Abstract
The balanced differentiation of naïve CD4+ T cells into either pro- or anti-inflammatory fates is a central regulator of immune homeostasis, dysregulation of which can lead to inflammatory disease or cancer. Accordingly, the development of diagnostics and therapeutics to measure and modulate this balance is of great interest. In this Review, we focus on the predominant anti-inflammatory subset, regulatory T cells (Tregs), discussing key concepts including development, function, antigen specificity and lineage stability. In particular, we highlight how these notions are shaping the evolution of therapeutics, especially in the context of the transfusion medicine specialist, and identify several key areas that urgently need to be addressed.
Keywords: Regulatory T cell, Immunotherapy, Cell processing, T cell differentiation, Antigen specificity, Lineage stability
Introduction
The immune system faces the fundamental challenge of protecting the individual from foreign pathogens while maintaining tolerance to self. The innate immune system uses a small number of receptors to recognize conserved pathogen-associated molecular patterns, such as lipopolysaccharide (LPS). In more advanced organisms, this is buttressed by the adaptive immune response, which recognizes a much larger diversity of antigens with both specificity and memory, leveraging the error-prone joining of variable region gene segments (i.e. V(D)J recombination) to generate a vast diversity of T- and B-cell receptors using limited genomic space [1]. Because these receptors are generated in an antigen-agnostic manner, they may recognize either self or foreign antigens. Thus, self-tolerance necessitates mechanisms to remove and/or attenuate self-reactive cells. In the thymus, medullary thymic epithelial cells display self-antigens encoded throughout the genome via the activity of AIRE, facilitating the negative selection of self-reactive T cells (i.e. clonal deletion) [2]. Loss of AIRE cripples this process, leading to the autoimmune syndrome known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in humans [3,4]. Importantly, AIRE can only promote tolerance to antigens encoded within the genome. At the least, our microbiota and food generate antigens that are extrinsic to our genome, and thus to AIRE, that must also be tolerated. Moreover, self-reactive clones can and do escape central deletion. These scenarios highlight the need for extrathymic mechanisms to promote tolerance, i.e. peripheral tolerance.
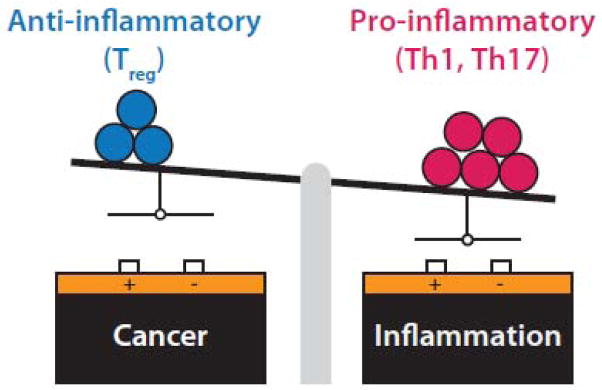
The CD4+ T helper (Th) cell regulates both B and T cell effector functions and is a key player in maintaining an appropriate balance between inflammation and tolerance. This is in large part achieved by the differentiation of naïve CD4+ T cells into either pro-inflammatory subsets (e.g. Th1, Th17) or anti-inflammatory subsets (Treg) (Fig. 1) [5]. This lineage decision is dictated by the local cytokine milieu in which naïve Th cells are activated by their cognate MHC-peptide ligands. Thus, T cell receptors are generated in an antigen-agnostic fashion, but their associated functional program (e.g. inflammation vs tolerance) is educated contextually. Appropriate and balanced differentiation into these fates critically impacts immune homeostasis, with excessive bias in either direction leading to pathologic inflammation or cancer [6].
Figure 1.
Immune homeostasis is maintained by balanced differentiation of naïve CD4+ T cells into pro-inflammatory (e.g. Th1, Th17) or anti-inflammatory (e.g. Treg) subsets. Excessive skewing leads to either pathologic inflammation or cancer.
The anti-inflammatory arm is largely represented by regulatory T cells (Tregs), although the contribution of other subsets, including Tr1 cells, is increasingly being recognized. The central role of Tregs in maintaining immune homeostasis is highlighted by patients with deleterious mutations in FOXP3, a hallmark transcription factor required for Treg development. These patients develop immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, which is characterized by a plethora of autoimmune processes that typically lead to early death without treatment [7,8]. Similar autoimmunity arises from FOXP3 defects in mice, as exemplified by the scurfy mutation [9]. In addition to Mendelian disorders like IPEX, a broader role of Tregs in complex human diseases is suggested by the recurring over-representation of Treg-related genes in genome-wide association studies of inflammatory diseases such as inflammatory bowel disease and type 1 diabetes (T1D). This may reflect a quantitative and/or qualitative defect in the Treg axis.
