Abstract
Transglutaminase 2 (TG2) expression is required for epidermal squamous cell carcinoma cancer stem cell survival. However, the molecular signaling mechanisms triggered by TG2 that mediate this survival action are not well understood. Here we show that TG2 is constitutively expressed in ECS cells where it interacts with α6/β4 integrin to stimulate FAK and Src signaling, leading to phosphoinositide 3 kinase (PI3K) activation of phosphoinositide dependent kinase 1 (PDK1). PDK1 inhibits Hippo signaling, leading to enhanced nuclear accumulation of YAP1, which interacted with and stabilized ΔNp63α to enhance ECS cell spheroid formation, invasion, and migration. Overall, these findings suggest that constitutive TG2 expression results in stabilization of ΔNp63α, leading to maintenance of cancer stem cell properties and enhanced tumor formation.
Keywords: squamous cell carcinoma, type II transglutaminase, stem cell, cancer, TG2, Transglutaminase 2, integrin signaling, Hippo signaling, YAP1, cancer stem cells, ΔNp63α
Introduction
Epidermal squamous cell carcinoma develops in response to exposure to mutagens such as UV irradiation and is a one of the most frequent human cancers (1). Treatment is by surgical removal of the primary tumor, but the recurrence rates range to thirty percent and the recurring tumors are aggressive and resistant to therapy (1). Increasing evidence suggests the existence of cancer stem cells that have a central role in tumor formation, and facilitate cancer recurrence and metastasis (2). We have characterized epidermal squamous cell carcinoma cancer stem cells (ECS cells) and showed that they express stem cell markers, form aggressive and highly vascularized tumors, and possess enhanced migratory and invasive potential as compared to non-stem cancer cells (3). Thus, these cells are important key targets for skin cancer prevention and therapy.
As part of a search for candidate intracellular targets, we have identified several proteins that are essential for ECS cell survival, migration and invasion (3). In the present, we focus on transglutaminase 2 (TG2), which we have identified as highly elevated in ECS cells, as compared to non-stem cancer cells, and required for ECS cell survival (4, 5). TG2 is a multifunctional protein that is involved in inflammation, tissue repair and cancer (6, 7). It possesses a number of activities including calcium-dependent transamidase activity, GTP binding activity, protein disulfide isomerase activity and serine/threonine kinase activity (8). TG2 also serves as a scaffold protein (9). The most important functions appear to be the TG2 GTP binding/GTPase activity, which is important in G-protein signaling, and the transamidase activity which is important in protein-protein crosslinking (9). We previously reported that TG2 knockdown, or pharmacologic inhibition of TG2 activity, results in reduced ECS cell survival, spheroid formation, migration and invasion (4, 5, 10). However, the mechanism of TG2 action is not well understood in epidermal squamous cell carcinoma.
ΔNp63α is an important member of the p63 family of proteins that regulate epithelial cell differentiation, stem cell status and fate (11, 12). Studies in murine models identify ΔNp63α as a key controller of differentiation in epidermis (11, 12). The function of p63 in epithelial development was first observed in p63 knockout mice which die shortly after birth due to defects in the epidermal barrier (13). Another study shows that TAp63 expression is required for initiation of epithelial stratification and the inhibition of terminal differentiation in mouse embryogenesis (14), while ΔNp63α counteracts TAp63 action to promote epidermal differentiation (14). Thus, ΔNp63α, which is the primary p63 form expressed in squamous epithelial tissues (15), is a key controller of normal epidermal development. ΔNp63α overexpression is a frequent event in some human cancers, including squamous cell carcinoma (16). Although its function has been studied, the role of ΔNp63α in tumors is not yet well understood. Moreover, knowledge of the mechanisms that control ΔNp63α function and expression is limited.
In the present study we show that TG2 and ΔNp63α are constitutively enriched in ECS cells as compared to non-stem epidermal cancer cells, that both proteins are required for ECS cell survival, migration and invasion, and the TG2 controls ΔNp63α level. We propose that TG2 associates with the α6/β4-integrins to increase FAK/Src signaling and PI3K/PDK1 activity, and that PDK1, in turn, suppresses Hippo signaling leading to enhanced YAP1 and ΔNp63α accumulation and enhanced ECS cell survival.
Materials and Methods
Antibodies and reagents
Sodium pyruvate (11360-070), DMEM (11960-077), 0.25% trypsin-EDTA (25200-056) and L-Glutamine (25030-164) were purchased from Gibco (Grand Island, NY). Heat inactivated fetal calf serum (FCS, F4135) and anti-β-actin (A5441) antibody was purchased from Sigma (St. Louis, Mo). Cell lysis Buffer (9803) was purchased from Cell Signaling Technology (Danvers, MA). Anti-TG2 (MAB3839) was purchased from EMD Milipore (Bedford, MA). Antibodies for Src (ab47411) and Src-P (ab321012) were purchased from Abcam. Antibodies for YAP1 (4912), YAP1-P (13008) and FAK-P (3283) were purchased from Cell Signaling Technologies. p63 (sc-8431), integrin-β4 (sc-9090) and integrin-α6 (sc-374057) were purchased from Santa Cruz. FAK (610087) antibody was purchased from BD Transduction Laboratories. Peroxidase-conjugated anti-mouse IgG (NXA931) and anti-rabbit IgG (NA934V) were obtained from GE healthcare (Buckinghamshire, UK). Alexaflour 555 (A21424) and Alexaflour 488 (A11034) were from Invitrogen. TG2- (sc-37514), p63- (sc-36161), FAK- (sc-29310), integrin α6- (sc-43129), integrin β4- (sc-35678) and control-siRNA (sc-37007) were from Santa Cruz (Dallas, TX). YAP1-siRNA (S102662954) was from Qiagen (Valencia, CA). Matrigel (354234) and BD Biocoat cell inserts (353097) were from BD Biosciences. SCC-13 and HaCaT cells were originally obtained from ATCC (17, 18). Cell line identity is routinely confirmed by short tandem repeat profiling and cells are assayed to assure absence of mycoplasma at six months intervals.
Lentivirus production
Lentivirus was produced using 293T cells maintained in DMEM with 1 mM L-glutamine, 1 mM sodium pyruvate and 10% fetal calf serum. 293T cells were harvested and plated in 100 mm dishes at 50% confluence 24 h prior to transfection. Media was removed and plates were washed with Hank’s Balanced Salt Solution before serum free media was added containing 1 μg pCMV-VSVG, 5 μg pCMV-dr8.91 and 5 μg shRNA encoding plasmid for co-transfection. After 3 h 10% FCS was added, and at 72 h after transfection the medium was collected, centrifuged for 15 min at 1500 rpm, sterile filtered (22 micron), and stored at −80 C in aliquots.
