Abstract
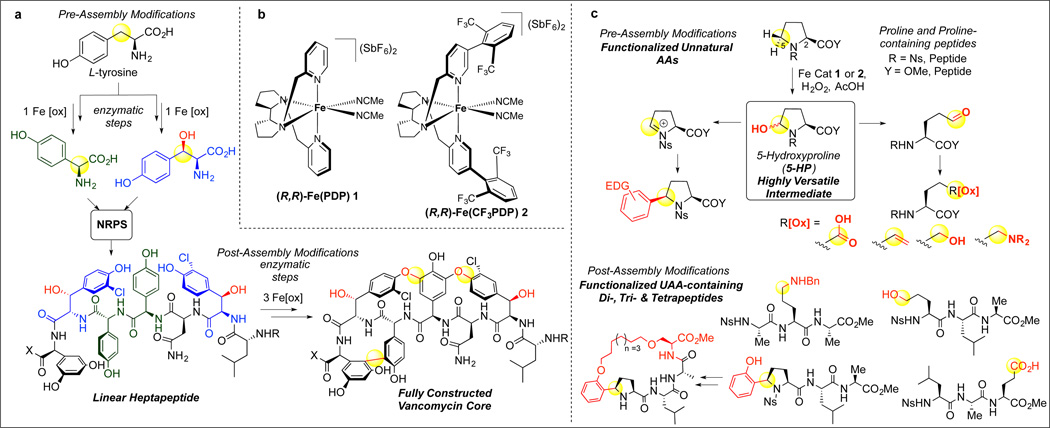
Secondary metabolites synthesized by nonribosomal peptide synthetases (NRPSs) display diverse and complex topologies and possess an impressive range of biological activities1,2 Much of this diversity derives from a synthetic strategy that entails the oxidation of both the chiral amino acid building blocks and the assembled peptide scaffolds pre-3 and post-assembly2. The vancomycin biosynthetic pathway is an excellent example of the range of oxidative transformations that can be performed by the iron-containing enzymes involved in its biosynthesis.4 However, because of the challenges associated with using such oxidative enzymes to carry out chemical transformations in vitro, chemical syntheses guided by these principles have not been fully realized outside of nature.5 In this manuscript, we report that two small-molecule iron catalysts are capable of facilitating the targeted C—H oxidative modification of amino acids and peptides with preservation of α-center chirality. Oxidation of proline to 5-hydroxyproline furnishes a versatile intermediate that can be transformed to rigid arylated derivatives or flexible linear carboxylic acids, alcohols, olefins, and amines in both monomer and peptide settings. The value of this C—H oxidation strategy is demonstrated in its capacity for generating diversity: four 'chiral pool' amino acids are transformed to twenty-one chiral unnatural amino acids (UAAs) representing seven distinct functional group arrays; late-stage C—H functionalizations of a single proline-containing tripeptide furnish eight tripeptides, each having different UAAs. Additionally, a macrocyclic peptide containing a proline turn element is transformed via late-stage C—H oxidation to one containing a linear UAA.
An NRPS-inspired synthetic strategy was envisioned wherein a small molecule mediated C—H oxidation of an amino acid in a monomer or peptide generates a versatile synthetic intermediate that may be transformed into numerous structural and functional group types with retained chirality. Analogous strategies have employed prefunctionalised pluripotent building blocks to generate structurally diverse compounds to great effect.6,7 Limited examples of C—H oxidations of amino acid derivatives are known and of these few have been demonstrated in peptides.8–11 Chelate-controlled C—H arylations are positionally limited to N-terminal residues9 and stoichiometric C—H hydroxylation methods suffer from operational difficulty, modest efficiency, and have no demonstrated chemoselectivity in peptide settings.10,11 A survey of the possible products of C—H oxidation at the side chains of the proteinogenic amino acids led us to reason that targeting hydroxylation at C5 of proline provides an excellent first example of our envisioned strategy (Fig. 1C). Oxidation of proline, a biomass chemical, to 5-hydroxyproline (5-HP) furnishes an intermediate having a highly synthetically versatile hemiaminal functional group that may be transformed to unnatural amino acids and UAA-containing peptides. 5-HP and 5-functionalized proline derivatives are currently accessed via multistep synthetic routes from pre-functionalized glutamic acid or pyroglutamic acid derivatives.12 Recently, methods have been developed to furnish α-aryl pyrrolidines via iron salts13 or photoredox catalysts14 and chiral α-nitrile pyrrolidines via biocatalysis.15 These α-amine functionalization methods proceed via generation of positively charged nitrogen via quaternization or amino radical cation formation followed by deprotonation or abstraction of the α-hydrogen of the homolytically and heterolytically weakest C—H bond. On a proline core, C—H abstraction will occur preferentially at the weakest α-(C2)-H (bond dissociation enthalpy (BDE) ~ 87 kcal/mol)16 bond versus the α-(C5)-H (BDE ~ 90 kcal/mol), leading to racemization or decarboxylation. Free hydroxyl radical oxidations17 and photoredox arylations14 of proline proceed via abstraction of the α-(C2)-H bond followed by decarboxylation to form 2-pyrrolidone and racemic 2-arylated derivatives, respectively.