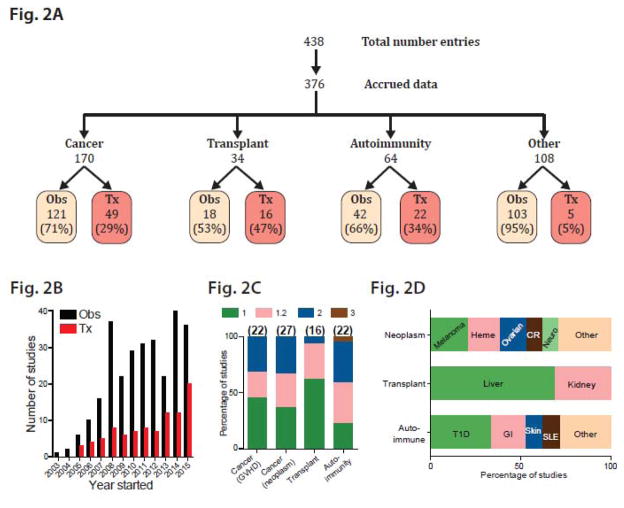
The dominant contribution of Tregs to the anti-inflammatory component makes this a very attractive subset to study, since enhancing Tregs is likely to be broadly relevant to autoimmunity and inflammation, while inhibiting Tregs might be of general utility in cancer. Consistent with this notion, searching registered studies at clinicaltrials.gov reveals 438 entries related to Tregs, equivalent to all other major subsets combined (Th1, Th2 and Th17 with 174, 172 and 93 entries respectively). Of these, 376 Treg-related study entries successfully enrolled patients and collected data. The most studied entities were cancer, autoimmunity and transplant (170, 64 and 34 studies respectively, Fig. 2A). Uniformly, the majority of studies have been observational, typically examining the relationship between peripheral blood Tregs and disease progression or standard therapeutic intervention (Fig. 2A). Importantly, studies aimed at the therapeutic manipulation of Tregs began only 2 years after the first observational studies were initiated, and have been steadily increasing since (Fig. 2B). These have so far been overwhelmingly phase 1 and/or 2 studies in diverse disease entities, reflecting early and general interest, with relative emphasis on T1D and colitis in autoimmunity as well as melanoma, hematologic malignancies and ovarian cancer in neoplastic diseases (Figs. 2C and 2D). Systemic drugs have been used both to deplete Tregs, such as the anti-CD25 agent denileukin diftitox (Ontak) and metronomic cyclophosphamide, as well as to enhance Tregs, most notably low-dose IL-2. Perhaps of greatest interest to the transfusion medicine practitioner is the extracorporeal manipulation of Tregs, including Treg depletion from donor lymphocyte infusions to increase anti-cancer effects, and Treg enhancement ex vivo, especially to promote transplant tolerance and minimize graft-versus-host disease. Ex vivo Treg differentiation and expansion has particular promise as an emerging tool in the cellular therapy portfolio. In this context, we review concepts of fundamental importance, including Treg identity, mechanism of action, stability and explore how qualitative and quantitative enhancement may be achieved to therapeutic utility. Notably, these concepts are broadly applicable to all endeavors exploring differentiation of Th and other subsets.
Figure 2.
Overview of Treg clinical trials documented in clinicaltrials.gov. 2A. Manual curation of all study entries involving Tregs, with further subdivision according to data accrual, related disease entity, and type of study (observational, Obs, versus therapeutic, Tx). 2B. Number of observational (Obs) and therapeutic (Tx) study entries initiated in each year. 2C. Percentage of clinical studies in each phase (1 to 3) according to disease category; total number of studies per category indicated in parentheses. 2D. Distribution of most-studied diseases/organ systems in each category. CR, colorectal.
Defining and identifying Tregs
As previously discussed, genetic studies in both humans and mice clearly point to Foxp3 as a key transcription factor required for Treg differentiation. Multiple lines of evidence support Foxp3 as a robust marker of Tregs in mice [6]. In humans, Foxp3 is also expressed by activated T cells, and may be less specific a marker of Tregs than in mice [10]. Another key limitation to the use of Foxp3 as a marker is that it is an intracellular protein, detection of which (in humans) requires fixation and intracellular permeabilization. Thus, detection of Foxp3 is not compatible with recovery of live cells in patients and compromises the usability of the labeled cells for in other assays, such as RNAseq studies [11].
To date, no single surface marker has been found that specifically defines Tregs. Tregs were classically identified as CD4+CD25hi cells, and many studies continue to use this method. However, activated T cells also express CD25, which may impact the specificity of this marker, especially in the setting of ongoing inflammation. At the least, CD4+CD127lowCD25hi should be used to define Tregs. Additional markers including CTLA4 and GITR may also be useful. Taken together, continuing work to identify markers, especially surface proteins, of Tregs in humans will be useful, and should be carefully validated with the recognition that there is likely to be heterogeneity within Tregs. Studies identifying CD15s and TIGIT as markers of suppressive human Tregs represent important efforts in this regard [12,13].
Treg development and function: interventional points
Our understanding of Treg ontogeny is largely informed by murine studies. Many Tregs develop in the thymus (thymic Tregs, tTregs) and subsequently circulate in the periphery. These tTregs likely develop in the context of relatively strong TCR-signaling in response to self-peptides presented in an AIRE-dependent manner [14]. Tregs that develop in the periphery (pTregs) are likely essential for peripheral tolerance and seem to have demonstrable roles in the gut and at the fetal-maternal interface, consistent with the notion that local commensals and paternal-specific proteins represent extra-genomic antigens that pTregs regulate tolerance of [15,16]. Whether tTregs and pTregs are functionally or mechanistically distinct remains unclear and will require tools to distinguish between these subsets. Expression of the surface marker neuropilin-1 helps distinguish tTregs from pTregs in mice, but appears less Treg-specific in humans [17–19]. Mice lacking the CNS1 regulatory region in the Foxp3 gene can generate tTregs but not pTregs, and might reveal additional ways to discriminate between these populations [20].
Induced Tregs (iTregs) are generated in vitro by stimulating naïve CD4+ T cells in the presence of TGFβ [21]. The ability to generate iTregs in large numbers ex vivo makes them of significant interest as cellular therapeutics; moreover, both iTregs and pTregs are thought to arise from the same precursor so that mechanistic dissection iTreg differentiation may more broadly inform our understanding of pTreg and tTreg differentiation [22]. Transfer of iTregs attenuates disease in multiple murine models, and human iTregs exert anti-inflammatory effects in mice with humanized immune systems, although studies suggesting that iTregs may lack the full genetic, epigenetic and functional signature of tTregs raise cautionary notes [22–25]. Accordingly, many groups have focused on expanding tTregs. However, data suggesting that iTregs play a non-redundant role in tolerance and studies showing that molecules like vitamin C can significantly enhance iTreg function point to the value of continued investigation into the therapeutic potential of iTregs [26,27].
