Introduction
Chronic pain due to nerve trauma is a significant health problem. Among the various origins of such pain, limb amputation stands out as particularly important. In the United States alone, more than 100,000 patients per year undergo amputation due to trauma or medical conditions including diabetes and peripheral vascular disease and the incidence of long-term morbidity due to chronic pain in this population ranges from 50–80% [12,13,34].
Although the precise pathogenesis of chronic pain due to nerve trauma still remains elusive, great progress has been made in recent years in our understanding of this condition. [7,10,23]. There is now abundant evidence from preclinical animal models that the immune system plays a critical role in driving chronic pain[1,6,11,17]. Several human studies have also corroborated the important role of the immune system in various chronic pain states, with a particular focus on systemic inflammatory mediators such as cytokines, chemokines and related molecules. For example, in one study of patients with Complex Regional Pain Syndrome (CRPS), pro-inflammatory mediators such as tumor necrosis factor (TNF) and interleukin-(IL-)2 were found to be significantly elevated compared to controls, while anti-inflammatory mediators such as IL-4 and IL-10 exhibited the opposite trend[28]. Similarly, a study comparing patients with painful vs. non-painful peripheral neuropathies demonstrated that patients with painful neuropathy had elevated systemic TNF and IL-2 (both protein and mRNA) compared to their non-painful counterparts[29].
Given these past findings, we hypothesized that a systemic pro-inflammatory profile is also associated with chronic pain after nerve trauma due to amputation. To address this question, we examined blood samples collected from a cohort of recent active duty military post-traumatic amputees in the Veterans Integrated Pain Amputation Evaluation Research (VIPER) study [4] This study employed a case-control design, with amputees experiencing chronic residual limb pain classified as cases, and amputees with little or no pain designated as controls. Unique features of the VIPER study include the lack of significant co-morbidities in the otherwise young and healthy study cohort, as well as the fact that both case and control groups experienced the same traumatic injury, minimizing the likelihood that injury status would confound results. The first aim of the present study was to investigate systemic inflammatory profiles in a subset of 76 patients from the VIPER cohort using multiplexed, high-sensitivity, electrochemiluminescent assays.
Pain catastrophizing shows ubiquitous associations with pain severity across various chronic pain conditions, including phantom limb pain post-amputation [27,31]. However, little is known about links between catastrophizing and chronic post-amputation residual limb pain. While a few studies using evoked pain models have examined possible links between catastrophizing and inflammatory status[8,9], this issue has received little study in the chronic pain setting [25]. A second aim of this study was therefore to examine associations between catastrophizing, chronic post-amputation pain, and systemic inflammatory profiles.
Methods
Design
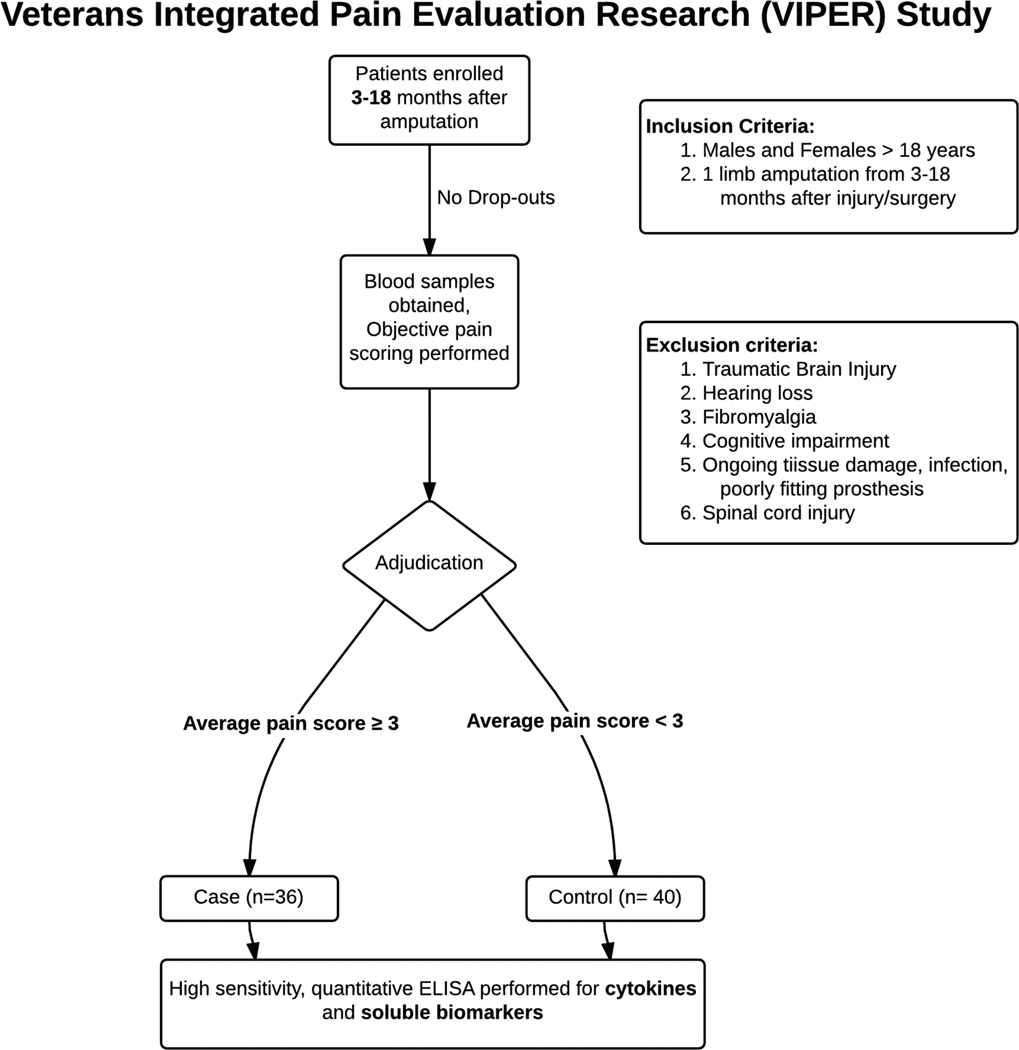
Data were obtained as part of a larger observational case-control study comparing young recent active duty military traumatic amputees with and without significant residual limb pain 3 to 18 months after injury. After enrollment, study subjects provided blood samples and psychometric data were collected. Patients were assigned case or control status based on average pain score over the week prior to enrollment (Figure 1). Blood plasma samples were then sent to the Duke Biomarker Core facility for inflammatory marker detection using the MesoScaleDiscovery System.