Figure 1. NRPS-inspired strategy for iron-catalysed C-H oxidative functionalization of amino acids and peptides.
(A) Oxidative tailoring iron enzyme pre- and post-assembly modifications in the biosynthesis of vancomycin. Iron enzymes diversify tyrosine into two unnatural amino acids hydroxyphenylglycine and β-hydroxytyrosine that are incorporated by the NRPS into a heptapeptide. Post-assembly oxidative tailoring by iron enzymes effects side-chain cross-linking to afford the vancomycin core. X = OH or peptidyl carrier protein; R = H or methyl (B) Small molecule non-heme iron C-H oxidation catalysts Fe(PDP) 1 and Fe(CF3PDP) 2. PDP = [N,N′-Bis(2-pyridylmethyl)]-2,2′-bipyrrolidine. (C) Iron catalysts 1 and 2 catalyzed pre-assembly oxidative modification of proline to afford numerous classes of unnatural amino acids. Post-assembly oxidative modifications by 1 and 2 of proline-containing polypeptides to furnish UAA-functionalized polypeptides. AA = amino acid; UAA = unnatural amino acid; Ns = 4-nitrophenylsulfonyl; Bn = Benzyl.
We sought a method for the direct (C5)—H hydroxylation of proline that would preserve its C2 stereocenter and those in every amino acid residue present in peptide settings. Additionally, we sought an oxidant that would be highly chemoselective for the target residue over the other amino acid side-chain C—H bonds. For these reasons, we evaluated small molecule non-heme iron catalysts Fe(PDP) (1)18,19 and Fe(CF3PDP) (2)20 (Fig. 1B). Such bulky, electrophilic C—H oxidation catalysts do not discriminate solely based on C—H bond dissociation energies, but rather select between C—H bonds based on their electronic, steric and stereoelectronic properties. This, along with observations of stereoretentive oxidations of an isoleucine derivative and dipeptide suggested that site selectivity for C5 proline oxidation would be likely, given that C2 is both sterically and electronically deactivated.20 Additionally, in complex molecule settings, Fe(PDP) 1 was shown to oxidize hyperconjugatively activated C—H bonds (e.g. ethereal C—H bonds) at faster rates than other aliphatic C—H bonds,19 suggesting chemoselectivity for α-(C5)-H proline, hyperconjugatively activated by the nitrogen lone pair, would effectively compete with C—H oxidation of aliphatic amino acid residues.
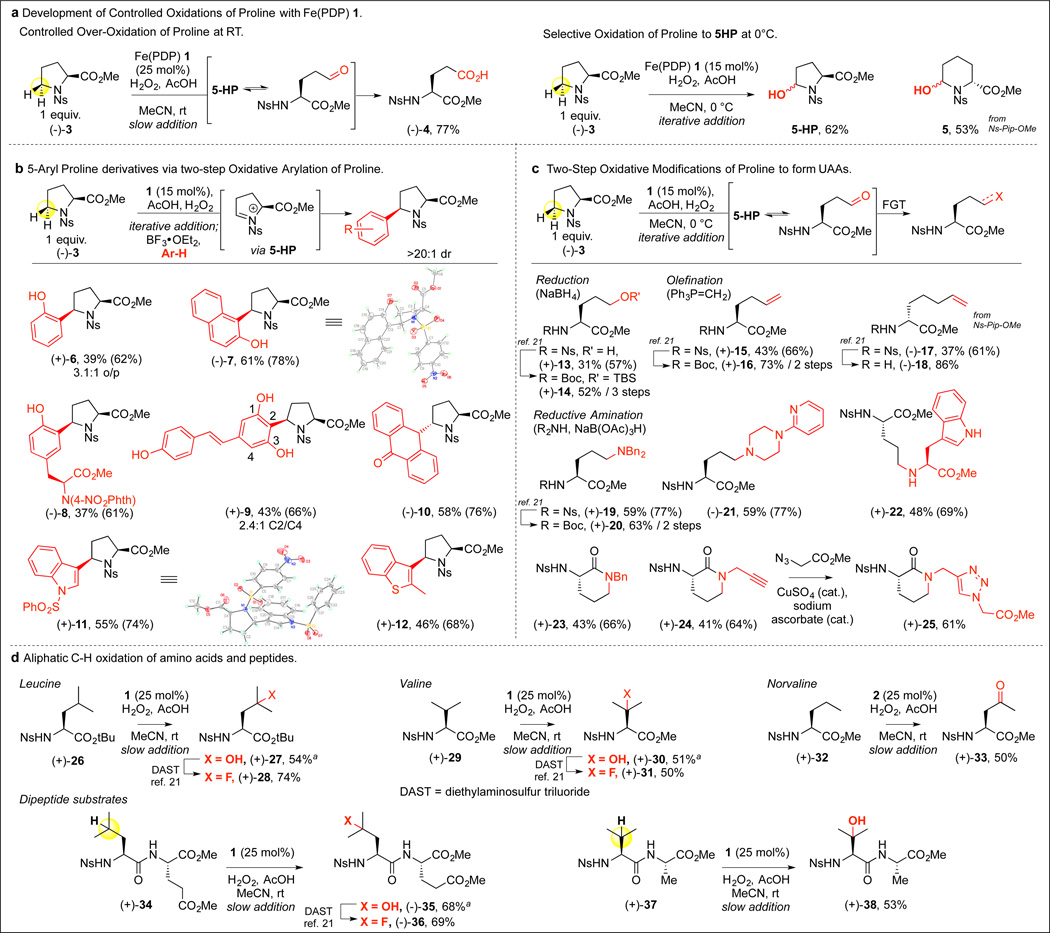
We began our investigations into this NRPS-inspired strategy with the evaluation of the oxidation reactivity of N-(4-nitrophenylsulfonyl)-(L)-proline methyl ester (−)-3 with 1 (Figure 2A). Subjection of (−)-3 to reported slow addition conditions19 with 1 (25 mol%), AcOH, and H2O2 at room temperature led to full oxidation at C5 of proline, affording glutamic acid derivative (−)-4 in 77% yield, presumably via over-oxidation of singly-oxidized 5-HP as its open-chain tautomer. We reasoned that a milder oxidation protocol may allow for selective oxidation of proline to the desired 5-HP, and found that by lowering the reaction temperature to 0 °C and decreasing the catalyst loading (iterative addition of 1, 15 mol%), it was possible to isolate 5-HP in good yield (62%).21 A similarly encouraging result was observed with the less rigid proline homologue pipecolic acid, affording 6-hydroxylpipecolic acid 5 in 53% yield. Interestingly, Boc-Proline methyl ester (Boc = tert-butoxylcarbonyl) gave oxidation to Boc-pyroglutamic acid methyl ester under the same conditions as the major isolated product.21 Gratifyingly, these experiments resulted in conditions for C5 oxidation of proline with control of the final oxidation state. Notably, we did not observe oxidation or racemization of the C2 stereocenter, even under the forcing conditions used to generate the glutamic acid analog (−)-4.