Stable TG2 knockdown lines
SCC-13 cells (1 × 105) were plated in 24 well cluster plates and allowed to attach overnight. The cells were then infected with 1 ml of medium containing lentivirus encoding TG2-specific shRNA. The infection was performed in serum-free growth media containing 8 μg/ml polybrene at 37 C for 5 h. The media was then changed to growth media supplemented with 5% fetal calf serum. Cells were then plated in 100 μM dishes and grown in the presence of 0.25 μg puromycin per ml for two weeks. The TG2 knockdown cells were then infected a second time with the same virus at a 1:1 dilution in serum free media with 8 μg/ml polybrene. The virus was left on for 72 h and cells were subsequently selected for two weeks with puromycin at 0.25 μg/ml. TG2 knockdown was confirmed by anti-TG2 immunoblot. These cells are referred to as SCC13-TG2-shRNA2. A control cell line was produced by double infection with control-shRNA encoding lentivirus using an identical protocol as above. These cells are referred to as SCC13-Control-shRNA.
Spheroid formation
Cancer cells were grown as spheroids as previously described (3). Only 0.15% of the cells grow as spheroids, and these cells are highly enriched in embryonic (Oct4) and epidermal keratinocyte stem cell (K19, CD200, ALDH1, K15) markers (3). We refer to these as cultures as ECS cells, but note that the cultures are highly enriched but not pure populations of ECS cells. Parallel cultures were plated in spheroid media on conventional plastic dishes for growth as monolayer cultures which contain a limited number (0.15%) of ECS cells. We refer to these as non-stem cancer cells. A spheroid is defined as a mass of cells, derived from a single cell, which grows as a cohesive cell assembly that increases in size with time in culture. Mature spheroids, grown for 8 d, contain 982 ± 136 cells (mean ± SEM, n = 73).
Electroporation of nucleic acids
Cancer cells (150,000) were plated on 60 mm plates in growth medium. After 24 h, when approximately 50% confluent, the cells were collected using 0.25% trypsin, centrifuged at 200 × g, washed with sterile phosphate-buffered saline (PBS, pH 7.5), suspended in 100 μl of keratinocyte nucleofection reagent VPD-1002 (Walkersville, MD), and electroporated. The cell suspension, containing either 3 μg of siRNA or 2 μg of plasmid DNA, was gently mixed and electroporated using the T-018 setting on the AMAXA Electroporator. Immediately after electroporation, pre-warmed spheroid media was added and the suspension was transferred to a 60 mm cell culture plate and media adjusted to a final volume of 4 ml with spheroid media. When siRNA was used, but no plasmid DNA, the cells were electroporated a second time, following the same protocol, 72 h after the initial electroporation.
Wound closure and matrigel invasion assays
The SCC13-Control-shRNA or SCC-13-TG2-shRNA2 wound closure and matrigel invasion assays were performed exactly as outlined previously (5).
Tumor xenograft growth assay
Spheroid-selected (ECS) cells were trypsinized to prepare single cell suspensions, resuspended in phosphate buffered saline containing 30% Matrigel and 100,000 cells, in 100 μl, was injected subcutaneously into the two front flanks of NOD/scid/IL2 receptor gamma chain knockout mice (NSG mice) using a 26.5 gauge needle. Five mice were used per data point (3). Tumor growth was monitored by measuring tumor diameter and calculating tumor volume = 4/3π × (diameter/2)3. Tumor experiments were repeated five separate times, each time using five mice per treatment group, and identical results were obtained in each experiment. Tumor samples were harvested to prepare extracts for immunoblot and sections for immunostaining. These studies were approved by the institutional boards and followed accepted national and international practices for the treatment and welfare of animals. NC9 (19) was dissolved at 300 mM in dimethyl sulfoxide and stored in small aliquots at −20 C. For animal experiments, NC9 was delivered in Captisol, a non-toxic delivery vehicle (20). Briefly, 40% Captisol (RC-0C7-020, CyDex Pharmaceuticals, Lawrence, KS) was prepared in sterile water by stirring overnight at 25 C followed by filter sterilization. The 40% captisol solution was diluted 1:1 in sterile water and supplemented with 0.5 ml of 1 N acetic acid to make 20% captisol. NC9 was prepared as a 2 mg/ml stock by mixing 7.17 μl of 300 mM NC9 with 742.83 μl of 20% captisol. NC9 was delivered by intraperitoneal injection, three times per week on alternate days, of 200 μl of the 2 mg/ml stock (20 mg/kg body weight). NC9 treatment was initiated two days after tumor cell injection and that last NC9 treatment was two days before tumor harvest. The animal studies were approved by the University of Maryland-Baltimore Animal Care and Use Committee and meet all national and international standards.
Results
TG2 stimulates integrin signaling to increase ΔNp63α level
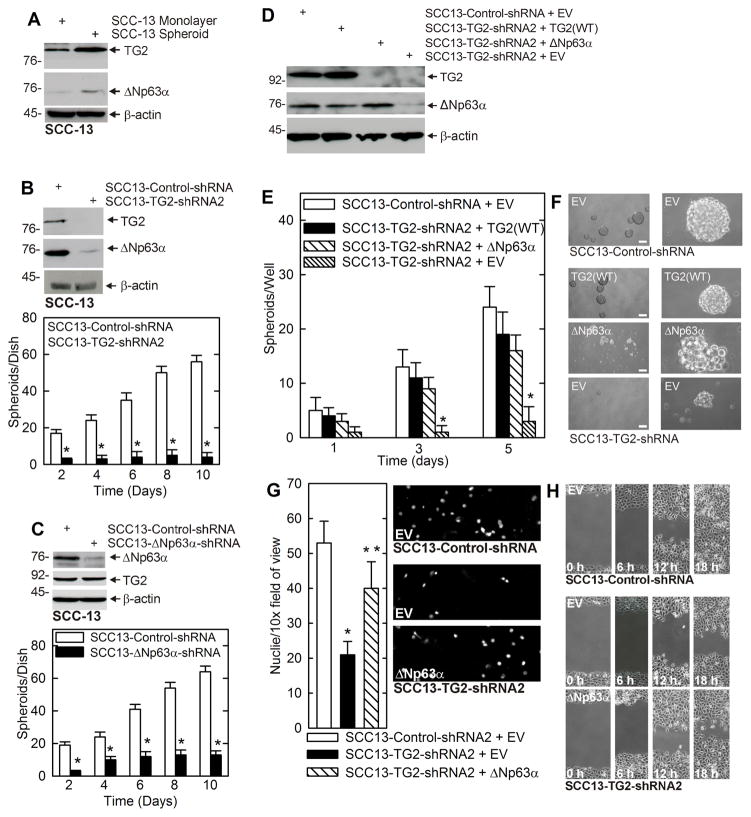
We recently showed that transglutaminase 2 (TG2) is required for ECS cell survival (3–5, 10). However, how TG2 contributes to survival is not well understood. In the present study we examine the role of ΔNp63α as a downstream mediator of TG2 action. We note that ECS cells, enriched by growth as spheroids, are enriched in both TG2 and ΔNp63α as compared to non-stem cancer (monolayer) cells (Fig. 1A), suggesting a role for each protein in mediating ECS cell survival. To understand the function of these proteins, we studied their role in ECS cell spheroid formation. Fig. 1B shows that TG2 knockdown is associated with reduced ΔNp63α expression and reduced ECS cell spheroid formation. ΔNp63α knockdown also reduces spheroid formation, but this is not associated with loss of TG2 (Fig. 1C). These findings suggest that TG2 maintains ΔNp63α level to drive ECS cell spheroid formation. To elucidate the relationship between TG2 and ΔNp63α, TG2 knockdown cells were assayed for ability to form spheroids following restoration of TG2 or ΔNp63α. Fig. 1D confirms that TG2 level is reduced in SCC13-TG2-shRNA2 cells, demonstrates that this is associated with reduced ΔNp63α levels, and shows that vector-mediated expression of TG2 restores ΔNp63α level. Consistent with the idea that ΔNp63α is downstream of TG2, vector-mediated expression of ΔNp63α does not restore TG2 expression (Fig. 1D). Fig. 1E shows the biological effect of manipulating TG2 and ΔNp63α, and demonstrates that restoring expression of TG2 or ΔNp63α enhances spheroid formation to a level approaching that observed in control (wild-type TG2 level) cells. Fig. 1F shows that there are subtle differences in the properties of the spheroids, with TG2-restored cells forming compact spheroids versus the less compact spheroids formed following restoration of ΔNp63α. These findings suggest that both proteins can drive spheroid formation, but that they have subtly different roles. Figs. 1G and H show that TG2 knockdown cells also display reduced matrigel invasion and migration, and that restoration of ΔNp63α partially restores these properties.