Figure 1.
VIPER study flow diagram defining inclusion/exclusion criteria and patient adjudication results.
Subjects
All study procedures were approved by the Institutional Review Board of Walter Reed National Military Medical Center (WRNMMC). Subjects included 36 cases (as defined below) and 40 controls who had undergone post-traumatic amputations while on active duty. All potential subjects were being treated at WRNMMC and the clinical research was supervised through the Defense and Veterans Center for Integrative Pain Management (DVCIPM – DVCIPM.org), part of the Uniformed Services University. Subjects were included if they were a military health care system beneficiary aged 18 years or older and undergoing treatment at WRNMMC with a diagnosis of post-injury amputation of all or part of one limb. Amputation injury must also have occurred between 3 and 18 months prior to enrollment. Patients were excluded if they were afflicted with severe traumatic brain injury, significant cognitive deficits, substantial hearing loss, spinal cord injury with permanent or persistent deficits, ongoing tissue damage that might cause pain, infection, heterotrophic ossification, poorly fitting prosthesis, or hip disarticulation.
We defined “Cases” as those with clinically significant residual limb pain, defined as an average pain score over the past week of greater than or equal to 3/10 on a numeric rating scale (NRS) (Figure 1). Those patients with clinically significant pain were further adjudicated into pain subtypes. Those subjects reporting no pain or pain less than 3/10 but greater than 0/10 were considered “Controls” (pain subtypes were not analyzed in the latter subgroup). This case/control methodology was chosen to facilitate the separate biomarker discovery and genomic analysis aims of the larger project.
Subject characteristics are summarized in Table 1. There were no significant differences between groups in subject age, BMI, ethnicity, smoking status, amputation site, amputation level or time between injury and enrollment. Patients defined as cases reported significantly higher levels of pain catastrophizing. By study design, cases also had significantly higher average pain scores. Cases also were more likely to use opioid medications of any kind. 94% of cases reported at least some degree of phantom limb pain.
Table 1.
Characteristics of sample participants from the Veterans Integrated Pain Evaluation Research (VIPER) study.
| Control (N=40) | Case (N=36) | p-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age | 25.2 (4.8) | 27.7 (9.1) | 0.153 |
| Body Mass Index | 25.7 (2.8) | 26.8 (3.6) | 0.143 |
| Time since amputation (months) | 7.6 (3.5) | 8.9 (5.9) | 0.224 |
| Smoking (ppd) | 0.6 (0.5) | 0.6 (0.54) | 0.817 |
| Average Pain Severity (0–10) | 1.2 (0.73) | 5.6 (1.38) | 0.000 |
| Pain Catastrophizing Scale | 2.6 (4.53) | 11.8 (11.1) | 0.000 |
| N (%) | N(%) | ||
| Male | 40 (100) | 35(97) | 0.958 |
| Smokers | 25 (63) | 21 (58) | 0.892 |
| Opioid use | 3 (8) | 19 (53) | 0.000 |
| Ethnicity: | N (%) | N(%) | |
| American Indian/Alaska Native | 0 (0) | 0 (0) | 1.000 |
| Asian | 2 (5) | 1 (3) | 1.000 |
| Native Hawaiian or Other Pacific Islander |
0 (0) | 0 (0) | 1.000 |
| Black or African American | 3 (7) | 3 (8) | 1.000 |
| White | 37 (93) | 32 (89) | 0.884 |
| Amputation site: | N (%) | N(%) | |
| Leg | 37 (92) | 32 (89) | 0.587 |
| Arm | 3 (8) | 4 (11) | 0.587 |
| Multiple | 0 (0) | 0 (0) | 1.000 |
| Proximal amputation | 12 (30) | 16 (44) | 0.192 |
Note: Continuous variables were analyzed using t-tests whereas categorical values were examined using a chi-squared test.