A species-specific facet of FOXP3 regulation may account in part for its differential association with Tregs in humans and mice. Although mouse and human FOXP3 are highly homologous, human CD4+CD25+ Tregs express both FOXP3 variants containing and missing exon 2 at equivalent levels, unlike mice, which express predominantly full-length Foxp3 [28]. Importantly, exon 2-containing FOXP3 is required for suppressive activity [28]. In support that this observation applies across species, mice forced to express Foxp3 lacking both exons 2 & 7 develop multi-organ inflammation comparable to that arising from complete loss of Foxp3 [29]. CD4+CD25+ cells in patients with relapsing-remitting multiple sclerosis and T1D have decreased suppressive activity and decreased expression of exon 2-containing FOXP3, consistent with decreased Treg numbers and/or activity [30]. Glycolysis helps regulate Treg differentiation; studies showing that the glycolytic enzyme enolase is recruited to the FOXP3 locus and affects exon 2 splicing suggest novel roles for this enzyme and point to the regulation of FOXP3 splicing as an interesting area of future study [30].
Several distinct mechanisms have been identified to mediate the suppressive effect of Tregs, including secretion of tolerogenic cytokines (e.g. IL-10, IL-35 and TGFβ), local consumption of IL-2 via preferential expression of the high-affinity IL-2 receptor, direct killing of responder T cells (e.g. granzyme B and perforin-1) and modulation of the function of innate immune cells (e.g. dendritic cells and NK cells) [31–35]. IL-10 is a pleiotropic pro-tolerogenic cytokine made by several cell types, but murine models specifically lacking IL-10 in Tregs point to a particular role for Treg-derived IL-10 in regulating mucosal inflammation[32]. Similarly, patients deficient in IL-10 or IL-10-receptor present primarily with early-onset immunosuppression-resistant enterocolitis responsive to hematopoietic stem cell transplant [36]. These and similar data point to the need to consider tissue-specific specializations of Treg function when contemplating their role in diagnosis and therapy.
Most of the assays currently used to assess Treg function largely focus on their suppressive effects on other T cells, and to a lesser extent, innate immune cells. The hallmark in vitro assay of Treg function tests their ability to suppress the proliferation of co-cultured naïve CD4+ T cells upon stimulation with anti-CD3 and anti-CD28 antibodies. Beyond these concepts, growing evidence points to Tregs also impacting B cell function. IPEX and DOCK8-deficient patients exemplify defects in Tregs associated with defective B cell tolerance, suggesting a role for Tregs in enforcing peripheral B cell tolerance [37,38]. The particular mechanisms involved remain to be clearly elucidated, and may involve enhancing antigen-specific T follicular helper responses via IL-2 sequestration, signaling to B cells through molecules like CTLA4 or other pathways yet to be described [39,40]. This relationship may explain the overrepresentation of Treg-associated genes in many autoimmune disease genome-wide association studies and impact how we think about mechanisms of autoantibody production.
Tregs may also have physiological functions beyond modulating inflammatory responses. Pioneering studies showing that Tregs in muscle overexpress amphiregulin and promote tissue repair have been buttressed by the finding that Treg-specific ablation of amphiregulin leads to increased tissue damage in an infectious lung injury model independent of Treg-suppressive effects [41,42]. These findings highlight not only potential clinical interest in and utility of Tregs beyond manipulating immune homeostasis, but also the broader importance of understanding compartment- and context-specific specializations of cellular function, that might reveal additional roles for Tregs. It will be important to develop corresponding assays that will not only enable diagnosis of Treg functional defects, but also development of therapeutics to enhance these functions.
Stability of Tregs
The stability of differentiated Th fates is an ongoing investigation, with controversial findings that have clinically important implications. If administered Tregs have significant capacity to subsequently adopt pro-inflammatory fates, therapeutic benefit may be muted or even negative. Lineage-tracing experiments using Foxp3-GFP-Cre-BAC transgenic Rosa26loxP-stop-loxP-YFP mice allowed indelible YFP labeling of all cells that expressed Foxp3 at any point in their ontogeny. In these mice, 10% – 15% of YFP+ cells (previously expressed Foxp3) did not maintain FOXP3 expression; furthermore these ex-Foxp3 cells drove pathogenic inflammation in models of T1D and multiple sclerosis [43,44]. A related suite of experiments used a tamoxifen-inducible Foxp3GFP-Cre-ERT2 to label Foxp3-expressing cells, finding fewer but detectable ex-Foxp3 cells 14 days or 5 months after tamoxifen treatment (2% – 5%); notably, sorted GFP+YFP+ Tregs maintained Foxp3 expression (>99%) in the face of multiple stresses [45]. One possible explanation for these differences arises from the observation that Treg and Th17 cells can arise from a common FOXP3+RORγT+ precursor, thus some of the exFoxp3 cells observed might reflect normal ontogeny of Th17 cells. Another contributing factor may be differing fidelity of the Foxp3-GFP-Cre-BAC transgene, as opposed to the Foxp3GFP-Cre-ERT2 knock-in allele.
Whereas the above studies examine the plasticity of tTregs, this may be more pressing in iTregs, which data suggest exhibit decreased stability due at least in part to decreased hypomethylation of key Treg-associated loci (including Foxp3, Tnfrsf18, Ctla4 and Ikzf4) which occurs in a Foxp3-independent fashion [25]. Recent studies identified the ten-eleven-translocation (TET) family of enzymes as key mediators of DNA demethylation; this family comprises 3 members, of which TET2 and TET3 act redundantly to demethylate Treg regulatory regions [27]. Loss of both TET2 and TET3 impairs Foxp3 expression stability, while enhancement with either vitamin C or hydrogen sulfide increases both Foxp3 expression stability and Treg function, suggesting immediate applications to improve iTreg therapeutics [27,46].