Fig. 1. TG2 and ΔNp63α are enriched in ECS cells, and required for ECS cell survival.
A ECS cells have elevated TG2 and ΔNp63α. SCC-13 cells (40,000 per well) were grown in spheroid medium in attached (monolayer) or non-attached (spheroid, ECS cell) conditions. At 10 d lysates were electrophoresed for detection of the indicated epitopes. B SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells were grown in spheroid culture for 10 d prior to counting of spheroid number and extract preparation for detection of TG2 and ΔNp63α. C SCC13-Control-shRNA and SCC13-ΔNp63α-shRNA cells were grown in spheroid culture for 10 d prior to counting of spheroid number and extract preparation for detection of TG2 and ΔNp63α. D SCC13-Control-shRNA or SCC13-TG2-shRNA2 cells were double-electroporated with empty vector (EV) or expression vectors encoding TG2 or ΔNp63α and after 5 d the indicated proteins were detected. E/F The indicated cell lines were electroporated with EV, TG2(WT) or ΔNp63α and grown as spheroids for 5 d prior to counting of spheroids and recording of images. G The indicated cell lines was electroporated with the indicated plasmid and after 48 h seeded onto a matrigel-coated membrane in a Millicell chamber for invasion assay. After 20 h the membrane was removed, fixed, and DAPI-stained for nuclei counting. H Cells were electroporated with the indicated plasmid, plated at high density to form confluent monolayers, the monolayers were scratched with a 10 μl pipette tip to create a wound and wound closure was monitored with time. The plotted values are mean ± SEM and asterisks indicate significant change compared to control, n = 3, p < 0.005. The double asterisk in panel G indicates a significant change compared to the single asterisk group, n = 3, p < 0.005.
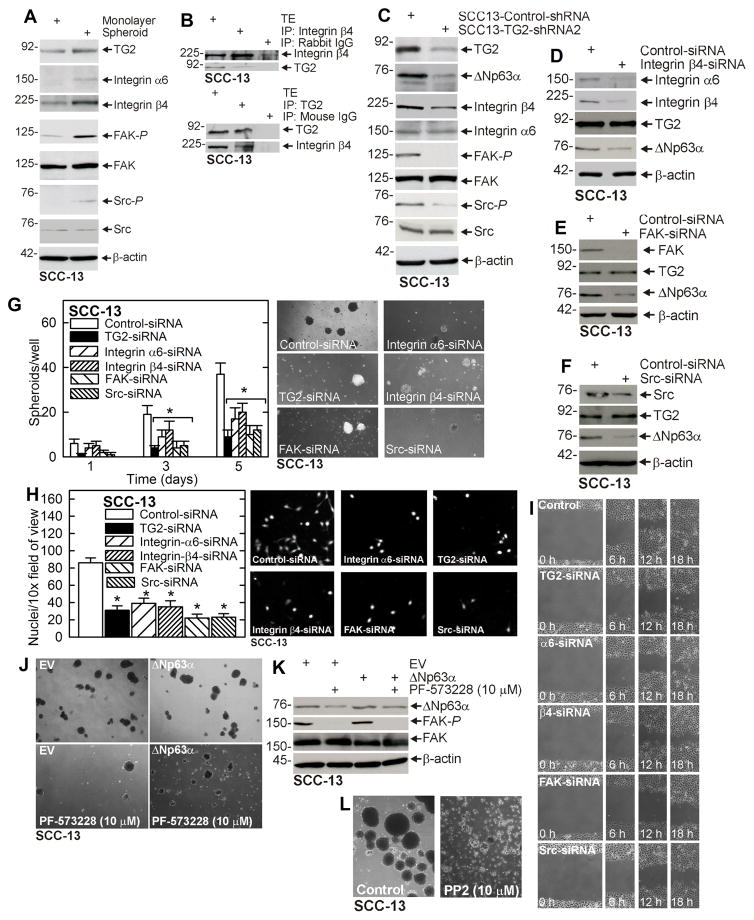
An important goal is identifying the TG2 activated signaling events that maintain ΔNp63α level. Recent reports suggest that TG2 interacts with integrins and stimulates integrin-related signaling (21–24). We therefore compared non-stem cancer cell and ECS cell integrin signaling. Fig. 2A shows TG2 level is elevated in ECS cells and that this is associated with increased α6/β4-integrin level and increased focal adhesion kinase (FAK) and src kinase activities, suggesting that the integrin/FAK/src cascade is selectively activated in ECS cells. This is consistent with immunoprecipitation experiment showing that TG2 associates with β4-integrin (Fig. 2B). We next examined the impact of TG2 knockdown in integrin-related signaling. Fig. 2C shows that TG2 knockdown reduces β4-integrin level, and FAK and src kinase activity, and that this is associated with reduced ΔNp63α level. Figs. 2D/E/F show that knockdown of β4-integrin, FAK or src reduces ΔNp63α level. Together, these findings strongly suggest that TG2 maintains ΔNp63α level via a mechanism that requires activity in this cascade. We next assessed the impact of TG2, α6-integrin, β4-integrin, FAK and src knockdown on ECS cell biological responses. Figs. 2G/H/I show that loss of these regulators reduces spheroid formation, matrigel invasion and migration. To further confirm that FAK kinase is involved in TG2 regulation of ΔNp63α, we electroporated SCC-13 cells with empty vector (EV) or ΔNp63α expression vector, plated the cells for growth as spheroids and then and added 10 μM PF-573288, a FAK kinase inhibitor, and monitored spheroid number at 6 d. Fig. 2J shows that PF-573228 treatment markedly reduces spheroid formation which is partially reversed by ΔNp63α expression. Fig. 2K confirms that PF-573228 reduces FAK kinase activity and that this is associated with reduced ΔNp63α level. Fig. 2L shows that treatment with src kinase inhibitor (PP2) also suppresses spheroid formation and causes spheroid disintegration.