Procedures
After written informed consent was obtained, blood samples were obtained from each patient at one time point for subsequent analysis. For preparation of plasma, 6ml of blood was collected in EDTA-containing K2 tubes and inverted to mix. Tubes were then spun at 3,000g for 20 minutes at 4 degrees C. Plasma fraction was collected with a pipette and aliquoted into 1.5ml cryovials and stored at −20 degrees C for 24 hours and subsequently at −80 degrees C.
After blood sample collection, subjects completed the pain and psychometric measures described below.
Measures
Ratings of average pain severity over the past week were provided by all subjects using the self-report version of the Leeds Assessment of Neuropathic Symptoms and Signs scale [2]. The S-LANSS is a validated measure of pain severity and neuropathic pain characteristics. Pain severity on the S-LANSS is rated on an 11-point numeric pain rating scale, anchored with “No Pain” and “Pain As Severe As It Could Be.” Given the current hypotheses and to minimize the number of analyses conducted, data regarding neuropathic pain characteristics from the S-LANSS are not reported here. All subjects also completed the Pain Catastrophizing Scale (PCS), a widely-used and validated measure of pain catastrophizing [18,26] Focus in the current study was on overall level of catastrophizing as reflected in total PCS scores.
Inflammatory Mediator Assays
The Neuroinflammation Panel 1 by MesoScaleDiscovery (MSD #K15210D) was used to quantify 37 acute inflammatory and injury markers in human serum. These sandwich immunoassays consist of five microplates, each pre-coated with capture antibodies on 4 to 10 independent spots and are grouped based on optimal performance in a multiplex panel as follows: Proinflammatory Panel 1 (IFNγ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, and TNF-α), Cytokine Panel 1 (IL-1α, IL-5, IL-7, IL-12/IL-23p40, IL-15, IL-16, IL-17a, TNF-β, and VEGF), Chemokine Panel 1 (Eotaxin, MIP-1β, Eotaxin-3, TARC, IP-10, MIP-1α, MCP-1, MDC, and MCP-4), Angiogenesis Panel 1 (VEGF-c, VEGF-D, Tie-2, Flt-1, PIGF, and bFGF), and Vascular Injury Panel 2 (SAA, CRP, VCAM-1, and ICAM-1). Each of these panels is a V-plex assay indicating it is fully validated according to fit-for-purpose principles and the FDA’s analytical validation guidelines, offering highly sensitivity and reproducible results from lot-to-lot. All assays were run according to the manufacturer and samples were run in duplicate. Values below LLOD were defined as ½ LLOD when determining significant differences in inflammatory mediator concentration between cases and controls but not for correlational analysis.
Statistical Analysis
All analyses were conducted using IBM SPSS for Statistics version 23. Initial examination of the distributions of the inflammatory mediators indicated most were not normally distributed. Because of this, we used the nonparametric Mann-Whitney U test for evaluating differences in inflammatory mediators between groups (Case vs. Control) and used nonparametric correlations (Spearman’s rho) for examining associations between residual limb pain levels, pain catastrophizing (PCS scores), and inflammatory mediators.
Because of the unique data available in this study, we evaluated a statistical mediation model in which the association of catastrophizing with chronic post-amputation residual limb pain severity was conveyed in part via indirect effects of inflammatory mediators. To evaluate this statistical mediation model, the approach of Preacher and Hayes (2004) was used to test the significance of the indirect effects[21]. Custom SPSS dialogue (the Indirect Procedure; http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html#sobel) was used to test the significance of indirect effects in these models using bootstrapping procedures[21]. This bootstrap methodology tested each model in a series of 1000 random subsamples repeatedly drawn from the full sample, generating 95% confidence intervals (bias corrected) around the indirect effect test statistic. If the 95% confidence interval for the indirect effect generated by the model did not include zero, this indicated that the hypothesized indirect (mediated) effect was significant at the p<.05 level. To minimize the risk of bias in estimation of indirect effects, inflammatory mediator values were normalized via log-transformations prior to conducting mediation analyses.
