Abstract
The default mode network (DMN) has been proposed as a biomarker for several chronic pain conditions. DMN functional connectivity (fcMRI) is typically examined during resting-state fMRI, in which participants are instructed to let thoughts wander. However, factors at the time of data collection (e.g., negative mood) that might systematically impact pain perception and its brain activity, influencing the application of the DMN as a pain biomarker, are rarely reported. The present study measured whether positive and negative moods altered DMN fcMRI patterns in chronic low back pain (CLBP) patients, specifically focusing on negative mood due to its clinical-relevance. Thirty-three participants (CLBP = 17) underwent resting-state fMRI scanning before and after sad and happy mood inductions, and rated levels of mood and pain intensity at the time of scanning. Two-way repeated measures ANOVAs were conducted on resting-state functional connectivity data. Significant group (CLBP > HC) X condition (sadness > baseline) interaction effects were identified in clusters spanning parietal operculum/postcentral gyrus, insular cortices, anterior cingulate cortex, frontal pole, and a portion of the cerebellum (pFDR < .05). However, only one significant cluster covering a portion of the cerebellum was identified examining a two-way repeated measures ANOVA for happiness > baseline (pFDR < .05). Overall, these findings suggest that DMN fcMRI is affected by negative mood in individuals with and without CLBP. It is possible that DMN fcMRI seen in chronic pain patients is related to an affective dimension of pain, which is important to consider in future neuroimaging biomarker development and implementation.
Keywords: default mode network, biomarker, chronic low back pain
Introduction
Neuroimaging biomarker development for chronic pain has become increasingly popular in recent years [2,13,29,46,48,56,73,74,76,80,91]. These studies have provided useful mechanistic information about pain perception, and their specific application to measure neurobiological processes underlying pain [89] is certainly warranted. However, potential issues arise in the clinical application of such biomarkers to classify (i.e., diagnose) individuals, which should be addressed before proposed biomarkers are clinically translated.
The initial rationale for pain biomarker development was largely based on the notion that pain self-report is “unreliable” [49], and “an imperfect measure of subjective experience” [80]. Although biomarker proponents have moved away from this rationale, the inherent assumption of this argument persists; namely, neuroimaging is presumed to be a more stable and informative measure of pain perception than self-report. Neuroimaging is undoubtedly valuable for understanding the complexity of pain ratings and mechanisms underlying chronic pain conditions; however, the stability of findings over time, an essential characteristic for biomarker implementation [52], remains questionable.
Aside from concerns about error impacting reproducibility of fMRI data [5,43,77], variables that systematically alter pain perception have received little attention in context of neuroimaging biomarkers for chronic pain. Factors that affect pain ratings and concomitant brain activity include mood [6,72,78,82], recall of autobiographical painful experiences [22,36,40], social support/distraction [11,21,23,87], and expectations of pain intensity/relief [19,39,47,67,86]. Given that such factors have been widely demonstrated to influence pain perception and its neural correlates, it is likely that these variables will similarly impact functional neuroimaging pain biomarkers.
Among ostensible biomarkers of chronic pain, the default mode network (DMN) has been named as a candidate marker for at least five conditions [8,13,48,55,73,91]. The DMN is a correlated set of brain regions showing increased activity during wakeful rest (i.e., resting-state) and self-referential tasks [62]. Although the exact function of the DMN is still debated, it is postulated to be involved in spontaneous cognition related to self-referential thought (e.g., mind wandering, autobiographical memory) and/or intrinsic neural dynamics [18,60,65,90]. DMN functional connectivity (fcMRI) is commonly captured using resting-state fMRI in which participants are typically asked to let their minds wander.
Critical reviews of the resting-state paradigm have cited modest reproducibility of results as the largest barrier to clinical translation [25]. One potential reason for replication issues is the lack of information regarding individuals’ mental state at the time of scanning [10,18]. In the case of previous studies naming the DMN as a potential pain biomarker, factors that influence mental state and/or pain perception itself during scanning are rarely considered. This information is vital to 1) understand exactly which aspects of a clinical pain condition are captured by the proposed neural signature (e.g., pain intensity/unpleasantness, clinical mood disturbance), and 2) inform the use of functional neuroimaging biomarkers for clinical decision-making by determining how a neural signature might change based on behavioral factors. The goal of the present study was to measure whether negative mood, a variable known to increase pain ratings and alter brain activity in individuals with and without CLBP [72,82], impacted DMN fcMRI during resting-state scanning.