Treg stability is important, exemplified by spontaneous focal inflammation in mice where this has been impaired by deletion of the Foxp3 regulatory element CNS2 [47]. However, transition of Tregs to other lineages may not necessarily be pathologic; stability appears dependent on full demethylation of CNS2, which is observed in mature but not immature Tregs [47–49]. This may allow evolving cytokine contexts a window to drive Th cells to the final desired effector state. In this regard, concomitant inflammation can condition naïve CD4+ T cells towards Treg polarization, so that if a subsequent infection took some time to “build up” pro-inflammatory cytokine levels, some degree of lineage flexibility might be physiologically useful [50].
Although these observations were made in murine models, the potential clinical ramifications have evoked concerns regarding human applications. Because of the increased concern of iTreg lineage instability, many early studies have focused on expanded tTregs as a cellular therapeutic. Tracking the fate of the transferred cells is clearly a key issue; methods used range from examining chimerism in the Treg population (using variable number tandem repeat analysis) to labeling transferred Tregs with deuterium [51,52]. As Th therapeutics expand beyond Tregs, it is useful to recognize that lineage stability is a broadly relevant issue, and transitions of Th2 to Th1 and Th17 to non-inflammatory regulatory fates have been described, amongst others [53–55].
Antigen specificity of Tregs
Most methods currently used assess Treg function independent of their MHC-peptide specificity, ranging from the standard in vitro suppression assay (used to assess both mouse and human Tregs) to animal models, such as colitis and airway inflammation [56–59]. Given the energetic expenditure to generate a TCR that passes thymic selection, specificity might be expected to impact Treg function. In further evidence that the TCR is not a vestigial feature of Tregs, loss of TCR and associated signaling leads to increased prevalence of inflammatory T cells despite persistence of Foxp3+TCR- cells, demonstrating a persistent requirement of the TCR for Treg function [60]. Consistent with this notion, studies of the BDC2.5 transfer model of diabetes revealed potent and consistent suppression of disease with transfer of 2×106 specific Tregs, as compared to modest delay of disease using 8×106 non-antigen-specific Tregs (no higher doses tested) [61]. Studies in a humanized model of alloimmune skin graft injury reveal that alloantigen-specific human Tregs (enriched by surface expression of CD69 and CD71) protect from injury more efficiently and potently than non-alloantigen-specific Tregs [62]. Together, these studies suggest that antigen-specific Tregs may represent a therapeutic with increased potency as compared to polyclonal Tregs.
Clues from human disease
Mendelian diseases pointing to the critical anti-inflammatory role of Tregs include IPEX and Wiskott-Aldrich syndrome, where defects in Treg development and function, respectively, lead to inflammation in multiple compartments including the gut [63]. Genome-wide association (GWA) studies suggest a broader role for Tregs in complex human diseases including inflammatory bowel disease and T1D, with numerous genes known to be important for Treg differentiation and/or function being associated with disease risk (IL-10, IL-10R, IL-2, STAT3, PTPN22, etc) [63]. FOXP3 is not typically implicated in these GWA studies, potentially because significant defects cause more devastating disease; sequencing studies may find rare disease-associated variants with subtler effects, such as a polymorphism in the 3′ untranslated region recently associated with both atopic and autoimmune disease in a smaller cohort [64].
These GWA studies may help prioritize genes to study for roles in Treg differentiation and/or function. The relative dearth of Treg-specific therapies is likely at least in part due to an insufficiently complete understanding of disease-relevant Treg pathways. Studies showing significant interindividual variation in CD4+ T cell responses to activation, only ~25% of which is ascribable to simple genetic effects, highlight the challenge and importance of these efforts [65]. Efforts to highlight regulatory elements conserved across species may help focus subsequent studies in conjunction with disease associations [66].
An interesting approach is the use of unbiased high-throughput methods to identify compounds that modulate Treg differentiation and/or function. Traditional genetic methods have been hampered by the resistance of naïve CD4+ T cells to viral transduction, and by the proliferation that accompanies Th differentiation, which limits the utility of siRNA approaches. Small molecule approaches offer the potential dual benefit of not only learning novel biology, but also the potential utility of the identified compound as a therapeutic lead. Such an approach identified DYRK1A as a novel regulator of Treg differentiation, suggesting an explanation for the hypofunctional Tregs seen in Down Syndrome (DYRK1A is on chromosome 21) and potential clinical utility of DYRK1A inhibitors [23].
An important consideration is that the majority of current approaches assess quantitative differences in Tregs in the peripheral blood, for technical and logistic reasons. This may not correlate well with observations in local tissues which is more relevant to disease pathobiology [67]. Better tools are needed to assess Tregs in tissues, especially those not typically biopsied. Furthermore, an enhanced suite of tools to assess Treg function is urgently needed. In diseases where Tregs are hypofunctional (e.g. Down syndrome) or normofunctional (e.g. relapsing-remitting multiple sclerosis) it is unclear that currently available approaches of quantitatively increasing Tregs, especially polyclonally, will be effective [68,69]. Additional tools, beyond the in vitro suppression assay, are needed to diagnose qualitative Treg defects and how they might be rescued.
Methods to manipulate Tregs
Taken together, defects in the Treg axis can broadly be classified into quantitative defects versus qualitative defects, layered on top of which are considerations of antigen specificity (a variant of quantitative defect) and stability (features of both quantitative and qualitative defects). Understanding the nature of the defect in a disease-, tissue- and patient-specific manner is necessary to devise the best intervention. Most of the interventions available in the foreseeable future are aimed at enhancing Treg numbers, but other tools may be more important in patients who generate hypofunctional Tregs or who cannot direct appropriate antigen specificities to the Treg lineage.