In preliminary analyses, time (in months) since amputation was examined as it related to the primary outcomes to determine whether it might confound primary analyses. Correlational analyses indicated that pain duration was not associated with either average residual limb pain severity (Spearman’s rho = −0.07, p = 0.572) or catastrophizing scores on the PCS (Spearman’s rho = −0.13, p = 0.274), nor with most inflammatory mediator values. Exceptions to the latter were: IL-12 (Spearman’s rho = −0.24, p = 0.035), IL-15 (Spearman’s rho = 0.26, p = 0.026), IL-16 (Spearman’s rho = −0.27, p = 0.022), IL-1alpha (Spearman’s rho = 0.27, p = 0.021), and VCAM-1 (Spearman’s rho = −0.23, p = 0.044). Examination of interactions between time from injury and pain intensity on all inflammatory markers revealed only one significant interaction (for IL-2; p<.04). Because of the general absence of relevant pain duration effects for key outcomes of interest, it was not included as a control variable in the analyses reported below.
Results
Multiple inflammatory mediators are upregulated in amputees with residual limb pain
To determine whether any differences in systemic inflammatory mediators were present between the case and control groups, we measured the levels of 37 inflammatory mediators in all 76 patients. Relative to patients defined as controls, patients defined as cases exhibited significantly higher levels of TNF-α, TNF-β, IL-8, ICAM-1, Tie2, CRP, and SAA (Table 2). Each of the elevated markers have mainly pro-inflammatory properties. Descriptive statistics for all of the mediators tested are shown in Table 2.
Table 2.
Systemic Inflammatory Mediator Concentrations in Cases vs. Controls.
| Mediator | Case (n=36) Median(IQR) |
Control (n=40) Median (IQR) |
Mann-Whitney U Test (p value) |
|---|---|---|---|
| IFN-γ | 4.1 (3.3–6.4) | 3.5(3.0–6.2) | 0.252 |
| IL-10 | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.113 |
| IL-13 | 0.8 (0.8–0.8) | 0.8 (0.8–0.8) | 0.099 |
| IL-1β | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.859 |
| IL-2 | 0.1 (0.1–0.3) | 0.1 (0.1–0.2) | 0.713 |
| IL-4 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.970 |
| IL-6 | 0.8 (0.4–1.2) | 0.5 (0.3–0.9) | 0.138 |
| IL-8 | 4.7 (3.8–6.1) | 4.0 (3.1–5.0) | 0.041 |
| TNF-α | 2.2 (1.8–2.6) | 1.9 (1.5–2.3) | 0.031 |
| IL-12 | 140 (103–191) | 130 (83–154) | 0.131 |
| IL-17 | 1.1 (1.1–2.3) | 1.1 (1.1–1.1) | 0.546 |
| IL-5 | 0.3 (0.1–0.6) | 0.3 (0.2–0.6) | 0.983 |
| IL-7 | 3.4 (2.1–5.6) | 3.5 (2.2–5.9) | 0.768 |
| TNF-β | 0.2 (0.1–0.2) | 0.1 (0.1–0.2) | 0.037 |
| VEGF | 43.4 (32.0–56.5) | 35.1 (28.3–44.1) | 0.065 |
| IL-15 | 1.9 (1.6–2.0) | 1.8 (1.5–2.0) | 0.914 |
| IL-16 | 183 (152–230) | 190 (165–272) | 0.349 |
| IL-1α | 0.9 (0.5–2.1) | 1.2 (0.5–1.9) | 0.971 |
| Eotaxin | 74.7 (62.7–104) | 86.4 (62.0–108) | 0.938 |
| Eotaxin-3 | 18.8 (14.1–26.4) | 22.3 (17.1–25.3) | 0.399 |
| IP-10 | 253 (170–361) | 210 (155–329) | 0.293 |
| MCP-4 | 45 (32.2–60.6) | 52.4 (38.6–69.7) | 0.182 |
| MDC | 660 (481–824) | 657 (532–746) | 0.979 |
| MIP-1α | 5.5 (5.5–12.7) | 5.5 (5.5–12.0) | 0.494 |
| MIP-1β | 53.8 (38.9–68.0) | 45.0 (37.8–62.0) | 0.302 |
| TARC | 58.5 (30.8–101.0) | 71.9 (38.9–124.0) | 0.163 |
| Flt1 | 47.5 (37.5–53.8) | 47.5 (38.1–57.8) | 0.881 |
| PIGF | 28.2 (22.5–34.2) | 25.0 (18.8–28.1) | 0.056 |
| Tie2 | 6380 (5680–7530) | 5550 (4830–6280) | 0.008 |
| VEGF-C | 43.5 (33.9–68.2) | 61.0 (42.9–93.7) | 0.112 |
| VEGF-D | 540 (382–1060) | 750 (453–2240) | 0.172 |
| bFGF | 7.7 (2.0–22.8) | 7.2 (4.4–32.3) | 0.298 |
| CRP | 4010 (2010–10600) | 2150 (775–5300) | 0.034 |
| SAA | 3880 (2420–8640) | 1980 (840–4160) | 0.002 |
| ICAM-1 | 421 (351–586) | 379 (310–421) | 0.007 |
| VCAM-1 | 415 (340–462) | 415 (344–484) | 0.873 |
All concentrations are expressed in pg/ml except with the exception of CRP, SAA, ICAM-1 and VCAM-1, which are reported in ng/ml. IQR = Interquartile Range (Q1–Q3)
Inflammatory mediators correlate with pain severity and catastrophizing in amputees with significant residual limb pain
A second aim of this study was to examine associations between systemic inflammatory mediators, post-amputation residual limb pain severity, and pain catastrophizing. Ratings of average residual limb pain severity and PCS scores were significantly correlated, r (74) = 0.62, p<.001. Table 3 summarizes associations between systemic inflammatory mediators and both residual limb pain severity and PCS scores. Higher pain severity was found to be associated with significantly higher levels of IL-8, IL-12, TNF-α, TNF-β, PIGF, Tie2, SAA, and ICAM-1, with inverse associations noted for IL-2, IL-13, and Eotaxin-3. Similarly, higher PCS scores were associated with significantly higher levels of IL-8, IL-12, TNF-β, PIGF, and ICAM-1, with an inverse association observed with IL-13. To address possible inflated type I error due to the number of inflammatory mediators examined, permutation testing (1000 permutations) was conducted to determine empirical probability values for the correlational analyses as a set. As indicated at the bottom of Table 3, set-wise associations with levels of inflammatory mediators (taking into account observed degree of intercorrelations among the mediators) were highly significant for both average pain severity and catastrophizing. These results indicate that the overall associations reported between these two variables and inflammatory status are unlikely to represent spurious findings.