Methods
Participants
Participants for the present study were recruited via flyers posted around the Gainesville community, as well as through HealthStreet, a UF-based organization designed to reduce health and research disparities in underrepresented populations. Specific inclusion criteria for the CLBP group included: 1) experiencing CLBP for greater than the past three months that meets at least one of the following Quebec Task Force on Spinal Disorders diagnostic criteria [71]:1c (CLBP without radiation below the gluteal fold), 2c (CLBP with proximal radiation to the knee), or 3c (CLBP with distal radiation below the knee), and 2) no history of psychological or neuropsychological disorder. Additionally, participants were included if they endorsed having vivid memories of 1) a past event in which they experienced extreme happiness, and 2) a past, isolated event in which CLBP caused sadness. Presence of such memories was necessary for the study’s mood induction procedures. Because the self-report of reduced quality of life is high in chronic pain patients, we included CLBP patients who endorsed a subclinical level of depressive symptoms on a questionnaire related to mood [defined as Beck Depression Inventory – II, (BDI-II) score < 21, based on a previous study [27]].
Specific inclusion criteria for the HC group included: 1) no history of chronic pain, psychological, or neuropsychological disorder, 2) a vivid memory of a past event in which they experienced extreme happiness, and 3) a vivid memory of a past, isolated event in which acute pain caused sadness. Participants were excluded if they endorsed: 1) use of analgesics that could not be stopped the day prior to the study, 2) use of serotonin reuptake inhibitors, serotonin antagonists, or tricyclic antidepressants at the time of the study, 3) positive result on a pre-MRI metal screening or pregnancy test, and 4) pain symptoms inconsistent with Quebec Task Force on Spinal Disorders diagnostic criteria mentioned above.
Data from 33 participants were used in the present study (n: CLBP = 17, HC = 16). To ensure that participants did not have a significant level of mood disturbance or neurocognitive deficits that could confound results, participants were screened using the BDI-II and Mini Mental Status Examination (MMSE). Groups did not specifically differ on age [t(31) = 1.52, p = 0.14], gender (CLBP females = 10; HC females = 9), global neurocognitive functioning, or level of endorsed depressive symptoms (Table 1). Ethnically, 18 participants identified as Caucasian (CLBP = 10), 14 identified as African-American (CLBP = 7), and one HC identified as Asian-American. The Institutional Review Board (IRB) at the University of Florida (UF) approved the study, and all participants provided written informed consent.
Table 1.
Comparison of descriptive statistics for demographic information and questionnaire scores between CLBP and HC.
| HC (n=16) Mean (SD) |
CLBP (n=17) Mean (SD) |
|
|---|---|---|
| Age (years) | 41.0 (12.11) | 47.82 (13.57) |
| Chronic Low Back Pain Duration (years) | - | 4.29 (2.76) |
| Average Pain Intensity (0–100) | - | 44.35 (18.19) |
| Beck Depression Inventory-II | 4.0 (3.46) | 7.65 (7.17) |
| Pain Vigilance and Attention Questionnaire | 37.44 (8.31) | 46.82 (12.39)a |
| Mini Mental Status Examination | 29.13 (.89) | 29.36 (.93) |
CLBP > HC (p<.05)
Data Collection Procedures
For the present study, participants completed two study visits, including a screening evaluation (Visit 1) and an MRI session (Visit 2). Visit 1 occurred one week prior to Visit 2.
Visit 1
After completing screening measures (BDI-II and MMSE), participants underwent a 10-minute mock MRI session. The mock scan was conducted to promote data quality by reducing participants’ scanner-related anxiety and movement while lying down, and was used to further screen for participants who were ineligible to complete Visit 2 (e.g., difficulty remaining still, elevated anxiety to the mock MRI environment that did not habituate). Participants deemed eligible for MRI scanning were provided with instructions to complete the mood induction during Visit 2 (see “Mood Induction Paradigm”). For the happy mood induction, both groups were asked to describe a time in which they felt particularly elated, such as a special occasion (e.g., wedding or birthday). For the sad mood induction, participants were asked to think of an affectively salient memory to describe aloud related to an autobiographical event in which pain caused sadness. Whereas HC participants were asked to think about an event involving acute pain (e.g., inability to complete an important athletic venture due to strained muscle), CLBP patients were asked to think about an event involving their clinical pain (e.g., missing an important family function due to CLBP).