Figure 2. Four amino acids transformed to twenty-one chiral unnatural amino acids via Fe(PDP)-catalysed C-H hydroxylations.
(A) Oxidations to glutamic acid and 5-HP. Slow addition: AcOH (0.5–5 equiv.) was added to a MeCN solution of (−)-3. 1 (0.25 equiv. in CH3CN, 0.2 M) and H2O2 (5–9 equiv. in CH3CN, 0.4–0.72 M) were added via syringe pump (75 minutes) simultaneously. Iterative addition: (−)-3 in MeCN was cooled to 0 °C. 1 (5 mol%) and AcOH (0.5 equiv.) were added, followed by dropwise addition (3 min) of 0 °C MeCN solution of H2O2 (1.9 equiv.). The addition of 1, AcOH, and H2O2 was repeated twice, every 10 minutes. Crude 5-HP was passed through a silica gel plug and concentrated prior to (B) arylation (C) reduction, olefination, or reductive amination. (D) Aliphatic C-H oxidation. a Starting material recycled 1×. Yields in parentheses are average yield per step.
We questioned if in situ derivatization of the hemiaminal functional group of 5-HP could effect pre-assembly oxidative tailoring modifications, diversifying proline into non-proteinogenic amino acids. Arylated proline motifs are prevalent in medicinal agents22. Direct arylation at the 5-position of proline could be effected by a sequential proline oxidation/arylation procedure: crude 5-HP generated by Fe(PDP) oxidation is treated with BF3OEt2 to afford a highly reactive N-sulfonyl iminium ion intermediate that undergoes diastereoselective nucleophilic attack by an electron-rich arene (Figure 2B). We first explored phenols as arenes in this transformation: phenol and 2-naphthol adducts (+)-6 and (−)-7 were formed in high yields with >20:1 syn-stereochemistry, and with generally high regioselectivity (3.1:1.0 o/p for 6, >20:1 for 7). Using this oxidative arylation procedure, novel crosslink (−)-8 between proline and tyrosine was efficiently forged, reminiscent of the side chain crosslinks between amino acids effected by oxidative tailoring enzymes (e.g. vancomycin). Additionally, intriguing natural product-amino acid conjugate (+)-9 was prepared when the polyphenol natural product resveratrol was employed as the arene. The scope of electron-rich arenes was not limited to phenols, as high yields and selectivites were observed with heteroarenes such as anthrone, indole, and benzothiophene, affording adducts 10–12. The adducts were generally formed in syn-stereochemistry- possibly due to steric factors introduced by the nosyl group21- confirmed by single crystal X-ray diffraction of adducts (−)-7, (+)-11, and (+)-12.21 Interestingly, anthrone adduct (−)-10 was furnished as the anti-diastereomer. Overall, this proline oxidation / arylation procedure efficiently generates stereochemically enriched (>20:1 dr) 5-arylproline derivatives presenting a unique array of structural features and functional groups.