Table 3.
Spearman correlations between inflammatory mediators, average residual limb pain intensity, and pain catastrophizing in the full sample (n=76).
| Systemic Mediator | Average Pain | P value | PCS | P value |
|---|---|---|---|---|
| IFN-γ | 0.11 | 0.339 | 0.18 | 0.119 |
| IL-10 | 0.15 | 0.191 | 0.03 | 0.804 |
| IL-13 | −0.45 | 0.000 | −0.29 | 0.010 |
| IL-1β | 0.06 | 0.604 | 0.13 | 0.280 |
| IL-2 | −0.24 | 0.038 | −0.15 | 0.184 |
| IL-4 | −0.22 | 0.054 | −0.06 | 0.612 |
| IL-6 | 0.17 | 0.137 | 0.10 | 0.374 |
| IL-8 | 0.26 | 0.024 | 0.25 | 0.030 |
| TNF-α | 0.30 | 0.008 | 0.18 | 0.129 |
| IL-12 | 0.31 | 0.006 | 0.24 | 0.037 |
| IL-17 | 0.21 | 0.065 | 0.10 | 0.369 |
| IL-5 | 0.04 | 0.706 | 0.08 | 0.517 |
| IL-7 | −0.03 | 0.774 | 0.12 | 0.293 |
| TNF-β | 0.43 | 0.000 | 0.39 | 0.001 |
| VEGF | 0.19 | 0.104 | 0.17 | 0.139 |
| IL-15 | −0.01 | 0.906 | 0.02 | 0.855 |
| IL-16 | 0.09 | 0.439 | 0.21 | 0.077 |
| IL-1a | 0.11 | 0.334 | 0.03 | 0.773 |
| Eotaxin | 0.02 | 0.856 | −0.04 | 0.775 |
| Eotaxin-3 | −0.27 | 0.017 | −0.10 | 0.385 |
| IP-10 | 0.07 | 0.557 | 0.02 | 0.887 |
| MCP-1 | −0.03 | 0.813 | 0.01 | 0.917 |
| MCP-4 | −0.08 | 0.496 | −0.06 | 0.628 |
| MDC | 0.04 | 0.728 | −0.04 | 0.763 |
| MIP-1α | 0.06 | 0.632 | −0.03 | 0.831 |
| MIP-1β | 0.14 | 0.238 | 0.11 | 0.358 |
| TARC | −0.11 | 0.366 | 0.01 | 0.947 |
| Flt-1 | 0.13 | 0.262 | 0.15 | 0.194 |
| PIGF | 0.31 | 0.008 | 0.34 | 0.003 |
| Tie2 | 0.35 | 0.002 | 0.22 | 0.059 |
| VEGF-C | −0.15 | 0.185 | −0.04 | 0.756 |
| VEGF-D | −0.05 | 0.664 | 0.20 | 0.087 |
| bFGF | −0.13 | 0.270 | 0.06 | 0.595 |
| CRP | 0.20 | 0.092 | 0.05 | 0.671 |
| SAA | 0.33 | 0.004 | 0.21 | 0.072 |
| ICAM-1 | 0.43 | 0.000 | 0.44 | 0.000 |
| VCAM-1 | 0.11 | 0.346 | 0.21 | 0.074 |
| Set-wise p value | --- | 0.008 | --- | 0.005 |
Do Inflammatory Mediators Contribute to Associations between Pain Catastrophizing and Residual Limb Pain Severity?