Systemic Treg therapies
Many existing tools are directed towards well-described core components of the T cell signaling machinery (e.g. CD3 and IL-2 signaling) that can be manipulated in relatively Treg-dominant manners, although more pleiotropic effects should always be considered. Treatment with anti-CD3 increases pTreg induction and induces remission of autoimmune diabetes in mice [70,71]. Encouragingly, treatment of 24 – 80 patients with new-onset T1D with anti-CD3 therapy in placebo-controlled phase II trials demonstrated improved metabolic control and residual insulin production 12 months to 4 years later [72–74]. Phase III trials have been less publicly successful, owing at least in part to the use of unvalidated end-points and drug dosages, although re-analysis of the teplizumab trial using the previously validated end-points showed benefit of therapy at 1 and 2 years post therapy [75–77]. Dosage was changed in part to avoid toxicity, again pointing to the value for continuing to search for more Treg-specific interventions. Results from revised phase III trials and testing in the context of other autoimmune diseases remain anticipated.
Although IL-2 is broadly essential for the maintenance and survival of most T cell subsets, Tregs are particularly dependent on exogenous IL-2 because FOXP3 represses IL2 expression [78–80]. Expression of the high-affinity IL-2 receptor CD25 facilitates Treg survival and may modulate local levels of IL-2 available for other cells, including NK cells [34,35]. This underlies the use of low-dose IL-2 (<1.5–3×106 IU daily, as compared to high dose 1.8×106 IU/kg daily in cancer) to attenuate the inflammation in graft-versus-host disease and HCV vasculitis [81–83]. These studies observed clinical improvement accompanied by increased circulating Tregs after IL-2 infusion, returning to baseline by 4 weeks after IL-2 discontinuation. Low-dose IL-2 (1.5–3×106 IU x5 days) has also shown early promise in alopecia areata, where the persistent increased Tregs specifically in skin biopsies (2 months after last treatment) point to the value of looking at target tissue, and in systemic lupus erythematosus, where progress in stratifying patients is likely to help select those most likely to benefit from Treg therapies [84–86]. In patients with T1D, IL-2 (4.5×106 IU 3 times/week × 1 month) was used together with rapamycin, resulting in increased circulating Tregs and NK cells but without improvement in islet β-cell function [87]. This was likely due in part to the need to further reduce IL-2 dosing; subsequent trial (phase I/II) of 3 lower IL-2 doses (0 – 3×106 IU/day x5 days) showed safe, dose-dependent Treg increase without negatively impacting β-cells [88].
The realization that the mTOR inhibitor rapamycin enhances Treg differentiation in vitro, in conjunction with its use in transplant for immunosuppression, has led to a broader consideration of its value as a pro-Treg agent [89,90]. Recent data suggest that rapamycin’s growth-inhibitory effects may dominantly contribute to the in vitro results and similarly impact Treg expansion in vitro and in vivo, although there may be ancillary benefits on stronger inhibition of other lineages and reducing production of pro-inflammatory cytokines [23,91,92]. Rapamycin can impair insulin signaling and may have contributed to the transient decrease in β-cell function in the IL-2/rapamycin T1D trial [87,93]. Together, these results exemplify some of the challenges in using broadly acting agents to achieve specific immune goals.
The microbiota has emerged as a potent modulator of immune function, notably with segmented filamentous bacteria (SFB) driving Th17 differentiation and certain clades of Clostridia driving Treg differentiation [94–96]. Although a human counterpart of SFB has not yet been identified, human strains of Clostridia have been identified with anti-inflammatory activity in murine models of colitis [96,97]. These microbes are thought to promote Treg differentiation through their natural production of short-chain fatty acids such as acetate, proprionate and butyrate, raising the notion of using microbes and microbial products as therapeutic agents [97–100].
Adoptive cellular therapy
The emerging field of Treg cellular therapy is perhaps of most obvious pertinence to the transfusion medicine physician, leveraging existing infrastructure to harvest, store, manipulate and deliver blood-based therapeutics. Most of these have been phase 1 studies exploring safety in the context of graft-versus-host prophylaxis. A University of Minnesota study used Tregs (3×106/kg) expanded from third party cord blood in 23 patients undergoing double-cord blood transplantation and found no Treg-associated toxicities [51]. Although not powered to examine efficacy given co-administration of standard GVHD prophylaxis, a trend towards decreased grade II-IV acute GVHD was observed (P=0.04) along with transient increase in peripheral blood Treg frequencies (up to 2 weeks); chimerism analyses were not performed to examine long-term persistence. A University of Perugia study tested freshly isolated donor Tregs (2 – 4×106/kg) as the sole immunosuppression in 28 haploidentical stem cell transplant recipients, leveraging predicted in vivo expansion of Tregs observed in mouse models [101]. Although no improvement in mortality was seen in this high-risk population, only 2 patients developed GVHD and 1 relapse with seen after median 12 months follow-up. A phase 2 follow-up expanded testing to 43 haploidentical stem cell transplant recipients receiving 2.5×106/kg donor Tregs as sole immunosuppression finding full engraftment in 95% and acute GVHD ≥ grade 2 in 15% with only 2 relapses at median 46 months follow-up [102]. These findings show great promise for the use of Tregs in GVHD prophylaxis – how this can best be applied to treat ongoing GVHD is more challenging and remains to be clarified.
The potential of Treg cellular therapy in T1D is also of active interest. At the Medical University of Gdansk, 10 new-onset patients were treated with autologous expanded Tregs (10 – 20×106/kg) with no toxicity was noted [103]. Long-term persistence of Tregs was not examined. Clinical parameters, including clinical remission, insulin need and C-peptide levels, were better in Treg-treated than in untreated patients and stayed improved up to 1 year later [103,104]. A study at University of California San Francisco and Yale, treated 14 recent-onset T1D patients with expanded deuterium-labeled autologous Tregs (5×106 – 2.6×109), which was tolerated well [52]. Deuterium labeling allowed tracking of the infused Tregs, demonstrating no instability to inflammatory fates (at least in peripheral blood), and persistence of a small proportion of infused Tregs in peripheral blood up to a year later. This study was not powered to detect metabolic improvements, but clearly did not exhibit the same precipitous C-peptide decline that accompanied IL-2/rapamycin treatment [52,87].