Visit 2
MRI scanning took place during Visit 2, and included four resting-state fMRI scans. For each resting-state scan, participants were instructed to fixate their eyes on a projected crosshair, remain as still as possible, and to let their minds wander. Figure 1 demonstrates fMRI scanning procedures. To minimize carryover effects between the two mood manipulations, inductions were conducted before the second and fourth resting-state scans using counterbalanced moods (i.e., happy and sad) across participants; results from the second baseline scan (i.e., scan 3) and happy mood induction were not included in the present analyses. Individuals were asked to rate their current mood before and after each scan and induction. Using a verbal rating scale (0 = “no level of the mood”, 100 = “highest amount of the mood imaginable”), participants rated the following emotions: happy, sad, angry, anxious, and neutral. Additionally, participants rated their current level of low back pain (0 = “no back pain”, 100 = “most back pain imaginable”). Only self-report data related to sadness, happiness, and pain were used in the present analyses.
Figure 1.
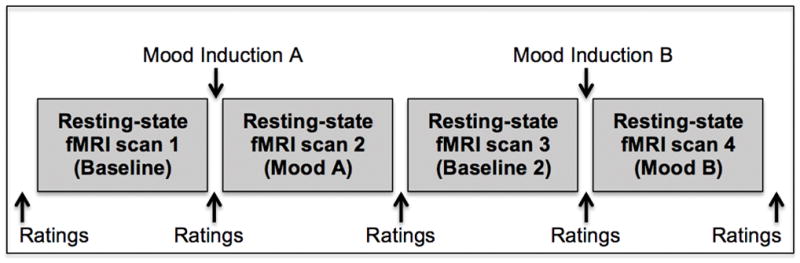
The protocol for fMRI scanning included four resting-state fMRI scans, with two mood inductions (i.e., happy and sad moods) completed between these scans. The two inductions occurred before the second and fourth resting-state scans, and were counterbalanced across participants. The second baseline (i.e., scan 3) and happy mood scans were not analyzed for the present study. Mood and pain ratings were collected before and after each scan and mood induction.
Mood induction paradigm
This study used a modified version of the mood induction paradigm described by Harrison and colleagues [31], which measured differences in DMN fcMRI among HC before and after undergoing sad mood induction. Additionally, this procedure has been used successfully in other PET and fMRI studies to significantly alter mood [16,51]. The version of this paradigm was modified, however, to include a sad mood induction specific to recall of a painful experience. We chose this induction specifically to act as a clinically-relevant model of fluctuations in pain-related affect. During Visit 1, participants were given instructions for the mood induction procedures used in Visit 2. Specifically, they were asked to think of two stories related to happy and sad autobiographical events, respectively, and attempt to re-experience the emotions during these events while telling the stories. Several steps were taken to increase the likelihood that the desired mood was induced during Visit 2: 1) participants were instructed to provide true autobiographical events, rather than contrive events, 2) participants were encouraged to describe very detailed aspects of the events including sensory experiences (e.g., smells, sounds, etc.) and thoughts at the time of the event (e.g., “My pain will never go away,” “I am a failure,” etc.), and 3) participants were asked to write down the detailed stories before Visit 2 and bring the written version to the scanning session.
Mood inductions were conducted before the second and fourth resting-state scans, while the participant was lying in the scanner, but without actual scanning taking place. As each participant recounted their stories, music played through MR-compatible headphones (Avotec, Stuart, FL). For the happy mood induction, the musical piece “Coppelia” by Debiles played during the recall period for both groups [50]. For the sad mood induction, the musical piece “Russia under the Mongolian Yoke” by Prokofiev played during the recall period for both groups [31,68]. Participants were instructed to continue telling their story for at least the duration of each musical piece (approximately 4–5 minutes), but could exceed this time if needed to finish the story. To maintain the integrity of the resting-state paradigm, no audio stimuli played during fMRI data collection, and participants were only provided with typical resting-state instructions prior to each scan (i.e., they were not instructed to try to remain in each mood for the duration of the scan).
Data acquisition parameters
Functional and structural MRI data were acquired with a research-dedicated whole-body scanner (Philips Achieva, 3.0T) using a standard head 32-channel RF coil. High-resolution, 3D anatomical images were collected using a T1-weighted MP-RAGE protocol (176 1mm-sagittal slices; repetition time = 7ms, echo time = 3.2ms, flip angle = 8°, 240 × 240mm matrix; field of view = 240 × 240 × 176mm). Functional images of the whole brain were collected using an echo planar imaging sequence (42 interleaved, transverse slices; repetition time = 2250ms; echo time = 30ms; flip angle = 90°; 80 × 80 matrix; field of view = 240 × 240 × 126mm; 3mm3 isotropic voxels with 0mm slice gap). Each scan lasted 8 minutes 12.7 seconds to collect a total of 213 volumes.