Fig. 2. α6/β4-Integrin signaling is essential for ΔNp63α expression.
A ECS cells display elevated α6/β4-integrin levels and elevated FAK/Src signaling. SCC-13 cells (40,000 per well) were grown in spheroid medium in attached (monolayer) or non-attached (spheroid, ECS cell). Cells were collected after 10 d and lysates were prepared for immunoblot. B SCC-13 cells spheroid were grown for 8 d and extracts were immunoprecipitated as indicated prior to immunoblot. C Integrin signaling is reduced in SCC13-TG2-shRNA2 cells. Cells were grown in spheroid medium in monolayer culture for 10 d prior to collection of lysates for immunoblot. D/E/F SCC-13 cells were double-electroporated with Control- or Integrin β4-, FAK-, or Src-siRNA and 48 h later extracts were prepared for immunoblot. G/H/I SCC-13 cells were double-electroporated with the indicated siRNA and 24 h later plated for growth as spheroids and for invasion and migration assays. J/K/L Cells were double-electroporated with indicated plasmid (EV, empty vector or ΔNp63α plasmid) and 24 h later plated for spheroid formation in the presence of the indicated inhibitor. Spheroids were grown for 5 d and then photographed and extracts prepared for immunoblot. The plotted values are mean ± SEM and asterisks indicate significant change compared to control, n = 3, p < 0.005.
A role for Hippo signaling
Hippo is a cell survival cascade that controls organ size during development (25). When an organ reaches a proper size, cell-cell contact activates hippo signaling to halt cell growth (25). The signal to halt proliferation involves activation of LATS1 kinase which phosphorylates YAP1 causing it to relocate to the cytoplasm where it undergoes proteasome-associated degradation. YAP1 is a transcriptional adaptor protein that interacts with nuclear factors to drive cell proliferation and survival (25). LATS1 activity is reduced in many tumor types leading to nuclear YAP1 accumulation to drive tumor formation (25). Integrin/FAK/src signaling has been reported to enhance PI3K/PDK1 signaling to inhibit LATS1 activity and enhance cell proliferation (26, 27). In addition, YAP1 has been reported to interact with ΔNp63α in airway epithelial cells and HaCaT cells (28, 29).
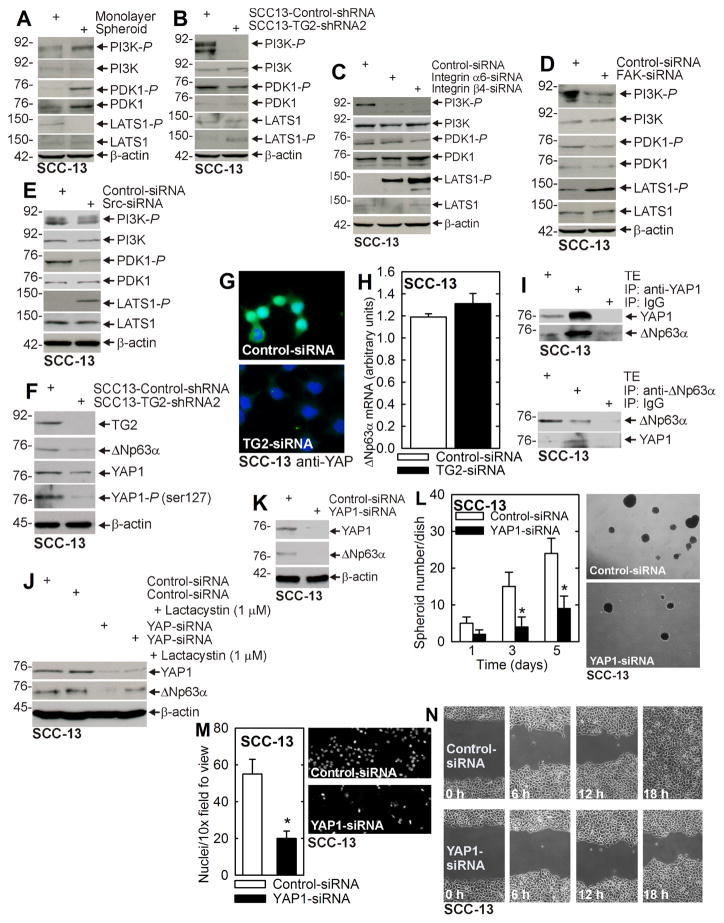
We therefore tested whether TG2, α6/β4-integrins, FAK and src regulate PI3K/PDK1 and LATS1 activity. Consistent with a potential role for this cascade in ECS cells, PI3K and PDK1 activities are increased, while LATS1 activity is reduced in ECS cells (Fig. 3A) as compared to non-stem cancer cells. Figs. 3B/C/D/E show that knockdown of TG2, α6/β4-integrin, FAK or src reduces PI3K/PDK1 activity and increases LATS1 activity, suggesting a role for TG2/integrin/FAK/src signaling in control of PI3K/PDK1 and LATS1. As noted above, LATS1 phosphorylates YAP1 causing it to localize in the cytoplasm as an inactive form that is degraded (25). We anticipated that TG2 knockdown would increase LATS1 activity to enhance YAP1 phosphorylation and mobilization to the cytoplasm. Fig. 3F shows that TG2 loss results in a marked reduction in YAP1 level which is associated with an apparent reduction in YAP1 phosphorylation. We propose that cytoplasmic YAP1-P is rapidly degraded in these cells as a mechanism of YAP1 inactivation. This reduction in YAP1 level in TG2-knockdown cells was confirmed by immunostaining. SCC-13 cells were electroporated with control- or TG2-siRNA, plated at 40% confluence and after 24 h stained to detect YAP1. Fig. 3G shows that YAP1 is present in both the nucleus and cytoplasm on control cells and that the YAP1 levels are substantially (but not completely) reduced in TG2-siRNA treated cells.
Fig. 3. TG2 Suppresses Hippo signaling to increase ΔNp63α level.
A Cells were grown in spheroid medium in monolayer or spheroid culture and after 10 d harvested for immunoblot. B SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells were grown in monolayer culture in spheroid medium and lysates were collected for immunoblot detection of the indicated epitopes. C/D/E SCC-13 cells were double electroporated with the indicated siRNA, grown as monolayer cultures for 2 d and lysates were collected for immunoblot. F SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells were grown as monolayers in spheroid medium and lysates collected at 6 d for marker detection. G SCC-13 cells were double electroporated with Control- or TG2-siRNA and given 24 h to recover before seeding on chamber slides. The following day, cells were fixed in 4% paraformaldehyde, stained with anti-YAP1 and appropriate secondary antibody, and co-stained with DAPI. H SCC-13 cells, double-electroporated with Control- or TG2-siRNA, were grown in spheroid medium for 48 h prior to extracts being prepared for assay of ΔNp63α mRNA by qRT-PCR. I SCC-13 cells were grown as spheroids and at day 8 lysates were collected for immunoprecipitation/immunoblot. J SCC-13 cells were double-electroporated with Control- or YAP1-siRNA followed by treatment with 1 μM lactacystin in monolayer culture. Extracts were prepared after an additional 24 h. K SCC-13 cells were double-electroporated with Control- or YAP-siRNA and after 2 d in monolayer culture extracts were prepared for immunoblot. L/M/N SCC-13 cells were electroporated with Control- or YAP1-siRNA and then seeded for spheroid formation, invasion and migration assay. The plotted values are mean ± SEM and asterisks indicate significant change compared to control, n = 3, p < 0.005.