To complement the synthetic versatility of 5-HP as a precursor to rigid proline derivatives, we envisioned that in situ transformations of the open-chain aldehyde tautomer of 5-HP could be a second avenue to access a variety of linear unnatural amino acid structures that remain difficult synthetic targets.21,23 A one-pot approach was developed starting with Fe(PDP) 1 oxidation of (−)-3 to 5-HP followed by either reduction, olefination, or reductive amination to furnish linear terminal hydroxyl, olefin, or amino-containing unnatural amino acids (Figure 2C). For example, (−)-3 was transformed to 5-hydroxy-L-norvaline dervative (+)-13 via Fe(PDP) 1 hydroxylation followed by in situ reduction with NaBH4. Alternatively, C—H hydroxylation followed by Wittig olefination of (L)- or (D)-proline furnished chiral (L)-2-aminohex-5-enoic acid dervative (+)-15 and its enantiomer21 (43% and 40%, respectively). Similarly, performing this transformation on proline homologue pipecolic acid, (D)-2-amino-6-heptenoic acid derivative (−)-17 was generated. The retention of stereochemistry at C2 of proline (−)-3 over these sequences was established by synthetic derivatization and comparison of optical activity of products (−)-4, (+)-13, and (+)-15 to known compounds.21
The Fe(PDP) catalyzed C—H hydroxylation followed by reductive amination afforded a general method for the installation of amines to furnish valuable unnatural amino acids, such as chiral ornithine derivative (+)-19. The diversity of functionalized secondary and primary amines that may be used renders this a powerful transformation; for example using 1-(2-aminopyridyl)-piperazine, a fluorescently labeled aminopyridine conjugated unnatural amino acid (UAA) (−)-21 may be generated directly in optically active form. The backbone amine of any suitably protected amino acid may be used to furnish backbone-to-side-chain linkages, e.g. tryptophan derivative (+)-22. Utilization of less sterically encumbered primary amines results in reductive amination followed by intramolecular cyclization to afford optically enriched 3-aminopiperidinone scaffolds like (+)-23. Notably, additional reactive functionality can be united with the proline-derived backbone: proline oxidation / reductive amination with propargylamine furnished alkyne-substituted (+)-24 that may undergo a Cu-catalyzed azide-alkyne cycloaddition (CuAAC) to afford optically enriched triazole (+)-25. Significantly, these unnatural amino acids can be readily denosylated under mild conditions to furnish chiral amino esters with N-protecting groups common to peptide synthesis (e.g. (+)-14, (+)-16, (−)-18, and (+)-20).
We additionally evaluated the generality of this method for the oxidation of chiral pool amino acids possessing oxidizable aliphatic side chain residues with stronger tertiary and secondary C—H bonds to enable direct routes to important UAAs (Figure 2D). For example, exposure of leucine (L), valine, and L-norvaline derived substrates to the reaction conditions with either 1 (tertiary oxidation) or 2 (secondary oxidation) at room temperature resulted in efficient aliphatic C—H oxidation, affording tertiary hydroxyl derivatives (+)-27 and (+)-30 and δ-oxo derivative (+)-33 in good yields. These chiral hydroxylated amino acids are widely used in medicinal chemistry and as synthetic intermediates.24 Importantly, the ability of catalysts 1 and 2 to selectively oxidize aliphatic side chain C—H bonds of amino acids was not diminished when this method was applied to dipeptides possessing these residues, as similarly efficient oxidation of a leucine and valine residue were observed in these settings [(−)-35, (+)-38]. The tertiary hydroxyl groups in (+)-27, (+)-30, and (−)-35 were converted to fluorinated amino acids (+)-28 and (+)-31 and fluorinated peptide (−)-36. Collectively, these results demonstrate a small-molecule catalyzed NRPS “pre-assembly” modification strategy, wherein a simple proline precursor and three other amino acids prone to oxidation are converted to twenty one chiral UAAs representing seven distinct functional group arrays: alcohols, fluorines, aryls, carboxylates, olefins, ketones, and amines.