Statistical Analyses
Functional MRI data preprocessing
SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) in MATLAB 2011b (MathWorks, Sherbon, MA, USA) was used to preprocess fMRI data. Preprocessing procedures included 1) slice-timing correction for interleaved data collection, 2) 3D motion correction with realignment to the middle volume of each scan, 3) coregistration to the individual’s structural MRI, 4) normalization to an MNI template, and 5) spatial smoothing [6mm3 Gaussian kernel (FWHM)].
Additionally, we used the Artifact Detection Tool (ART, www.nitrc.org/projects/artifact_detect/) to identify outlier time points in the data that might confound fcMRI results. Outliers were defined as rotational displacement greater than .02 radians from the prior volume, or head displacement greater than .4mm from the prior volume [14]. Average motion [Euclidean norm = t(31) = −1.2, p = .24] and data outliers [t(31) = −.9, p = .38] across all runs were not significantly different between groups.
Importantly, resting-state fcMRI can be confounded by physiological nuisance variables, such as respiration and cardiac output [54]. Although this has traditionally been corrected through regressing out cardiac and respiration data collected at the time of scanning, more recent work has demonstrated that applying anatomical segmentation in a general linear model is more effective in regressing out physiological noise [4]. As such, structural data were segmented into grey matter, white matter, and cerebrospinal fluid masks to be used as regressors in the fcMRI analysis pipeline.
Functional connectivity analyses
To assess DMN fcMRI, we used the CONN toolbox [84] implemented through MATLAB. This toolbox predominantly uses seed-based correlations to assess task-based or resting-state fcMRI among regions. Preprocessed structural and functional images were entered into the toolbox’s processing pipeline, which includes temporal processing (i.e., denoising), first-level analyses, and second-level analyses. Additionally, conditions were specified by the mood associated with each fMRI run (e.g., baseline, sad). Temporal processing was conducted using CONN’s CompCor algorithm to remove physiological noise, such as outlier data detected in ART and signal within white matter/CSF (i.e., proxy for cardiac and respiration confounds) [4]. Using principal component analysis, the following nuisance variables were regressed out: five principal components from white matter and CSF masks, head motion parameters with first-order temporal derivatives, outliers detected during ART, and linear trends. Data were also bandpass filtered (0.008 to 0.09 Hz) [84].
Processed time series data were subsequently used in first-level fcMRI analyses. CONN’s default processing pipeline includes an atlas of a priori regions of interest (ROIs), from which time series data were extracted. Specifically, time series data were extracted from 10mm spheres around peak coordinates of midline DMN hubs (i.e., mPFC and PCC) to be used in the seed-based fcMRI analyses [14]. We chose the mPFC and PCC as our DMN seed regions given their roles as key hubs in the DMN [9], which we combined into one ROI for seed-to-voxel analyses (MNI coordinates: mPFC = −1, 47, −4, PCC = −5, −49, 40). The ROIs used in the present study are standard within the CONN toolbox and were generated from a previous study by Fox and colleagues, which examined intrinsic organization of functional networks [26].
Seed-to-voxel analyses measure fcMRI strength between seed a priori ROIs and all other voxels in the brain. For first-level seed-to-voxel analyses, bivariate temporal correlations were conducted among individuals’ time series data from a priori ROIs and all other voxels in the brain for each fMRI run. As standardized within the toolbox, correlation coefficients were Fisher’s Z-transformed to improve assumptions of normality [84].
Second-level seed-to-voxel analyses were then completed to allow for group-level comparisons. We conducted two repeated-measures ANOVA (two-factorial design) using group (CLBP > HC) and condition (sadness > baseline; happiness > baseline) as factors. Significance thresholds were estimated via Monte Carlo simulations (Alphasim, http://afni.nih.gov/afni/docpdf/AlphaSim.pdf) using the resultant F-maps (cluster connection radius: 12.1mm, iterations: 10,000). Significance with multiple comparisons correction (pFDR < .05) was determined as a voxel threshold of p < .01 with a contiguous cluster size > 118 voxels. Individual-level values for clusters identified at this significance threshold using the omnibus model were extracted and entered into SPSS to determine the main effects of group and condition, as well as group X condition interaction effects.