Whereas the above studies focus on polyclonal Tregs, there is likely to be additional benefit to using antigen-specific Tregs. Human trials are in progress to assess the efficacy of such antigen-specific Tregs in kidney and liver transplantation (the ONE study and NCT02474199). Systemic approaches include peptide-pulsed apoptotic cells and peptide-linked microparticles, which target tolerogenic antigen-presenting cells to induce pTregs in murine models of multiple sclerosis and T1D [105,106]. With regards to cellular therapy-type approaches, ovalbumin-specific Treg clones were generated by incubating PBMCs with ovalbumin and Drosophila S2 cells expressing anti-CD3, CD80, CD58, IL-2 and IL-4 in a phase 1/2a study of 20 patients with refractory Crohn’s disease [107]. These Tregs were well-tolerated and demonstrated dose-related efficacy. Whether this approach can be effectively generalized using other autoimmune-relevant antigens and/or patient antigen-presenting cells remains to be demonstrated. Antigen-specific Tregs could be sorted using HLA/peptide multimers for subsequent expansion, although this is likely to be useful only in diseases with limited pathogenic HLA/peptide combinations [108,109]. One emerging hybrid approach to specificity uses chimeric antigen receptors, which confer specificity using an extracellular antibody domain that recognizes antigen in a non-MHC-specific manner. Murine studies demonstrate antigen-specific disease attenuation in models of colitis and multiple sclerosis [110,111]. Because specificity is conferred by antibodies, this approach is likely most useful to enhance localization of Tregs, rather than true TCR-MHC/peptide specificity.
Forced expression of FOXP3 can help generate Tregs, either in a polyclonal or antigen-directed manner (if pre-selected for specificity). Early efforts showed retroviral-driven FOXP3 expression in conventional CD4+ T cells confers suppressive activity, albeit not to normal Treg levels [28]. More recent efforts showed lentiviral-driven expression of FOXP3 in CD4+ T cells from IPEX patients drives differentiation into functional and stable Tregs [112]. Some concern regarding the general sufficiency of FOXP3 (outside IPEX) is raised by the finding that forced expression of FOXP3 does not necessarily recapitulate the entire Treg genetic signature although efforts to identify ancillary factors like transcription factors that help lock in the Treg signature may propel these efforts [113,114].
One of the more prominent technical barriers to using adoptive Treg cellular therapies is available purification techniques; fluorescence-associated cell sorting (FACS) is routinely used in research and allows for simultaneous positive/negative sorting based on multiple markers but standard sorters are not GMP-compliant [115]. For these reasons, magnetic sorting is typically substituted, but GMP-compliant sorters are eagerly awaited, development of which may be accelerated by recent philanthropic efforts [116]. Constant evolution of GMP reagents and methods is required to attain therapeutic Treg doses – this is currently achieved by expanding polyclonal tTregs, although iTregs can be generated in even larger quantities and may become more attractive, as the ability to enhance their stability and relevance of antigen-specific cellular therapy advance [27]. Regulatory issues also present a burden, especially in a context of a patient-specific therapeutic; studies that demonstrate efficacy of shipped products point to feasible ways to centralize necessary expertise [52,115]. Finally, especially given concerns regarding lineage stability, methods to track transferred Tregs will be of great value. Deuterium labeling is of demonstrable value for peripheral blood analysis, as is VNTR chimerism analysis in allogeneic recipients, but noninvasive techniques like single photon emission computed tomography (SPECT) may eventually allow tracking of Technetium-99-radiolabeled cells in target organs of patients [117].
Further considerations
As previously alluded to, Treg therapies are an aspect of a broader endeavor to modulate immune homeostasis. It is in this context that safety should continue to be evaluated. In this regard, ipilimumab is an antibody against the inhibitory co-receptor CTLA-4 currently used predominantly in melanoma to “break” tolerance to the tumor. Significant risk of immune-related adverse events accompanies ipilimumab use (72% all grades, 24% high grade), especially colitis [118]. In the converse context of pro-Treg therapy, key side effects of concern include infection, decreased graft-versus-leukemia (GVL) effect and impaired immune surveillance leading to neoplastic disease. In non-cancer-related studies, no increased susceptibility to infection has been noted, perhaps best exemplified by the absence of increased viral load in HCV-positive patients treated with low-dose IL-2 [52,83,103,104]. Post-transplant patients are immune-compromised and do develop infections, albeit at similar levels to historical rates where tracked [51]. Settings where Treg therapy may allow decreased immunosuppression may even reduce risk of infection, although this remains to be demonstrated. In the setting of using Tregs to treat GVHD, an attendant concern is inhibition of the GVL effect post-transplant; this was found to be maintained in one study and is worth tracking in subsequent studies [102].
With regards to immune surveillance, it bears explicating that patients with cancer or inflammatory disease do not typically exhibit robust perturbations of Treg numbers in peripheral blood. This highlights the importance of considering (Treg) location, specificity and functionality in the context of immune homeostasis, and the value of tools that allow measurement of these parameters. Tumor microenvironments may be particularly supportive of Treg development and the role of Tregs in initiating and/or propagating defects in immune surveillance in individual patients remains to be clearly delineated [119,120]. The risk of neoplastic disease may increase with prolonged pro-Treg regimens, although the chronic inflammation typically being treated is notably itself a risk factor.