We next assessed the mechanism whereby TG2 and YAP1 increase ΔNp63α level. As shown in Fig. 3H, TG2 knockdown does not reduce ΔNp63α mRNA, suggesting that TG2/YAP1 do not regulate ΔNp63α via transcriptional or RNA stability mechanisms. We next determined whether YAP1 may stabilize ΔNp63α against proteasomal degradation. Such a mechanism implies formation of a YAP1/ΔNp63α-containing complex. To assess this, we monitored YAP1/ΔNp63α interaction. Fig. 3I shows that immunoprecipitation of YAP1 or ΔNp63α results in co-precipitation of the other protein. We next determined whether YAP1 loss leads to proteasome degradation of ΔNp63α. To test this we treated cells with control- or YAP1-siRNA in the presence or absence of the proteasome inhibitor, lactacystin. Fig. 3J shows that YAP1-siRNA reduces YAP1 and ΔNp63α level and that the ΔNp63α reduction is partially reversed by lactacystin treatment.
YAP1 enhances ECS cell spheroid formation, invasion and migration
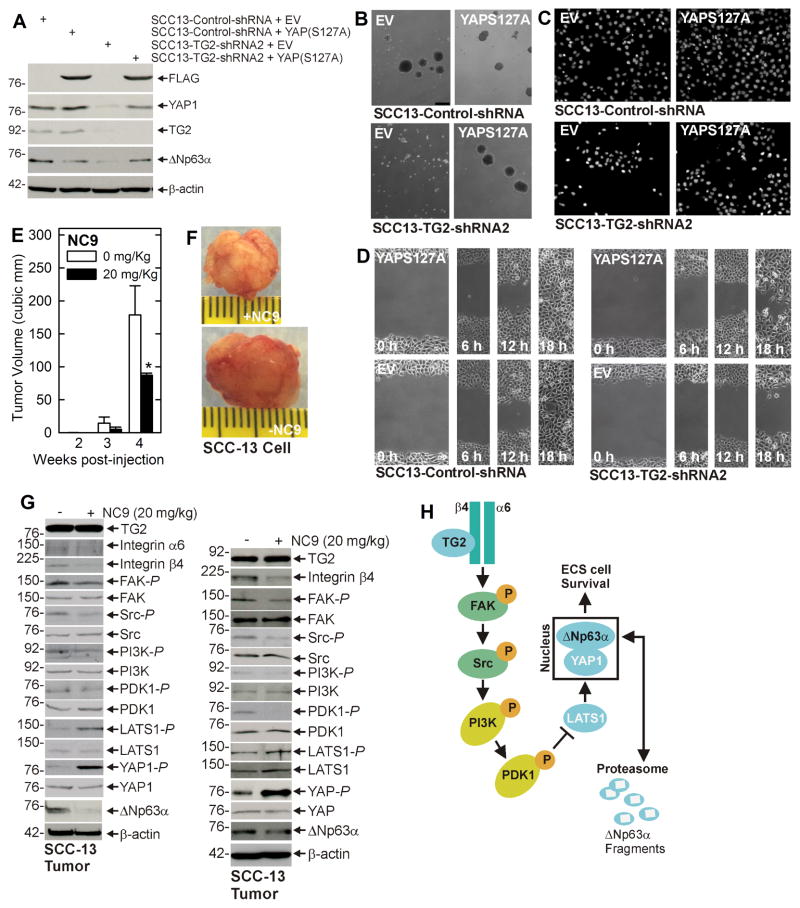
The above studies suggest that TG2 regulates YAP1 level which regulates ΔNp63α, and predict that YAP1 knockdown should reduce ΔNp63α level and ECS cell biological response. Fig. 3K shows that YAP1 knockdown is associated with reduced ΔNp63α expression and Figs. 3L/M/N show that this is associated with reduced spheroid formation, matrigel invasion and migration. We next examined whether constitutively active YAP1, YAP(S127A), expression in TG2 knockdown cells can restore ΔNp63α expression and the biological responses. Fig. 4A shows that YAP1 and ΔNp63α levels are reduced in SCC13-TG2-shRNA2 cells, and that vector-mediated expression of YAP(S127A), a constitutively active form of YAP1, restores ΔNp63α level and spheroid formation, matrigel invasion and migration (Figs. 4B/C/D). These findings indicate a role for YAP1 in maintaining ΔNp63α level and thereby driving ECS cell survival.
Fig. 4. YAP1 enhances ECS cell survival and TG2 inhibitor suppresses tumor formation.
A SCC13-Control-shRNA and SCC13-TG2-shRNA2 cells were double electroporated with empty vector (EV) or YAP(S127A)-encoding vector and at 48 h post-electroporation lysates were collected for immunoblot. B/C/D The indicated cell lines were electroporated with EV or YAP(S127A)-encoding vector and then plated for spheroid formation, invasion and migration assay. E/F ECS cells (100,000 cells derived from SCC-13) were injected into each front flank in NSG mice. At 1 d post injection, NC9 was delivered by intraperitoneal injection, three times per week on alternate days, of 200 μl of a 2 mg/ml stock (20 mg/kg body weight). Images represent appearance and size of typical control and NC9 treated tumors harvested on week four. The plotted values are mean ± SEM and asterisks indicate significant change compared to control, n = 5 mice (10 tumors), p < 0.001. G Tumors were harvested at 4 wk and extracts were prepared for assay of indicated proteins. Blots from two representative tumors are shown. H Proposed TG2 signaling scheme. TG2 interacts with α6/β4 integrin to enhance integrin (FAK/Src) signaling which increases PI3K/PDK1 activity and PDK1 suppresses activity in the Hippo signaling cascade (LATS1). This leads to reduced LATS1 phosphorylation of YAP1 which then interacts with and stabilizes ΔNp63α. This signaling pathway enhances ECS cell survival, invasion and migration. Loss of TG2 function reduces YAP1 which leads to degradation of ΔNp63α and reduced ECS cell survival, invasion and migration. Similar results were observed in extract prepared from additional tumors.
TG2 regulation of tumor signaling
An important goal is to assess whether this cascade functions in vivo in tumors. To assess this, we injected 100,000 ECS cells per each front flank in NSG mice and the initiated treatment with 20 mg/kg body weight NC9, a TG2 inhibitor (19, 30). Figs. 4E/F show that NC9 treatment markedly suppresses tumor formation. Extracts were prepared from representative tumors to assess the impact of NC9 on integrin and Hippo signaling. Fig. 4G shows that NC9 treatment reduces integrin-β4 level, and FAK and Src activity, and increases LATS1-P and YAP1-P, indicating that the HIPPO signaling cascade is activated in two separate tumors. We have previous shown that NC9 treatment is not necessarily associated with a reduction in TG2 level (5). Indeed, TG2 level is not reduced in the tumors (Fig. 4G). Based on these finding, we propose the signaling pathway outlined in Fig. 4H which will be described in the Discussion section.