Results
Behavioral Ratings and Questionnaires
A two-way repeated measures ANOVA (group X condition) of sadness ratings indicated that there was a significant main effect of condition (MBaseline=11.37, SD=17.81; MSadness= 40.12, SD=31.88; F1,31=32.02, p<0.001, η2p=0.5;), so that sadness ratings increased in both groups following sad mood induction. However, there was not a significant group X condition interaction effect (MCLBP=32.62, SD=20.58; MHC=18.88, SD=20.59; F1,31=.79, p=0.38), or main effect of group (F1,31=.2.88, p=0.1). The impact of group (CLBP > HC) and condition (baseline > happiness) on happiness ratings was also measured using a 2×2 ANOVA (Baseline: MCLBP=70.69, SD=19.33; MHC=77.32, SD=19.33; Happiness: MCLBP=83.71, SD=20.26; MHC=83.38, SD=24.30). Across all participants, there was a significant main effect of condition (F1,31=4.51, p=0.04), so that happiness ratings increased following the induction; however, there were no significant group (F1,31=.33, p=0.57) or group X condition interaction effects (F1,31=.49 p=0.49).
Finally, we conducted a 2×2 mixed ANOVA (group X condition) for pain ratings. As expected, there was a significant main effect of group, so that participants with CLBP reported significantly higher LBP at baseline (MCLBP=36.88, SD = 29.72; MHC=5.00, SD=5.58) and following sad mood induction (MCLBP=35.88, SD=32.67; MHC=2.93, SD=6.88; F1,31=17.22, p<.001, η2p =.36). However, the group X condition interaction effect for pain ratings did not reach significance (F1,31=.07, p>.05).
DMN fcMRI Following Sad Mood Induction
A 2×2 mixed ANOVA was also conducted for fcMRI data using combined MPFC and PCC ROIs as a seed representing the DMN with group (CLBP > HC) and condition (sadness > baseline) as factors. Seven significant clusters were identified in the omnibus model as having significantly different fcMRI to the DMN seed (Figure 2). No significant main effects of group were identified in any of these clusters.
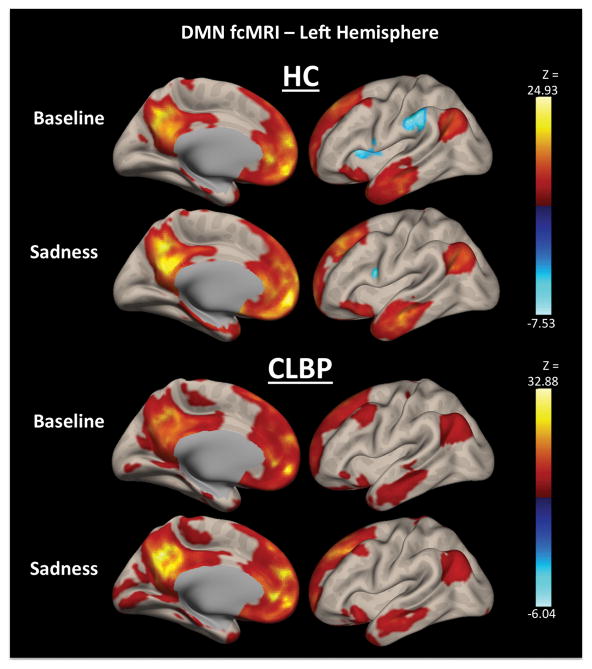
Figure 2.
Left-hemisphere default mode network (DMN) functional connectivity (fcMRI) broken down by groups and conditions. The top two DMN fcMRI maps show mean static fcMRI in healthy controls (HC) both in the baseline and sadness conditions, whereas the bottom two DMN fcMRI maps show mean static fcMRI in participants with chronic low back pain (CLBP) during both conditions.
Two clusters were identified as having a significant main effect of condition only: 1) ventral anterior cingulate cortex [(vACC); F1,31=23.35, p<.001, η2p =.43], and 2) right posterior insula [(pINS); F1,31=23.54, p<.001, η2p =.43]. In both groups, DMN-vACC was positively correlated in the baseline condition and showed a similar magnitude of change in fcMRI following sad mood induction [group X condition effect: F1,31=.01, p=.98, η2p =.0]. However, both groups showed anti-correlated DMN-right pINS fcMRI at baseline that became positively correlated following sad mood induction. Comparing the magnitude of change between groups, there was a trend for a greater increase in DMN-right pINS fcMRI within the CLBP group [group X condition effect: F1,31=3.0, p=.09, η2p =.09].