Durability of any given pro-Treg regimen is an issue of interest that better tools may help assess. This is typically monitored using the peripheral blood Treg count (which typically returns to baseline within 2–8 weeks after treatment) but this does not inform about location- or antigen-specific responses [51,81–83,103]. In the setting of adoptively transferred cellular therapy, deuterium labeling is a promising approach that shows detectable persistence of some transferred Tregs in the circulation of many patients up to one year later [52]. Developing quantitative tools will be helpful to monitor both persistence and stability of transferred Tregs, although this method may still be limited to accessible tissues such as blood and perhaps colon and skin. The ability to assess Tregs in other organs such as pancreas is greatly anticipated. These tools may significantly impact regimen design and development of next-generation therapies of increased durability.
Concluding remarks
Tregs are a critical regulator of immune homeostasis, manipulation of which is broadly relevant to both inflammation and cancer. Several studies show great promise of this therapy and progress in key areas will greatly advance our capabilities. There remains significant room and need to identify novel pathways that regulate Treg development and function, especially in the context of human disease. This will help better identify patients who might benefit from Treg therapies, as well as suggest how current Treg therapies might be improved, particularly with autologous therapies. Development of Treg-related interventions must include considerations not only of quantitative and qualitative defects, but also of tissue-localization, specificity and lineage stability. Diagnostically, major challenges include finding improved ways to identify Tregs, especially in tissues, as well as developing better assays to assess Treg function, ideally in a manner that lends itself to current testing platforms.
Current therapeutic modalities focus on increasing Tregs quantitatively either using systemic therapies or adoptive cellular therapies, and show promise in early studies. It is important to keep in mind that these Treg therapies need not and should not be considered in isolation, but rather as a component of a synergistic, coordinated manipulation of the immune system away from the pathologic state. For example, whereas adoptively transferred Tregs may be useful in early-onset type 1 diabetes, more advanced cases may require a different regimen to enable induction of tolerance, followed by a pro-Treg therapy and islet transplant. Adoptive cellular therapies offer unique opportunities to select T cells based on antigen specificity and even modulate their functionality using agents at concentrations that might not be tolerated when administered systemically. Certainly, Treg therapies exemplify the tip of an exciting chapter of therapeutic manipulations of immune homeostasis, in which the transfusion medicine physician plays a key role.
Supplementary Material
Highlights.
Core concepts of Treg differentiation and function.
Antigen-specificity and lineage stability are important considerations in Tregs.
Quantitative and qualitative defects in Tregs lead to disease.
Systemic and cellular therapies can address Treg defects.
Unique potential of cellular Treg therapies.
Acknowledgments
We would like to thank Cristina Penaranda and Kara L Conway for helpful discussions and critical comments.
Funding
This work was supported by the National Institutes of Health (grant K08 DK104021A).
Abbreviations
- GWA
Genome-wide association (study)
- T1D
Type 1 diabetes
- TCR
T cell receptor
- Th
T helper
- Treg
Regulatory T cell
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–61. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 4.Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 5.O’shea JJ, Paul WE. Mechanisms Underlying Lineage Commitment and Plasticity of Helper CD4+ T Cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 9.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 10.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Okello JBA, Zurek J, Devault AM, Kuch M, Okwi AL, Sewankambo NK, et al. Comparison of methods in the recovery of nucleic acids from archival formalin-fixed paraffin-embedded autopsy tissues. Anal Biochem. 2010;400:110–7. doi: 10.1016/j.ab.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci USA. 2015;112:7225–30. doi: 10.1073/pnas.1508224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg Cells Expressing the Coinhibitory Molecule TIGIT Selectively Inhibit Proinflammatory Th1 and Th17 Cell Responses. Immunity. 2014;40:569–81. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal T(H)2 inflammation. Nature. 2012 doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic Generation of Regulatory T Cells in Placental Mammals Mitigates Maternal-Fetal Conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–42. S1. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav M, Louvet C, Davini D, Gardner JM, Martínez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–22. S1–19. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milpied P, Renand A, Bruneau J, Mendes-da-Cruz DA, Jacquelin S, Asnafi V, et al. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol. 2009;39:1466–71. doi: 10.1002/eji.200839040. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–12. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Jin W, Hardegen N, Lei K-J, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khor B, Gagnon JD, Goel G, Roche MI, Conway KL, Tran K, et al. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. Elife. 2015:4. doi: 10.7554/eLife.05920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feuerer M, Hill JA, Kretschmer K, Boehmer von H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919–24. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–99. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–22. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue X, Trifari S, Aijö T, Tsagaratou A, Pastor WA, Zepeda-Martínez JA, et al. Control of Foxp3 stability through modulation of TET activity. J Exp Med. 2016;213:377–97. doi: 10.1084/jem.20151438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–84. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joly A-L, Liu S, Dahlberg CIM, Mailer RKW, Westerberg LS, Andersson J. Foxp3 lacking exons 2 and 7 is unable to confer suppressive ability to regulatory T cells in vivo. J Autoimmun. 2015;63:23–30. doi: 10.1016/j.jaut.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 30.De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16:1174–84. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D. Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med. 2013;210:1153–65. doi: 10.1084/jem.20122248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med. 2013;210:1179–87. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelhardt KR, Shah N, Faizura-Yeop I, Kocacik Uygun DF, Frede N, Muise AM, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131:825–30. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121:1595–603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen E, Morbach H, Ullas S, Bannock JM, Massad C, Menard L, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134:1365–74. doi: 10.1016/j.jaci.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.León B, Bradley JE, Lund FE, Randall TD, Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–39. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–89. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey-Bucktrout SL, Martínez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39:949–62. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–71. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity. 2015;43:251–63. doi: 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158:749–63. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–63. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 50.Thompson LJ, Lai J-F, Valladao AC, Thelen TD, Urry ZL, Ziegler SF. Conditioning of naive CD4(+) T cells for enhanced peripheral Foxp3 induction by nonspecific bystander inflammation. Nat Immunol. 2016;17:297–303. doi: 10.1038/ni.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Löhning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Höfer T, et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–5. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, et al. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. The Journal of Immunology. 2014;193:4988–99. doi: 10.4049/jimmunol.1401776. [DOI] [PubMed] [Google Scholar]