TG2 regulation of signaling in HaCaT cells
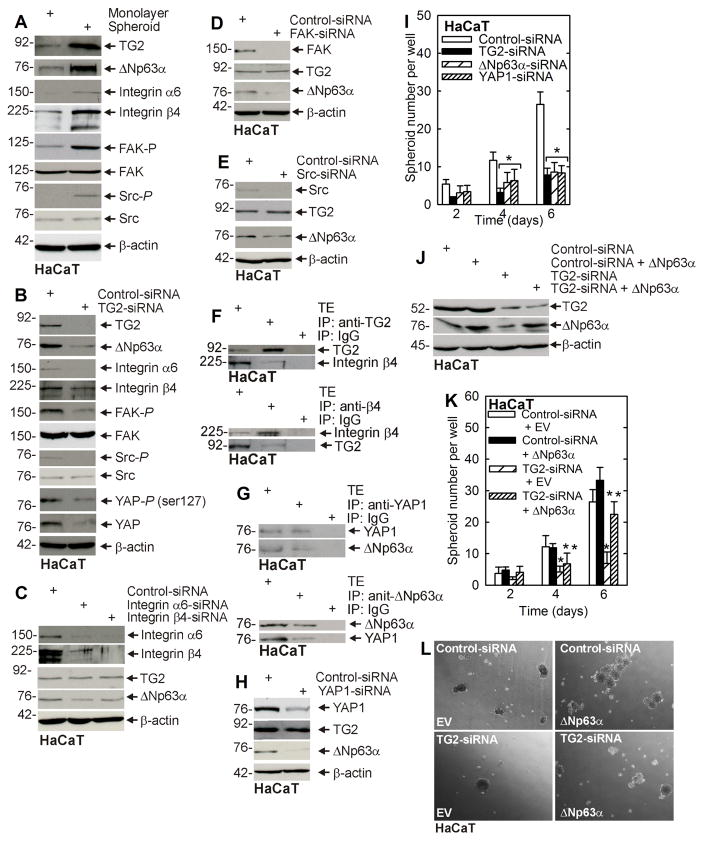
To assess whether this signaling pathway functions in other epidermis-derived cells, we used HaCaT cells, an immortal but non-transformed line isolated from epidermis. HaCaT cells were grown as monolayer or spheroid cultures and signaling kinase activity was monitored. A fraction of HaCaT cells (0.05%) survive culture in ultra-low attachment conditions (31). Fig. 5A shows that TG2 and ΔNp63α levels are elevated in HaCaT-derived ECS cells (spheroid cultures), and that this is associated with increased integrin level, and FAK and src signaling activity. In addition, TG2 knockdown reduces FAK and src signaling, and ΔNp63α level (Fig. 5B), and knockdown of α6/β4-integrin, FAK or src also reduces ΔNp63α (Figs. 5C/D/E). These findings suggest that TG2 control of ΔNp63α level occurs via a similar signaling mechanism as observed in SCC-13 cells. We also confirmed that TG2/integrin and YAP1/ΔNp63α interaction occurs in HaCaT cells (Figs. 5F/G). Since YAP1 and ΔNp63α interact, a key determination is whether YAP1 controls ΔNp63α level. Fig. 5H shows that YAP1 knockdown reduces ΔNp63α, but not TG2, level. We next studied the impact of these proteins on HaCaT cell phenotype. Fig. 5I shows that HaCaT cell spheroid formation is dependent upon TG2, ΔNp63α and YAP1. Finally, Figs. 5J/K/L show that restoration of ΔNp63α in TG2 knockdown cells partially restores spheroid formation.
Fig. 5. TG2 stimulated signaling in HaCaT cells.
A HaCaT cell-derived ECS cells display elevated integrin signaling and ΔNp63α level. HaCaT cells (40,000 per well) were grown in spheroid medium in monolayer or spheroid culture and at 10 d lysates were prepared for immunoblot. B/C/D/E HaCaT cells were double-electroporated with the indicated siRNA and then maintained in spheroid medium in monolayer culture for 48 h before extracts were prepared for detection of the indicated proteins. F/G HaCaT cells were grown in non-attached conditions as spheroids for 8 d. After day 8, lysates were prepared for immunoprecipitation and immunoblot. H HaCaT cells were double-electroporated with the indicated siRNA and then maintained in spheroid medium in monolayer culture for 48 h before extracts were prepared for detection of the indicated proteins. I Cells were double-electroporated with the indicated siRNA and then seeded to monitor for spheroid formation. J/K/L HaCaT cells were double-electroporated with the indicated siRNA or expression vector and at 48 h extracts were prepared for immunoblot. In parallel, cells were plated to assess ability to form spheroids and images were recorded. The plotted values are mean ± SEM and asterisks indicate significant change compared to control, n = 3, p < 0.005. The double asterisk in panel K indicate a significant change compared to the single asterisk group, n = 3, p < 0.005.
Discussion
TG2 is an ECS cell survival protein
We recently identified the ECS cell population resident in tumors from epidermal squamous cell carcinoma (3). These cells represent 0.15% or less of the tumor population, display enhanced invasion and migration, and are enriched for expression of stem cell markers as determined by cell sorting and magnetic bead selection including ALDH1, CD200, Sox2, Oct4, K15, K19 and α6-integrin) (3). They are also enriched in transcription factors associated with epithelial-mesenchymal transition (EMT) (Snail, Slug, and Twist, HIF-1α) and mesenchymal structural proteins including vimentin, fibronectin and N-cadherin (5). These cells are remarkable in that they form highly aggressive, invasive and vascularized tumors following injection of as few as 100 cells into immune compromised mice (3, 32). As an approach to identify ECS cell-enriched proteins for anti-ECS cell cancer therapy, we identified a number of proteins, including transglutaminase 2 (TG2), that are elevated in ECS cells and are required for ECS cell survival (3, 5, 10, 33).
TG2 is an important pro-inflammatory regulator in cancer cells (9, 34–36). Our studies show that TG2 level is markedly elevated in ECS cells as compared to non-stem cancer cells, and that TG2 is required for ECS cell survival, invasion, EMT, migration and tumor formation (4, 4, 5). Transient or stable TG2 knockdown results in a marked reduction in ECS cell survival, EMT, matrigel invasion, migration and tumor formation (4, 5). Moreover, treatment with TG2-specific inhibitors causes destruction of pre-established ECS cell spheroids, suppresses spheroid growth and inhibits tumor formation (4, 5).