Three clusters showed a significant main effect of condition, as well as a group X condition interaction effect: 1) left pINS [F1,31=5.59, p=.03, η2p =.15], 2) left parietal operculum/postcentral gyrus [(ParOper/PostGyr); F1,31=16.73, p<.001, η2p =.35], and 3) right ParOper/PostGyr [F1,31=8.84, p=.006, η2p =.22]. Both groups showed anti-correlated fcMRI among these three clusters and the DMN at baseline. Following sad mood induction, HC showed a greater magnitude of change for DMN-left pINS and ParOper/PostGyr fcMRI compared to CLBP participants. Conversely, participants with CLBP showed a greater magnitude of change in DMN-right ParOper/PostGyr fcMRI compared to HC following sad mood induction.
Finally, two clusters showed significant group X condition interaction effects only: 1) frontal pole [F1,31=26.05, p<.001, η2p =.46] and 2) cerebellum [F1,31=18.8, p<.001, η2p =.38]. Across both clusters, groups showed inverse patterns of change in fcMRI following sad mood induction. Whereas HC showed a decreased in DMN-cerebellum and frontal pole fcMRI comparing sad mood to baseline conditions, CLBP showed an increase in fcMRI between the DMN and these two clusters.
DMN fcMRI Following Happy Mood Induction
A 2×2 mixed ANOVA was also conducted using group (CLBP vs. HC) and condition (baseline vs. happiness). One significant cluster was identified, which spanned a portion of the cerebellum (Fig. 3). This region showed a main effect of condition, so that there was decreased DMN-cerebellum fcMRI following happy mood induction in both groups [F1,31=24.18, p<.001, η2p =.44]. The magnitude of change was not significantly different between groups [F1,31=2.29, p=.14, η2p =.07].
Figure 3.
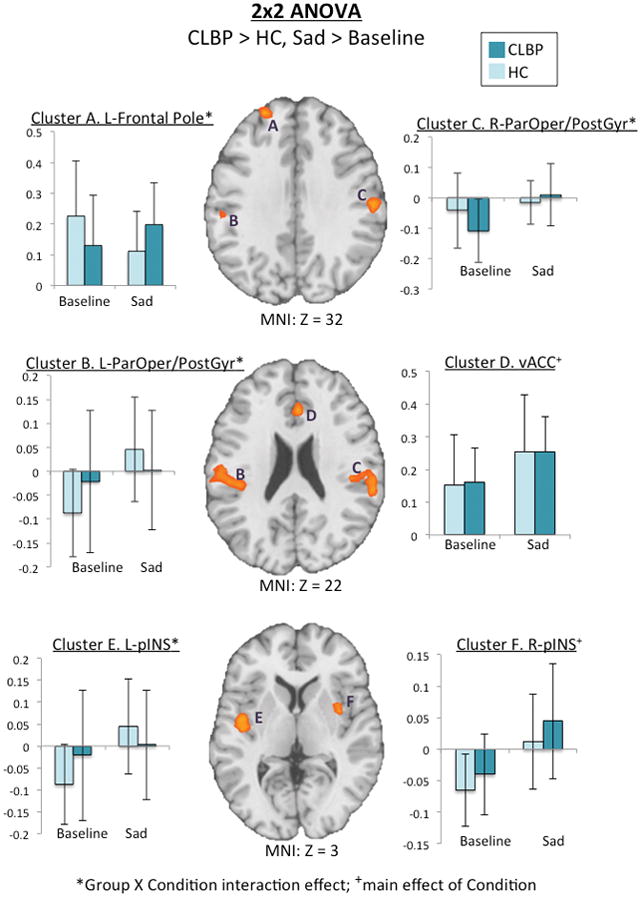
Axial slices of omnibus results from a 2×2 repeated-measures ANOVA using the factors group (CLBP > HC) and condition (sadness > baseline). Level is indicated by the Z MNI coordinate below each slice. Bar graphs represent mean fcMRI values of each cluster, which are labeled by letters. Light bars represent HC and dark bars represent CLBP. Group X condition interaction effects were identified in clusters A (frontal pole), B (left ParOper/PostGyr), C (right ParOper/PostGry), E (left pINS), and cerebellum (not pictured)], so that CLBP showed increased fcMRI between these clusters and the DMN following sad mood induction. Additionally, there was a main effect of condition only in clusters D (vACC) and F (right pINS).