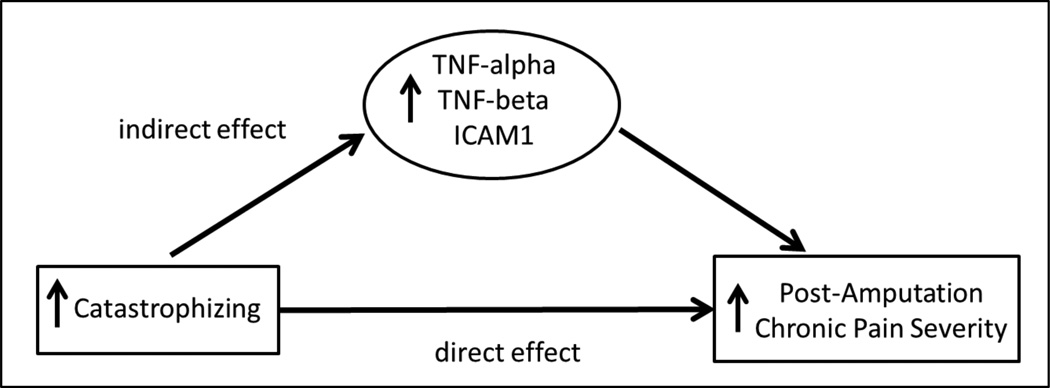
We considered the possibility that the positive association between pain catastrophizing levels (PCS) and residual limb pain severity might be accounted for in part by indirect effects conveyed through systemic inflammatory mediators. While the only requirement for conducting analyses to test this model was that catastrophizing needed to be associated with pain severity, we restricted our analyses to only those inflammatory mediators showing significant associations with the outcome of interest (residual limb pain severity) to limit the number of analyses conducted.
Results using bootstrapped mediation tests indicated significant indirect effects between PCS scores and residual limb pain severity via TNF-α (95% CI: 0.0004 – 0.0308), TNF-beta (95% CI: 0.0027 – 0.0309), SAA (95% CI: 0.0005 – 0.0351), and ICAM-1 (95% CI: 0.0011 – 0.0515). In each case, there were also significant direct effects of PCS scores on residual limb pain severity independent of systemic inflammatory mediators (p’s<.001). Because both significant direct and indirect effects were observed for these mediators, results can be interpreted as indicating that the positive association between pain catastrophizing and residual limb pain severity was statistically-mediated in part (rather than fully mediated) by TNF-α, TNF-beta, SAA, and ICAM-1 levels in plasma (model summarized in Figure 2). Tests of indirect effects for other systemic inflammatory mediators showing associations with residual limb pain severity in correlational analyses were all nonsignificant (p’s > .05).
Figure 2.
Model in which effects of catastrophizing on residual limb pain severity are conveyed through indirect effects of systemic inflammatory mediators.
Discussion
In this study, we found elevated systemic levels of several pro-inflammatory mediators in amputees with residual limb pain (Cases) compared to those without clinically significant residual limb pain (Controls). Specifically, the pro-inflammatory mediators TNF-α, TNF-b, IL-8, CRP, SAA, Tie2 and ICAM-1 were significantly elevated in cases compared to controls.
Across both patient groups, severity of residual limb pain was associated positively with levels of several pro-inflammatory mediators (IL-8, TNF-α, IL-12, TNF-β, PIGF, Tie2, SAA and ICAM-1), and inversely with concentrations of the anti-inflammatory mediator IL-13, as well as IL-2 and Eotaxin-3. Eotaxin-3, initially thought to have mainly pro-inflammatory properties through agonism of CCR3, more recently was found to be an antagonist for multiple CCR receptors whose blockade prevents chemotaxis [19]. Similarly, IL-2 was initially thought to be mainly pro-inflammatory, stimulating cytotoxic T-cells and NK cells, but was later found to be an important stimulator of Treg cells[3]. Taken together, these findings demonstrate an overall pro-inflammatory signature in amputees with chronic pain. To the best of our knowledge, this is the first comprehensive study of systemic inflammatory mediators in human subjects with residual limb pain following amputation.
This study also appears to be the first to examine associations between post-amputation residual limb pain and levels of pain catastrophizing, a cognitive factor previously shown to exacerbate chronic pain severity across a variety of other pain conditions[22,31]. Results, not surprisingly, indicated that elevated catastrophizing was correlated with greater residual limb pain severity. Although the causal nature of these effects cannot be ascertained due to the design of this study, the results nonetheless add to the existing literature by extending the apparent negative effects of pain catastrophizing into the post-amputation residual limb pain population.