These findings, and findings in other tumor models (10), have propelled efforts to develop TG2 inhibitors as cancer therapeutics (30, 37). Although TG2 has been shown to drive pro-inflammatory NFκB signaling (34, 38–44), our studies show that NFκB signaling is not relevant in ECS cells (4, 5). Thus, how TG2 targets downstream signaling mechanisms to maintain cancer stem cell properties is not well understood (10).
Integrin, PI3K/PDK1 and Hippo/YAP1/TAZ signaling
In the present studies, we identify a signaling cascade that mediates TG2-dependent ECS cell survival (Fig. 4H). Immunoprecipitation studies identify a TG2/α6/β4-integrin complex in ECS cells and show that TG2 impacts integrin level. The elevated TG2 expression detected in ECS cells is associated with enhanced α6/β4 integrin level. Consistent with a role for TG2 in maintaining integrin expression, reduced integrin levels are observed in TG2 knockdown cultures, and in TG2 inhibitor-treated tumors. This suggests that TG2 acts to maintain integrin level. Moreover, TG2 interaction with and maintenance of integrin level is associated with increased integrin signaling. In particular, FAK and Src kinase activity are elevated in TG2-positive cells and reduced in TG2 knockdown cultures, suggesting that TG2 interacts with integrins to enhance FAK/Src signaling, a finding also reported in pancreatic cancer cells (43). TG2 interaction with integrins has also been noted in other cell types (45–47). Although elevated FAK/Src activity is a property of many cancer cell types, a key property is that FAK/Src signaling is markedly enhanced in ECS cells as compared to non-stem cancer cells. Moreover, it appears to be required for survival of these cells. Thus, we propose that this is one of many pathways potentially involved in ECS cell survival.
The observation that FAK/Src activity can stimulate PI3K signaling (43) prompted us to measure TG2 impact on PI3K/PDK1 activity in ECS cells. We show that TG2, FAK or Src knockdown, or treatment with pharmacologic inhibitors of these activities, reduces PI3K/PDK1 activity in ECS cell cultures, suggesting that FAK/Src signaling triggers PI3K/PDK1 activation. A recent study indicates that PDK1 can inhibit Hippo signaling by direct inhibition of LATS1 function (27). The LATS1 kinase is a key regulator in the Hippo signaling cascade (25). Reduced LATS1 kinase activity is associated with enhanced cell proliferation (25), and LATS1 activity is often constitutively reduced in cancer cells (25). We show that LATS1 activity is reduced in TG2-expressing cells and increased following TG2 knockdown or pharmacologic inhibition. Moreover, inhibition of TG2, integrin, FAK, Src, PI3K or PDK1 function increases LATS1 activity (Fig. 4H). Thus, our studies suggest that TG2-dependent integrin/FAK/Src signaling enhances PI3K/PDK1 signaling to inhibit LATS1 kinase activity. PDK1 inhibition of LATS1, as observed in our study, has also been observed in MCF-10A cells (27).
YAP1 and TAZ
LATS1 reduces cell proliferation by phosphorylating the pro-proliferation/survival transcription adaptor proteins, YAP1 and TAZ, resulting in their movement to the cytoplasm and subsequent degradation (25). In contrast, non-phosphorylated YAP1 and TAZ interact in the nucleus to stimulate cell survival and proliferation (48, 49). Activation of YAP1 is widespread in cancer (25) and YAP1 activity is associated with enhanced stem cell survival in epidermis and other tissues (48–50). Moreover, induced expression of YAP1 triggers transition of epithelial cells to a metastatic state and confers stem cell characteristics. We demonstrate that TG2 and YAP1 levels are elevated in ECS cells and that maintenance of YAP1 level requires TG2.
TG2 acts via YAP1 to regulate ΔNp63α turnover
ΔNp63α is a key regulator of epithelial cancer stem cell survival (12, 51, 52). The present study shows that ΔNp63α level is elevated in ECS cells and is required for maintenance of ECS cell survival, spheroid formation, invasion and migration. YAP1 has been suggested to maintain ΔNp63α level (28, 29, 53, 54). Our studies reveal an important and unexpected link between TG2, YAP1 and ΔNp63α. TG2, YAP1 and ΔNp63α are elevated in ECS cells as compared to non-stem cancer cells and TG2 knockdown is associated with loss of ΔNp63α. In contrast, ΔNp63α does not regulate TG2 level, implying that ΔNp63α is downstream of TG2. Moreover, forced expression of ΔNp63α partially reverses the reduction in ECS cell spheroid formation, invasion and migration associated with TG2 knockdown, suggesting it can mediate TG2 biological activity.
Studies in other models describe YAP1/ΔNp63α complex formation (28, 29, 53) which involves binding of the YAP1 WW-domain to the PPPPY motif of p63α (53). Some studies report that complex formation is increased by ΔNp63α phosphorylation (29). Our studies demonstrate that YAP1 acts through ΔNp63α to mediate TG2 biological responses. Indeed, expression of constitutively active YAP1, YAP(S127A), can restore ΔNp63α level, and spheroid formation, invasion and migration in TG2-deficient cells. Our studies further suggest that YAP1 controls ΔNp63α level. We show that the reduction in ΔNp63α level observed in TG2 deficient cells is not associated with a change in the level of ΔNp63α-encoding mRNA. Instead, TG2-stimulated signaling appears to control ΔNp63α protein level. YAP1 appears to be a key mediator, as YAP1 knockdown reduces ΔNp63α level. The reduction in ΔNp63α observed in TG2- or YAP1-knockdown cells is proteasome-dependent, as it is reversed by lactacystin treatment. This finding is consistent with a previous studies showing that YAP1 can regulate ΔNp63α stability (55). Based on these findings, we propose that formation of a YAP1/ΔNp63α complex protects ΔNp63α from degradation.
TG2 and signaling in tumors
Tumor xenograft experiments show that treatment with NC9, an irreversible TG2 inhibitor (19), reduces tumor formation. This is consistent with our previous demonstration that NC9 blocks ECS cell spheroid formation, EMT, invasion and migration (4, 5). A key question is whether NC9 treatment produces signaling changes in tumors that are consistent with our observations in cell culture. Characterization of tumor extract from multiple tumors indicates that NC9 inhibition of TG2 reduces β4-integrin level, and FAK, Src and PI3K/PDK1 activity, and increases LATS1 kinase activity and YAP1-P formation leading to enhanced ΔNp63α degradation, consistent with the model in Fig. 4H.
A TG2-stimulated signaling cascade maintains ECS cell survival
Based on these studies, we propose that TG2 associates with the α6/β4-integrins to increase FAK/Src signaling and that this leads to PI3K/PDK1 activation, and that PDK1, in turn, suppresses LATS1 (Hippo) signaling leading to enhance nuclear accumulation of YAP1 which forms a complex with and stabilizes ΔNp63α to enhance ECS cell survival (Fig. 4H). We propose that this pathway helps explain how TG2, via maintenance of the ΔNp63α stem cell survival factor, functions to maintain ECS cell survival, matrigel invasion, migration and tumor formation. These studies further suggest that drugs designed to inhibit TG2 may be suitable for treating squamous cell carcinoma.