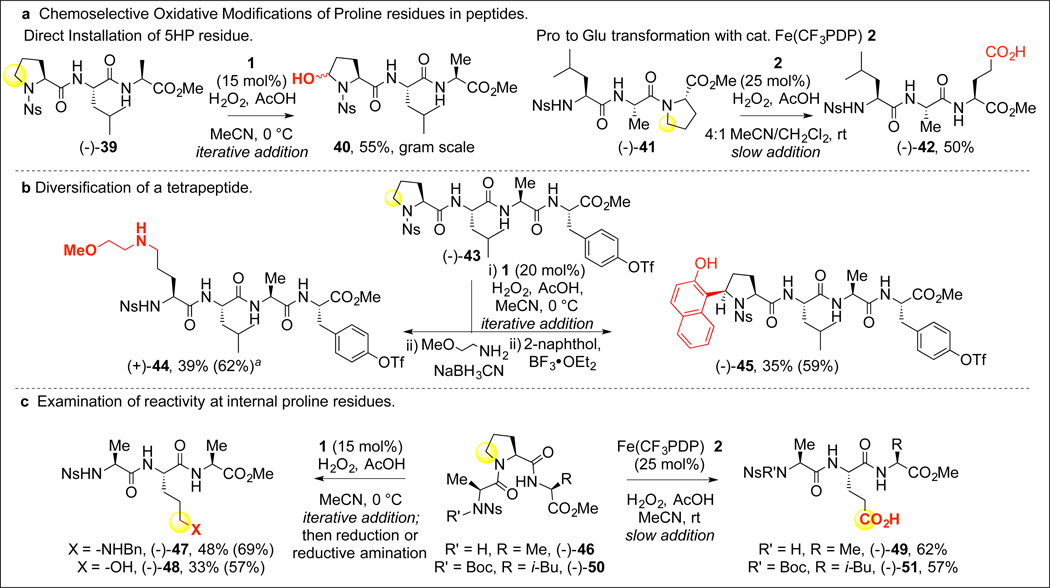
“Post-assembly” oxidative tailoring modifications in the more complex setting of a peptide were possible with catalysts 1 and 2 because of high functional group tolerance for amides, as well as high chemoselectivity in C5 oxidation of proline preferentially over other aliphatic C—H oxidations (Figure 3A). For example, subjecting tripeptide (−)-39 to oxidation with 1 at 0 °C led to the direct hydroxylation of the proline residue, with no observed off-site oxidation at the leucine residue. The use of Fe(CF3PDP) 2 for proline over-oxidation in peptides was superior to Fe(PDP) 1 possibly due to the increased steric bulk around the iron center of 2 that minimizes off-site tertiary oxidation and deleterious coordination with the peptide. Underscoring the site- and chemoselectivity that can be achieved with Fe(CF3PDP) 2, it is noteworthy that a +4 change in oxidation state of a methylene carbon in (−)-41 to a carboxylic acid in (−)-42 could be effected in the presence of an oxidizable tertiary C—H bond of an adjacent leucine residue. The Fe(PDP) C—H hydroxylation/functionalization (i.e. arylation or reductive amination) sequences were further tested in a challenging tetrapeptide setting (−)-43 that included potentially oxidizable leucine, alanine, and tyrosine residues (Figure 3B). Proline oxidation occurred with high site-selectivity, and functionalization proceeded to efficiently furnish amine (+)-44 and naphthol adduct (−)-45. We additionally examined the positional flexibility of proline oxidation, and found that Fe(CF3PDP) 2 controlled over-oxidation of tripeptides containing an internal proline furnished the corresponding glutamic acid derivatives (−)-49 and (−)-51 in excellent overall yields (62% and 57% yield, respectively, Figure 3C). Internal proline of tripeptide (−)-46 could also be transformed to amine-containing residue (−)-47 and bishomoserine residue (−)-48 via Fe(PDP) 1 oxidation followed by either reductive amination or reduction, respectively.
Figure 3. Direct oxidative modification of N-, C-terminal and internal proline residues in peptides by Fe(PDP) catalyzed C-H hydroxylation.
(A) Chemoselective oxidative modifications of N- and C-terminal proline-containing peptides. (B) Diversification of a tetrapeptide via chemoselective oxidation / functionalization sequences. a, Starting material recycled 1×. (C) Direct oxidative opening of internal proline residues in tripeptides affords UAA- or glutamic acid-containing tripeptides. Yields in parentheses note the average yield per step. All slow additions run with AcOH (0.5 equiv.)/H2O2 (5 equiv.).