Finally, the current findings, to our knowledge, are among the first to systematically examine possible links among catastrophizing, chronic pain severity, and a comprehensive array of inflammatory mediators. While a limited number of studies have examined associations between catastrophizing and selected inflammatory mediators in the context of laboratory evoked pain stimuli, this issue has received little study in the chronic pain setting[8,9]. The present results revealed that in the context of post-amputation residual limb pain, elevated catastrophizing levels were associated with higher levels of IL-8, IL-12, TNF-β, PIGF, and ICAM-1, and lower levels of IL-13. These findings are consistent with generally pro-inflammatory influences of catastrophizing. Interestingly, the positive association between catastrophizing and residual limb pain severity was statistically-mediated by TNF-α, TNF-β, SAA, and ICAM-1 levels. While causation cannot be inferred from this study, results are consistent with the possibility that catastrophizing might be linked with an elevated pro-inflammatory profile, which in turn produces elevated chronic pain severity. Definitive conclusions regarding this causal model must await replication using a design with evaluation of pain catastrophizing, inflammatory mediator levels, and chronic pain severity over time. Extending these results to other chronic pain conditions would also be worthwhile.
Exaggerated pro-inflammatory response in amputees with chronic pain
Our results demonstrate that there is an exaggerated and enduring pro-inflammatory response in amputees with chronic residual limb pain compared to amputees without significant pain. This finding is consistent with a substantial body of preclinical evidence suggesting that several of the mediators that were associated with pain in this study may be implicated in the pathogenesis of neuropathic pain. Of these, TNF and IL-6 are the most studied, with these pro-algesic cytokines having pleiotropic effects on neurons and immune cells throughout the neuraxis following nerve injury[14,15,32,33]. IL-8, while not examined directly in nerve injury, has been shown to cause hyperalgesia when administered exogenously to rodents, and has been associated with widespread tenderness to palpation in a large clinical study of TMD sufferers and in patients with interstitial cystitis[16,24]. IL-2 and IL-12, have both been shown to be pro-algesic in animal models, and in clinical studies of CRPS and painful small fiber neuropathy, IL-2 was shown to be elevated at the protein and mRNA level from blood samples[28,29].
One finding of particular interest relates to CRP, an acute phase reactant that is widely used in clinical practice as a general marker of inflammation. The elevation of CRP in amputees with chronic residual limb pain is consistent with a systemic pro-inflammatory state in this group. There is also evidence that patients with other pain states, such as lumbar radiculopathy and stenosis, have high normal CRP levels [30]. Recent work in a large community-based sample of women has reported small but significant positive associations between CRP levels and persistently elevated bodily pain, further supporting the pain relevance of CRP elevations [5]. Of note, CRP is an important risk factor for cardiovascular morbidity, with a value greater than 3 mg/L considered high risk [20]. The median CRP value of ~ 4 mg/L in the case group, though still within the range of normal CRP values, is high enough to suggest these individuals could be at increased risk for future cardiovascular disease.
Limitations
As a cross-sectional, observational study, this trial was not designed to determine causation with regards to any of the inflammatory mediators that were measured. Furthermore, since we only gathered information on this patient cohort at a single time point, several months after they had suffered injury and experienced chronic pain, we could not examine the temporal dynamics of the inflammatory mediators under study. Time from injury and pain intensity did not interact to show any consistent influence on inflammatory markers. Despite this, the possibility that the differential mediator profiles in these patients may have been present before injury cannot be excluded. Another possible limitation is that the degree of wound healing at the time of enrollment could have affected inflammatory mediator levels. However, active infection, heterotopic ossification, or signs of poor wound healing on physical examination were all exclusion criteria in this study, which should have minimized any effects of these potential confounds. Not surprisingly, opioid use in patients with significant residual limb pain was higher than controls. Opioids have been shown to favor an anti-inflammatory environment and could have affected inflammatory mediator concentrations in this patient cohort. We would expect, however, the anti-inflammatory effects of opioids to favor the null hypothesis, potentially strengthening the results presented here. The conclusions from this study arise from a modest sized cohort of primarily young and male military veterans (n=76), potentially limiting the generalizability of our findings. Also, because a large proportion of the plasma concentration values for IL-13, IL-2, IL-4 and IL-1β were below the lower level of detection of the inflammatory mediator assay, it is possible that the significant correlations for IL-13 and IL-2 may be spurious; however, this limitation is not present for the seven other mediators found to correlate with pain score. Finally, given the modest sample size and the relatively large number of associations examined, concerns might be raised as to whether the effects reported might simply be due to inflated Type I error. Results of permutation testing indicating highly significant set-wise correlations between levels of all inflammatory mediators and both residual limb pain severity and catastrophizing levels argues against our reported findings being spurious.
Conclusion
Amputees suffering from residual limb pain exhibit an overall pro-inflammatory signature when compared with amputees without significant pain. A pro-inflammatory profile is associated with both greater pain severity and higher pain catastrophizing levels. These results generate intriguing hypotheses regarding the links between causation and resolution of the inflammatory state and chronic pain following nerve trauma. The mediators measured here may have utility as potential biomarkers of nerve injury-induced pain.
Acknowledgments
Grant support from Congressionally Directed Medical Research Programs and the Department of Defense awards MR130082, W81XWH-12-2-0129 and W81XWH-15-2-0046. Partial support was also provided by NIH grant 2T32GM008600 and a grant from the Reflex Sympathetic Dystrophy Syndrome Association.