Discussion
Pain perception is always subjective [53], and myriad trait (e.g., personality characteristics, chronic mood disturbance) and state (e.g., negative mood, distraction) factors have been shown to influence pain self-report and associated brain activity [12,23,24,72,78,81,82,85,87]. However, studies proposing functional neuroimaging biomarkers of chronic pain have not adequately addressed these variables in their design, raising questions about the practical application of results. Our findings demonstrate that variables known to influence pain perception are highly important to consider in the development of functional neuroimaging biomarkers. Specifically, we found that mood altered fcMRI of the DMN, which has been named as a candidate biomarker for a wide variety of clinical conditions [3,8,28,37,61,79,92], including at least five chronic pain conditions [13,48,55,73,91].
Although consistent resting-state networks have been identified across numerous studies, manipulation of mental state at the time of scanning has been shown to systematically influence patterns of connectivity within these networks in healthy individuals. Experimentally manipulated factors include cognitive focus prior to scanning [44,45,75,83], maintaining eyes opened or closed during data collection [57,90], and elevated level of temporary sad mood. Harrison and colleagues [31] demonstrated that increased reported sadness following negative mood induction was associated with decreased within-DMN fcMRI in HC. The authors concluded that assessing mental state at the time of scanning is imperative for the interpretation of results in both scientific and clinical applications. Additionally, experimental pain stimulation prior to resting-state scanning in patients with fibromyalgia subsequently resulted in increased fcMRI between the thalamus and regions within the DMN (i.e., precuneus/PCC) [33]. The authors concluded that experimental pain altered the “neural signature of chronic pain” collected during resting-state fMRI, similarly highlighting the susceptibility of brain fcMRI to behavioral manipulations, and raising concerns for the practical application of pain neuroimaging biomarkers.
Given that such factors have been shown to alter DMN fcMRI in HC and fibromyalgia patients, we aimed to determine whether alterations in a clinically relevant aspect of pain (i.e., negative pain affect) could similarly alter the DMN in individuals with and without CLBP. Specifically, we used an empirically-based mood induction protocol modified to function as a clinically relevant manipulation (i.e., recall of an autobiographical story in which pain caused sadness). We chose to manipulate mood given the high prevalence of mood disturbance among chronic pain patients [1,24,32], impact of mood on pain perception [72,78,82], and previous findings linking a key hub of the DMN to pain-related affect (i.e., rumination [42]).
Sadness ratings significantly increased following negative mood induction, suggesting individuals experienced a heightened level of sadness during this condition compared to baseline. Further, participants with CLBP reported increased LBP intensity associated with this change in mood, which is consistent with previous studies noting a relationship between mood and pain. It is important to note that because participants recalled autobiographical stories related to pain, attentional focus prior to resting-state scanning was specific to a previous pain experience across all participants.
Overall, we found differences in DMN fcMRI based on condition (i.e., baseline vs. sadness, and baseline vs. happiness) and group X condition interaction effects for sadness only. There was increased DMN fcMRI to the vACC, bilateral pINS, and bilateral ParOper/PostGyr across both groups following sad mood induction. Whereas HC participants showed a greater magnitude of change in DMN fcMRI to the left pINS and ParOper/PostGyr, there a greater magnitude of change in DMN fcMRI to right ParOper/PostGyr (and trend for right pINS) in participants with CLBP. Two additional clusters were identified showing opposite patterns of fcMRI with the DMN between groups (i.e., cerebellum and frontal pole), so that HCs showed a decrease in fcMRI following sad mood induction, and participants with CLBP showed an increase in fcMRI with the DMN. Following the happiness induction, there was decreased DMN fcMRI with a portion of the cerebellum across both groups.
Previous research has described vACC, pINS, ParOper/PostGyr, and cerebellum in the context of pain processing [34,38,58], as well as imagined pain [22,36]. Both vACC and INS are included within limbic [64] and paralimbic circuitry [30], respectively. Specifically, vACC has been linked to the affective-motivational dimension of pain [63], and is activated during the retrieval of autobiographical pain memories [36]. The pINS has been associated with pain processing [69] and negative affect [7]. Among subregions of the INS, pINS shows resting-state fcMRI with primary and secondary somatosensory cortices, which is consistent with our findings of increased DMN-ParOper (i.e., S2, [20]) and PostGyr (i.e., S1, [35]) fcMRI following sad mood induction. Further, both vACC and ParOper/PostGyr activation were linked to modulation of affect in sensory perception [66], suggesting that the pattern of fcMRI identified in the present study was associated with modulated affect.
