chem Soc. 2008;130:4978–4983. doi: 10.1021/ja710922h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shizuka M, Snapper ML. Catalytic enantioselective Hosomi–Sakurai conjugate allylation of cyclic unsaturated ketoesters. Angew Chem Int Edn. 2008;47:5049–5051. doi: 10.1002/anie.200800628. [DOI] [PubMed] [Google Scholar]
- 14.Kuang Y, Liu X, Chang L, Wang M, Lin L, Feng X. Catalytic asymmetric conjugate allylation of coumarins. Org Lett. 2011;13:3814–3817. doi: 10.1021/ol201312y. [DOI] [PubMed] [Google Scholar]
- 15.Yanagida Y, Yazaki R, Kumagai N, Shibasaki M. Asymmetric synthesis of isothiazoles through Cu catalysis: Direct catalytic asymmetric conjugate addition of allyl cyanide to α,β-unsaturated thioamides. Angew Chem Int Edn. 2011;50:7910–7914. doi: 10.1002/anie.201102467. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikai N, Nakamura E. Mechanisms of nucleophilic organocopper(I) reactions. Chem Rev. 2012;112:2339–2372. doi: 10.1021/cr200241f. [DOI] [PubMed] [Google Scholar]
- 17.Keith JA, Behenna DC, Mohr JT, Ma S, Marinescu SC, Oxgaard J, Stoltz BM, Goddard WA. The inner-sphere process in the enantioselective Tsuji allylation reaction with (S)-t-Bu-phosphinooxazoline ligands. J Am Chem Soc. 2007;129:11876–11877. doi: 10.1021/ja070516j. [DOI] [PubMed] [Google Scholar]
- 18.Ardolino MJ, Morken JP. Congested C–C bonds by Pd-catalyzed enantioselective allyl–allyl cross-coupling, a mechanism-guided solution. J Am Chem Soc. 2014;136:7092–7100. doi: 10.1021/ja502280w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornillos V, Pérez M, Fañanás-Mastral M, Feringa BL. Copper-catalyzed enantioselective allyl–allyl cross-coupling. J Am Chem Soc. 2013;135:2140–2143. doi: 10.1021/ja312487r. [DOI] [PubMed] [Google Scholar]
- 20.Meng F, McGrath KP, Hoveyda AH. Multifunctional organoboron compounds for natural product synthesis. Nature. 2014;513:367–374. doi: 10.1038/nature13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quasdorf KE, Overman LE. Catalytic enantioselective synthesis of quaternary carbon stereocenters. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelz NF, Woodward AR, Burks HE, Sieber JD, Morken JP. Palladium-catalyzed enantioselective diboration of prochiral allenes. J Am Chem Soc. 2004;126:16328–16329. doi: 10.1021/ja044167u. [DOI] [PubMed] [Google Scholar]
- 23.Denmark SE, Beutner GL. Lewis base catalysis in organic synthesis. Angew Chem Int Edn. 2008;47:1560–1638. doi: 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]
- 24.Domíngues de María P, García-Burgos CA, Bargeman G, van Gemert RW. Pig liver esterase (PLE) as biocatalyst in organic synthesis: From nature to cloning and to practical applications. Synthesis. 2007:1439–1452. [Google Scholar]
- 25.Becker J, Butt L, von Kiedrowski V, Mischler E, Quentin F, Hiersemann M. Total synthesis of (–)-ecklonialactone B. Org Lett. 2013;15:5982–5985. doi: 10.1021/ol4028418. [DOI] [PubMed] [Google Scholar]
- 26.Krautwald S, Schafroth MA, Sarlah D, Carreira EM. Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis. J Am Chem Soc. 2014;136:3020–3023. doi: 10.1021/ja5003247. [DOI] [PubMed] [Google Scholar]
- 27.Yuki K, Shindo M, Shishido K. Enantioselective total synthesis of (–)-equisetin using a Me3Al-mediated intramolecular Diels–Alder reaction. Tetrahedron Lett. 2001;42:2517–2519. [Google Scholar]
- 28.Jang H, Zhugralin AR, Lee Y, Hoveyda AH. Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC–Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J Am Chem Soc. 2011;133:7859–7871. doi: 10.1021/ja2007643. [DOI] [PubMed] [Google Scholar]
- 29.Takai K, Shinomiya M, Kaihara H, Yoshida N, Moriwake T, Utimoto K. Transformation of aldehydes into (E)-1-alkenylboronic esters with a geminal dichromium reagent derived from a dichloromethylboronic ester and CrCl2. Synlett. 1995:963–964. [Google Scholar]
- 30.Johansson Seechurn CCC, Kitching MO, Colacot TJ, Sniekus V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew Chem Int Edn. 2012;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.