- 56.Collison LW, Vignali DAA. In vitro Treg suppression assays. Methods Mol Biol. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–41. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 60.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–8. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42–2. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pacheco-Gonzalez RM, Avila C, Dávila I, García-Sánchez A, Hernández-Hernández L, Benito-Pescador D, et al. Analysis of FOXP3 gene in children with allergy and autoimmune diseases. Allergol Immunopathol (Madr) 2016;44:32–40. doi: 10.1016/j.aller.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Ye CJ, Feng T, Kwon H-K, Raj T, Wilson MT, Asinovski N, et al. Intersection of population variation and autoimmunity genetics in human T cell activation. Science. 2014;345:1254665–5. doi: 10.1126/science.1254665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arvey A, van der Veeken J, Plitas G, Rich SS, Concannon P, Rudensky AY. Genetic and epigenetic variation in the lineage specification of regulatory T cells. Elife. 2015;4:455. doi: 10.7554/eLife.07571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol. 2015;45:333–43. doi: 10.1002/eji.201344205. [DOI] [PubMed] [Google Scholar]
- 68.Pellegrini FP, Marinoni M, Frangione V, Tedeschi A, Gandini V, Ciglia F, et al. Down syndrome, autoimmunity and T regulatory cells. Clin Exp Immunol. 2012;169:238–43. doi: 10.1111/j.1365-2249.2012.04610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider A, Long SA, Cerosaletti K, Ni CT, Samuels P, Kita M, et al. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive T(regs) involves IL-6-mediated signaling. Sci Transl Med. 2013;5:170ra15–5. doi: 10.1126/scitranslmed.3004970. [DOI] [PubMed] [Google Scholar]
- 70.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. The Journal of Immunology. 2011;187:2015–22. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–7. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 73.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 74.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–23. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 75.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hagopian W, Ferry RJ, Sherry N, Carlin D, Bonvini E, Johnson S, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–8. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bach J-F. Anti-CD3 antibodies for type 1 diabetes: beyond expectations. Lancet. 2011;378:459–60. doi: 10.1016/S0140-6736(11)60980-X. [DOI] [PubMed] [Google Scholar]
- 78.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 80.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–5. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koreth J, Matsuoka K-I, Kim HT, McDonough SM, Bindra B, Alyea EP, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuoka K-I, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5:179ra43. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–77. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 84.Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748–51. doi: 10.1001/jamadermatol.2014.504. [DOI] [PubMed] [Google Scholar]
- 85.Spee-Mayer von C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. 2016;75:1407–15. doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- 86.Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell. 2016;165:551–65. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes. 2012;61:2340–8. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 89.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41–1. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Camirand G, Lin Y, Froicu M, Deng S, Shlomchik WD, et al. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. The Journal of Immunology. 2011;186:2809–18. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di Paolo S, Teutonico A, Leogrande D, Capobianco C, Schena PF. Chronic inhibition of mammalian target of rapamycin signaling downregulates insulin receptor substrates 1 and 2 and AKT activation: A crossroad between cancer and diabetes? J Am Soc Nephrol. 2006;17:2236–44. doi: 10.1681/ASN.2006030196. [DOI] [PubMed] [Google Scholar]
- 94.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 2010 doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013:1–7. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 97.Narushima S, Sugiura Y, Oshima K, Atarashi K, Hattori M, Suematsu M, et al. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes. 2014;5:333–9. doi: 10.4161/gmic.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 101.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 102.Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124:638–44. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 103.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juścińska J, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–20. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juścińska J, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153:23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, Nakatsukasa H, et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci Transl Med. 2014;6:241ra78. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- 106.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30:1217–24. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, et al. Safety and Efficacy of Antigen-Specific Regulatory T-Cell Therapy for Patients With Refractory Crohn’s Disease. Gastroenterology. 2012:1–13. doi: 10.1053/j.gastro.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 108.Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320:119–31. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 109.Huang J, Zeng X, Sigal N, Lund PJ, Su LF, Huang H, et al. Detection, phenotyping, and quantification of antigen-specific T cells using a peptide-MHC dodecamer. Proc Natl Acad Sci USA. 2016;113:E1890–7. doi: 10.1073/pnas.1602488113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134:2014–24. doi: 10.1053/j.gastro.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 111.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. Journal of Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Passerini L, Rossi Mel E, Sartirana C, Fousteri G, Bondanza A, Naldini L, et al. CD4 T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci Transl Med. 2013;5:215ra174–4. doi: 10.1126/scitranslmed.3007320. [DOI] [PubMed] [Google Scholar]
- 113.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 114.Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS, et al. A multiply redundant genetic switch “locks in” the transcriptional signature of regulatory T cells. Nat Immunol. 2012;13:972–80. doi: 10.1038/ni.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, Brinke ten A, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015;7:304ps18–8. doi: 10.1126/scitranslmed.aaa7721. [DOI] [PubMed] [Google Scholar]
- 116.Kaiser J. Q&A: What can $250 million achieve? Chief of new cancer immunotherapy institute explains. Science. 2016 doi: 10.1126/science.aaf9928. [DOI] [Google Scholar]
- 117.Sharif-Paghaleh E, Sunassee K, Tavaré R, Ratnasothy K, Koers A, Ali N, et al. In vivo SPECT reporter gene imaging of regulatory T cells. PLoS ONE. 2011;6:e25857. doi: 10.1371/journal.pone.0025857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bertrand A, Kostine M, Barnetche T, Truchetet M-E, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol. 2015;33:101–11. doi: 10.1016/j.coi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 120.Liu C, Workman CJ, Vignali DAA. Targeting Regulatory T Cells in Tumors. Febs J. 2016 doi: 10.1111/febs.13656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.