Footnotes
All the authors have no financial interests in this study.
Copyright Protection: Our team is comprised partly of military service members and employees of the US Government. This work was prepared as part of our official duties. Title 17 United States Code (USC) 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Disclaimer: The views expressed in this publication are those of the authors, and do not necessarily reflect the official policy of the Department of the Army, the Department of Defense, or the United States Government.
Authors have no financial conflicts of interest to report.
References
- 1.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. The Journal of Pain. 2005;6:149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 4.Buchheit T, Van de Ven T, John Hsia HL, McDuffie M, MacLeod DB, White W, Chamessian A, Keefe FJ, Buckenmaier CT, Shaw AD. Pain Phenotypes and Associated Clinical Risk Factors Following Traumatic Amputation: Results from Veterans Integrated Pain Evaluation Research (VIPER) Pain Medicine. 2015 doi: 10.1111/pme.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns JW, Quartana PJ, Bruehl S, Janssen I, Dugan SA, Appelhans B, Matthews KA, Kravitz HM. Chronic pain, body mass index and cardiovascular disease risk factors: tests of moderation, unique and shared relationships in the Study of Women's Health Across the Nation (SWAN) J Behav Med. 2015;38:372–383. doi: 10.1007/s10865-014-9608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo M, Dawes JM, Bennett DLH. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–642. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656–f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Almeida Y, King CD, Wallet SM, Riley JL. Immune biomarker response depends on choice of experimental pain stimulus in healthy adults: a preliminary study. Pain Res Treat. 2012;2012:538739–7. doi: 10.1155/2012/538739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. PAIN. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hehn von CA, Baron R, Woolf CJ. Deconstructing the Neuropathic Pain Phenotype to Reveal Neural Mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji R-R, Xu Z-Z, Gao Y-J. Emerging targets in neuroinflammation- driven chronic pain. Nat Rev Drug Discov. 2014:1–16. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. The Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 13.Kooijman CM, Dijkstra PU, Geertzen JHB, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. PAIN. 2000;87:33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 14.Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Research. 2000;866:15–22. doi: 10.1016/s0006-8993(00)02190-9. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzetti BB, Poole S, Veiga FH, Cunha FQ, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-13. European Cytokine Network. 2001;12:260–267. [PubMed] [Google Scholar]
- 17.Marchand F, Perretti M, McMahon SB. Role of the Immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 18.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 19.Petkovic V, Moghini C, Paoletti S, Uguccioni M. Eotaxin-3/CCL26 is a natural antagonist for CC chemokine receptors 1 and 5 A human chemokine with a regulatory role. J Biol Chem. 2004;279(22):23357–23363. doi: 10.1074/jbc.M309283200. [DOI] [PubMed] [Google Scholar]
- 20.Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus - PubMed - NCBI. Diabetes Technology & Therapeutics. 2006;8:28–36. doi: 10.1089/dia.2006.8.28. [DOI] [PubMed] [Google Scholar]
- 21.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 22.Richardson C, Glenn S, Horgan M, Nurmikko T. A prospective study of factors associated with the presence of phantom limb pain six months after major lower limb amputation in patients with peripheral vascular disease. The Journal of Pain. 2007;8:793–801. doi: 10.1016/j.jpain.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 24.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine biomarkers and chronic pain: Association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. PAIN. 2011;152:2802–2812. doi: 10.1016/j.pain.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturgeon JA. Psychological therapies for the management of chronic pain. Psychol Res Behav Manag. 2014;7:115–124. doi: 10.2147/PRBM.S44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 27.Theunissen M, Peters ML, Bruce J, Gramke H-F, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. The Clinical Journal of Pain. 2012;28:819–841. doi: 10.1097/AJP.0b013e31824549d6. [DOI] [PubMed] [Google Scholar]
- 28.Uçeyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. PAIN. 2007;132:195–205. doi: 10.1016/j.pain.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Uçeyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- 30.Uher T, Bob P. Neuropathic Pain, Depressive Symptoms, and C-Reactive Protein in Sciatica Patients. Int J Neurosci. 2012;123:204–208. doi: 10.3109/00207454.2012.746335. [DOI] [PubMed] [Google Scholar]
- 31.Vase L, Nikolajsen L, Christensen B, Egsgaard LL, Arendt-Nielsen L, Svensson P, Staehelin Jensen T. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. PAIN. 2011;152:157–162. doi: 10.1016/j.pain.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Wei X-H, Na X-D, Liao G-J, Chen Q-Y, Cui Y, Chen F-Y, Li Y-Y, Zang Y, Liu X-G. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Experimental Neurology. 2013;241:159–168. doi: 10.1016/j.expneurol.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Berta T, Xu Z-Z, Liu T, Park JY, Ji R-R. TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. PAIN. 2011;152:419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Archives of Physical Medicine and Rehabilitation. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]