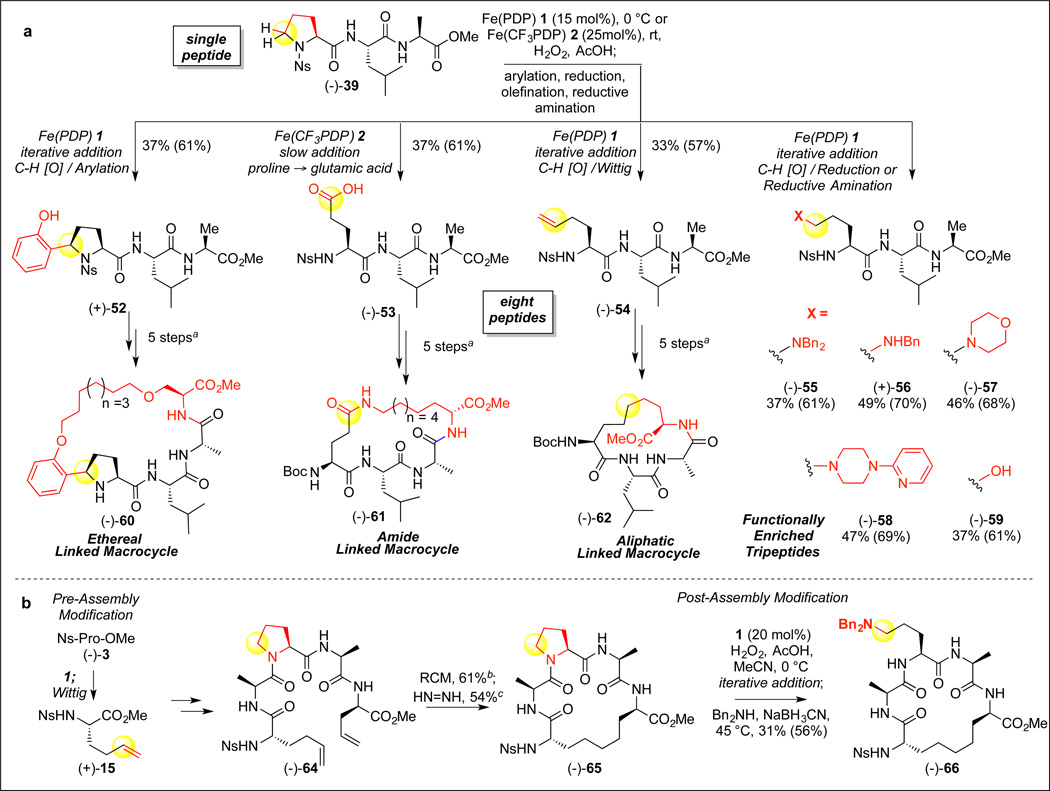
We sought to test our hypothesis that the ability to selectively install 5-HP residues into proline-containing precursor peptides with Fe(PDP) 1 or Fe(CF3PDP) 2 would enable a small-molecule catalyzed “post-assembly” oxidative strategy, affording late-stage diversification of peptides to new structures containing natural or unnatural amino acids (Figure 4A). Tripeptide (−)-39 was subjected to the full suite of proline oxidative modification reactions to install a phenol (oxidative arylation), carboxylic acid (controlled over-oxidation with cat. 2), alkene (Wittig olefination), alcohol (reduction), and four different amine functionalities (reductive amination), in good overall yields (average 40%, 63% per step) without observing epimerization of α C-H bonds (analysis of (−)-53 indicated no epimerization to D-configuration of any residues21). Strikingly, eight novel peptide sequences (52–59) were rapidly constructed from one peptide in one to two steps, underscoring the potential for such reactions to enable efficient diversification of native residues in a preassembled peptide setting. Alternative routes to make all eight peptides would involve eight separate syntheses from the respective amino acid building blocks, including the synthesis of unnatural residues.
Figure 4. Fe(PDP)-catalyzed oxidative diversification of tripeptides and macrocycles.
(A) Fe(PDP) 1 and Fe(CF3PDP) 2 oxidative modifications of a single tripeptide enables synthesis of eight functionally diverse UAA-containing tripeptides. Slow addition with AcOH (0.5 equiv.)/H2O2 (5 equiv.). a Macrocycles 60–62 were prepared from tripeptides 52–54 using 5-step transformations involving: alkene appendage to the UAA residue, coupling of a fourth alkene-containing amino acid to the C-terminus, conversion of Nosyl to a Boc group, ring-closing metathesis, and hydrogenation. Individual routes vary in order. See the SI for full details. (B) Late-stage diversification of a proline-containing peptide macrocycle via post-assembly oxidation / reductive amination. RCM = ring closing metathesis. b Hoveyda-Grubbs Catalyst, second generation (5 mol%) c Dipotassium azodicarboxylate (40 equiv.), AcOH (80 equiv.). Values in parentheses indicate the average yield per step.