Acknowledgments
Grant Support: National Institutes of Health (RLE - CA131074 and CA184027)
Footnotes
The authors declare no conflict of interest.
References
- 1.Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi: 10.1136/bmj.f6153.:f6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikary G, Grun D, Kerr C, Balasubramanian S, Rorke EA, Vemuri M, et al. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One. 2013;8:e84324. doi: 10.1371/journal.pone.0084324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher ML, Keillor JW, Xu W, Eckert RL, Kerr C. Transglutaminase is required for epidermal squamous cell carcinoma stem cell survival. Mol Cancer Res. 2015;13:1083–94. doi: 10.1158/1541-7786.MCR-14-0685-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher ML, Adhikary G, Xu W, Kerr C, Keillor JW, Eckert RL. Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition. Oncotarget. 2015;6:20525–39. doi: 10.18632/oncotarget.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SY. Transglutaminase 2 in inflammation. Front Biosci. 2006;11:3026–35. doi: 10.2741/2030. [DOI] [PubMed] [Google Scholar]
- 7.Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther. 2007;115:232–45. doi: 10.1016/j.pharmthera.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gundemir S, Colak G, Tucholski J, Johnson GV. Transglutaminase 2: a molecular Swiss army knife. Biochim Biophys Acta. 2012;1823:406–19. doi: 10.1016/j.bbamcr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, et al. Transglutaminase regulation of cell function. Physiol Rev. 2014;94:383–417. doi: 10.1152/physrev.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert RL, Fisher ML, Grun D, Adhikary G, Xu W, Kerr C. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol Carcinog. 2015;54:947–58. doi: 10.1002/mc.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koster MI, Huntzinger KA, Roop DR. Epidermal differentiation: transgenic/knockout mouse models reveal genes involved in stem cell fate decisions and commitment to differentiation. J Investig Dermatol Symp Proc. 2002;7:41–5. doi: 10.1046/j.1523-1747.2002.19639.x. [DOI] [PubMed] [Google Scholar]
- 12.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–13. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 14.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–31. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 16.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517–23. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- 17.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–63. [PubMed] [Google Scholar]
- 18.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keillor JW, Chica RA, Chabot N, Vinci V, Pardin C, Fortin E, et al. The bioorganic chemistry of transglutaminase - from mechanism to inhibition and engineering. Can J Chem. 2008;86:271–6. [Google Scholar]
- 20.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 21.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–38. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J. 2011;278:4704–16. doi: 10.1111/j.1742-4658.2011.08346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006;11:1057–76. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids. 2012;42:939–49. doi: 10.1007/s00726-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 25.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 26.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA. 2013;110:2569–74. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–15. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30:151–65. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlinson V, Gudmundsdottir K, Luong P, Leung KY, Knebel A, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:e29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keillor JW, Apperley KY, Akbar A. Inhibitors of tissue transglutaminase. Trends Pharmacol Sci. 2015;36:32–40. doi: 10.1016/j.tips.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Adhikary G, Grun D, Balasubramanian S, Kerr C, Huang JM, Eckert RL. Survival of skin cancer stem cells requires the Ezh2 polycomb group protein. Carcinogenesis. 2015;36:800–10. doi: 10.1093/carcin/bgv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grun D, Adhikary G, Eckert RL. VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors. Oncogene. 2016 doi: 10.1038/onc.2015.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher M, Adhikary G, Grun D, Kaetzel D, Eckert R. The Ezh2 polycomb group protein drives an aggressive phentype in melanoma cancer stem cells and is a target of diet derived sulforaphane. Mol Carcinog. 2015 doi: 10.1002/mc.22448. in press. [DOI] [PMC free article] [PubMed]
- 34.Agnihotri N, Kumar S, Mehta K. Tissue transglutaminase as a central mediator in inflammation-induced progression of breast cancer. Breast Cancer Res. 2013;15:202. doi: 10.1186/bcr3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol. 2010;80:1921–9. doi: 10.1016/j.bcp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, et al. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008;14:1997–2005. doi: 10.1158/1078-0432.CCR-07-1533. [DOI] [PubMed] [Google Scholar]
- 37.Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther. 2007;115:232–45. doi: 10.1016/j.pharmthera.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893–900. doi: 10.1093/carcin/bgn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yakubov B, Chen L, Belkin AM, Zhang S, Chelladurai B, Zhang ZY, et al. Small molecule inhibitors target the tissue transglutaminase and fibronectin interaction. PLoS One. 2014;9:e89285. doi: 10.1371/journal.pone.0089285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yakubov B, Chelladurai B, Schmitt J, Emerson R, Turchi JJ, Matei D. Extracellular tissue transglutaminase activates noncanonical NF-kappaB signaling and promotes metastasis in ovarian cancer. Neoplasia. 2013;15:609–19. doi: 10.1593/neo.121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–95. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 42.Verderio EA, Scarpellini A, Johnson TS. Novel interactions of TG2 with heparan sulfate proteoglycans: reflection on physiological implications. Amino Acids. 2009;36:671–7. doi: 10.1007/s00726-008-0134-6. [DOI] [PubMed] [Google Scholar]
- 43.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–33. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 44.Han AL, Kumar S, Fok JY, Tyagi AK, Mehta K. Tissue transglutaminase expression promotes castration-resistant phenotype and transcriptional repression of androgen receptor. Eur J Cancer. 2014;50:1685–96. doi: 10.1016/j.ejca.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–38. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta K, Fok JY, Mangala LS. Tissue transglutaminase: from biological glue to cell survival cues. Front Biosci. 2006;11:173–85. doi: 10.2741/1789. [DOI] [PubMed] [Google Scholar]
- 47.Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007;26:2459–70. doi: 10.1038/sj.onc.1210035. [DOI] [PubMed] [Google Scholar]
- 48.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–46. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–83. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA. 2011;108:2270–5. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koster MI, Roop DR. Transgenic mouse models provide new insights into the role of p63 in epidermal development. Cell Cycle. 2004;3:411–3. [PubMed] [Google Scholar]
- 52.Koster MI, Roop DR. The role of p63 in development and differentiation of the epidermis. J Dermatol Sci. 2004;34:3–9. doi: 10.1016/j.jdermsci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Strano S, Fontemaggi G, Costanzo A, Rizzo MG, Monti O, Baccarini A, et al. Physical interaction with human tumor-derived p53 mutants inhibits p63 activities. J Biol Chem. 2002;277:18817–26. doi: 10.1074/jbc.M201405200. [DOI] [PubMed] [Google Scholar]
- 54.Parikh AA, Liu WB, Fan F, Stoeltzing O, Reinmuth N, Bruns CJ, et al. Expression and regulation of the novel vascular endothelial growth factor receptor neuropilin-1 by epidermal growth factor in human pancreatic carcinoma. Cancer. 2003;98:720–9. doi: 10.1002/cncr.11560. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee A, Sen T, Chang X, Sidransky D. Yes-associated protein 1 regulates the stability of DeltaNp63alpha. Cell Cycle. 2010;9:162–7. doi: 10.4161/cc.9.1.10321. [DOI] [PubMed] [Google Scholar]