Macrocyclic peptides are highly prevalent among NRPS natural products, and are valued as therapeutic candidates relative to their linear analogues due to their increased stability to chemical and enzymatic degradation, increased receptor selectivity, and pharmacokinetic properties.25–28 We sought to explore how the rapid installation of new functional groups in peptides from a simple proline residue could allow for the rapid construction and elaboration of macrocycles.7 The phenol-, carboxylic acid- and olefin-derived tripeptides (52–54, vide supra) could be rapidly transformed into three macrocycles containing ethereal (−)-60, amide (−)-61, and aliphatic (−)-62 linkers respectively via short synthetic sequences (Figure 4A).21 The presence of functional groups on the linkage of stapled peptide-like structures like these has been shown to modulate the biological properties of the overall product.29 Collectively, the small library of molecules rapidly synthesized from tripeptide (−)-39 demonstrates the breadth of functionally and structurally enriched molecules that can be accessed using our post-assembly oxidative strategy.
Proline has been utilized by synthetic chemists to enforce turn-like reactive conformations of polypeptide chains to promote macrocyclizations.30 We were interested to explore if the NRPS-inspired C—H oxidation/functionalization strategy would enable internal proline residues, which serve as turn elements within a linear peptide sequence, to be transformed into a range of natural and unnatural acyclic amino acids. Encouraged by the high positional flexibility of proline oxidation (vide supra), we assembled a proline-containing linear pentapeptide (−)-64 –using our post-synthetically modified unnatural amino acid (+)-15 that was rapidly produced by C—H oxidation / olefination of proline (−)-3, and subjected it to ring-closing metathesis that proceeded in good yield (61%) to furnish an 18-membered macrocycle. Reduction of the internal olefin with diimide provided the macrocyclic tetrapeptide (−)-65. Application of the post-assembly C—H oxidation / functionalization with 1 to this macrocycle resulted in the late-stage conversion of the proline conformational element to a dibenzylornithine derivative (−)-66. This example underscores the potential for proline residues as diversifiable structural elements that may be functionally and structurally transformed at late-stages in complex peptide settings.
In summary, the NRPS-inspired oxidation strategy described herein represents a powerful foray into direct diversification of amino acids and peptides via C—H oxidation. We anticipate that this strategy will benefit small-peptide therapeutics by enabling the rapid exploration of key physical properties (e.g. charge, polarity, steric, and stereochemical effects) and inspire the continued invention of non-directed, chemoselective C—H oxidation reactions that unmask the potential for pluripotent reactivity of C—H bonds in complex molecule settings.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the NIH/NIGMS (1R01 GM112492A) and a grant from Pfizer to study the modification of natural products and medicinal compounds. T.J.O. is a Springborn Graduate Fellow. We acknowledge Dr. Lingyang Zhu for assistance with NMR spectroscopy, Dr. Danielle Gray and Dr. Jeffrey Bertke for X-ray crystallographic studies, Dr. Chao Jiang on preliminary studies of amino acid oxidations, and Gregory S. Snapper for substrate synthesis. The data reported in this paper are tabulated in the Supplementary Information.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions T.J.O. and D.C.R. conducted the experiments and analyzed the data. M.C.W. and T.J.O. wrote the manuscript. M.C.W., J.T.K., A.F.S. T.J.O., and D.C.R. conceived and/or designed the project. All authors provided comment on the experiments and manuscript during its preparation.
Author Information The crystal data have been deposited in The Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk) under accession numbers 1478939, 1478940, and 1478941. The authors declare no competing financial interest.
References and Notes
- 1.Schwarzer D, Finking R, Mahariel M. Nonribosomal peptides: from genes to products. Nat. Prod. Rep. 2003;20:275–287. doi: 10.1039/b111145k. [DOI] [PubMed] [Google Scholar]
- 2.Walsh CT, et al. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Chem. Biol. 2001;5:525–534. doi: 10.1016/s1367-5931(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Gao X, Tang Y. Complexity generation during natural product biosynthesis using redox enzymes. Curr. Opin. Chem. Biol. 2012;16:362–269. doi: 10.1016/j.cbpa.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubbard BK, Walsh CT. Vancomycin Assembly: Nature’s Way. Angew. Chem. Int. Ed. 2003;42:730–765. doi: 10.1002/anie.200390202. [DOI] [PubMed] [Google Scholar]
- 5.White MC. Adding aliphatic C-H bonds to synthesis. Science. 2012;335:807–809. doi: 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]
- 6.Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 7.Beckmann HG, et al. A strategy for the diversity-oriented synthesis of macrocyclic scaffolds using multidimensional coupling. Nature Chem. 2013;5:861–867. doi: 10.1038/nchem.1729. [DOI] [PubMed] [Google Scholar]
- 8.Dangel BD, Johnson JA, Sames D. Selective functionalization of amino acids in water: a synthetic method via catalytic C—H bond activation. J. Am. Chem. Soc. 2001;123:8149–8150. doi: 10.1021/ja016280f. [DOI] [PubMed] [Google Scholar]
- 9.Gong W, Zhang G, Liu T, Giri R, Yu J-Q. Site-selective C(sp3)-H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J. Am. Chem. Soc. 2014;136:16940–16946. doi: 10.1021/ja510233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saladino R, et al. A new and efficient synthesis of unnatural amino acids and peptides by selective 3,3-dimethyldioxirane side chain oxidation. J. Org. Chem. 1999;64:8468–8474. [Google Scholar]
- 11.Rella MR, Williard PG. Oxidation of peptides by Methyl(trifluoromethyl)dioxirane: The protecting group matters. J. Org. Chem. 2007;72:525–531. doi: 10.1021/jo061910n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najera C, Yus M. Pyroglutamic acid: A versatile building block in asymmetric synthesis. Tetrahedron: Asymmetry. 1999;10:2245–2303. [Google Scholar]
- 13.Ratnikov MO, Xu X, Doyle MP. Simple and sustainable iron-catalyzed aerobic C—H functionalization of N,N-dialkylanilines. J. Am. Chem. Soc. 2013;135:9475–9479. doi: 10.1021/ja402479r. [DOI] [PubMed] [Google Scholar]
- 14.Zuo Z, MacMillan DWC. Decarboxylative arylation of α-amino acids via photoredox catalysis: one-step conversion of biomass to pharmacophores. J. Am. Chem. Soc. 2014;136:5257–5260. doi: 10.1021/ja501621q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner NJ. Enantioselective oxidation of C-O and C-N bonds using oxidases. Chem. Rev. 2011;111:4073–4087. doi: 10.1021/cr200111v. [DOI] [PubMed] [Google Scholar]
- 16.Rauk A, Yu D, Taylor J, Shustov GV, Block DA, Armstrong DA. Effect of structure on αC-H bond enthalpies of amino acid residues: relevance to H-transfers in enzyme mechanism and protein oxidation. Biochemistry. 1999;38:9089–9096. doi: 10.1021/bi990249x. [DOI] [PubMed] [Google Scholar]
- 17.Uchida K, Kato Y, Kawakishi S. A novel mechanism for oxidative cleavage of prolyl peptides induced by the hydroxyl radical. Biochem. Biophys. Res. Comm. 1990;169:265–271. doi: 10.1016/0006-291x(90)91463-3. [DOI] [PubMed] [Google Scholar]
- 18.Chen MS, White MC. A predictably selective aliphatic C—H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 19.Chen MS, White MC. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 20.Gormisky PE, White MC. Catalyst-controlled aliphatic C—H oxidations with a predictive model for site-selectivity. J. Am. Chem. Soc. 2013;135:14052–14055. doi: 10.1021/ja407388y. [DOI] [PubMed] [Google Scholar]
- 21.See the Supporting Information for details
- 22.Scola PM, et al. The discovery of Asunaprevir (BMS-650032), an orally efficacious NS3 protease inhibitor for the treatment of Hepatitis C virus infection. J. Med. Chem. 2014;57:1730–1752. doi: 10.1021/jm500297k. [DOI] [PubMed] [Google Scholar]
- 23.Stevenazzi A, Marchini M, Sandrone G, Vergani B, Lattanzio M. Amino acidic scaffolds bearing unnatural side chains: an old idea generates new and versatile tools for the life sciences. Bioorg. Med. Chem. Lett. 2014;24:5349–5356. doi: 10.1016/j.bmcl.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Seiple IB, Mercer JAM, Sussman RJ, Zhang Z, Myers AG. Stereocontrolled synthesis of syn-β-hydroxy-α-amino acids by direct aldolization of pseudoephenamine glycinamide. Angew. Chem. Int. Ed. 2014;53:4642–4647. doi: 10.1002/anie.201400928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery – an underexploited structural class. Nature Reviews Drug Discovery. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 26.Yudin AK. Macrocycles: lessons from the distant past, recent developments, and future directions. Chem. Sci. 2015;6:30–49. doi: 10.1039/c4sc03089c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafmeister CE, Po J, Verdine GL. An all-hydrocarbon crosslinking system for enhancing the helicity and metabolic stability of peptides. J. Am. Chem. Soc. 2000;122:5891–5892. [Google Scholar]
- 28.Miller SJ, Blackwell HE, Grubbs RH. Application of ring-closing metathesis to the synthesis of rigidified amino acids and peptides. J. Am. Chem. Soc. 1996;118:9606–9614. [Google Scholar]
- 29.Lau YH, et al. Functionalized staple linkages for modulating the cellular activity of stapled peptides. Chem. Sci. 2014;5:1804–1809. [Google Scholar]
- 30.White CJ, Yudin AK. Contemporary strategies for peptide macrocyclization. Nature Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.