Due to the pervasiveness of mood disturbance in chronic pain patients [17], it is imperative to ensure that biomarkers asserted to be pain intensity-specific are not resultant from changes in mood or pain-related affect. For example, patients with major depression show increased DMN fcMRI with the vACC [70], suggesting that mood impacts fcMRI of the DMN to this region. Similarly, approximately 60% of neuroimaging studies manipulating mood via recall of an emotional autobiographical memory resulted in INS activation, which was suggested to support this region’s role in evaluation of distressing cognitions and emotional processing [59].
Previous work aimed at identifying patients with chronic pain (i.e., biomarker development) has not examined the multi-component nature of pain, and instead focused more on pain intensity, to the exclusion of affective or other components of clinical pain. The present findings are consistent with most definitions of clinical pain in that changes in mood or pain-related affect also impact pain intensity ratings. Clinically then, it is important to note that if the purpose of a biomarker is as a diagnostic tool, fluctuations in mood (from any cause) or pain-related affect can impact DMN fcMRI. If the purpose of the biomarker is to provide mechanistic information and aid in treatment planning, then it is important to consider whether negative pain-related affect is contributing to the pattern of brain fcMRI.
Implications for Functional Neuroimaging Biomarker Development
The present results support previous research demonstrating that brain fcMRI associated with pain perception is not a static phenomenon [41], but rather, a dynamic process that is susceptible to varied conditions at the time of scanning. With the rise in number of purported pain biomarkers, future studies should experimentally test whether such factors known to influence pain perception and its neural correlates also impact these markers, which will ultimately improve their clinical application. It is entirely possible that previously (or future) proposed biomarkers are representative of the clinical phenomenon in question; however, few have undergone substantial experimental testing to warrant such a label. It is not enough to conclude that a certain fcMRI pattern is a pain-specific biomarker simply because two predetermined groups differ in fcMRI. Empirical evaluation of biomarkers under different conditions and across time points is encouraged as common practice [88].
Specific to studies using resting-state as a paradigm to derive biomarkers, it should be noted that the largest barrier to clinical translation of resting-state fMRI is moderate reproducibility of results within specific populations [25]. Consistent with previous research, our findings demonstrate that even “control” samples, used to make conclusions about aberrant fcMRI in clinical samples, can show altered DMN fcMRI based on varied conditions, such as increased negative affect and changes in prior cognitive task [31,44,45,75,83,90]. In a clinical setting, it is entirely possible that some patients might be primed to think about pain intensity or pain-related affect through clinical measures (e.g., questionnaires) completed immediately prior to scanning, whereas others might be distracted from focusing on their clinical pain. Studies proposing biomarkers should account for the impact of prior cognitive task as a potential confound for results.
Of note are the study’s limitations that future work should expand upon. First, the CLBP patients used in this study were specifically chosen to have subclinical symptoms of depression to avoid confounds of mood disorder; however, our sample was not fully representative of CLBP patients seeking treatment for pain (i.e., 52% meet criteria for clinical depression [15]). Future studies should determine whether CLBP patients with comorbid mood disorders show further DMN fcMRI differences. Additionally, we only manipulated one variable known to influence pain perception for the present study (i.e., negative mood). Future studies should determine whether proposed biomarkers endure other factors that influence pain perception, such as attention or expectations for relief. Finally, we examined static, seed-based fcMRI within the DMN; however, it will be important to determine whether potential chronic pain biomarkers derived from other analytic techniques (e.g., graph theory, dynamic fcMRI, machine-learning) would similarly be affected by changes in behavioral factors.
Conclusion
The present findings suggest that behavioral factors that influence pain perception can influence DMN fcMRI in both HC and CLBP patients, raising practical concerns for the application of the DMN as a biomarker of pain intensity. Thorough, empirical testing of brain fcMRI under varying conditions is necessary in claiming candidate biomarkers of chronic pain.
Table 2.
Significant clusters identified in an omnibus test for two 2×2 repeated measures ANOVA using group (CLBP > HC) and condition (Sadness > Baseline; Happiness > Baseline) as factors. These clusters were shown to have altered fcMRI with key hubs of the DMN (i.e., MPFC and PCC) following mood inductions.
| Model | Hemisphere | Cluster Coordinates | Cluster Size | F(1, 31) | Cluster p-val (<.05 FDR) | Cluster Regions | Voxels per Region |
|---|---|---|---|---|---|---|---|
| CLBP > HC, sadness > baseline | Left | −44 −28 20 | 453 | 11.59 | .000 | Parietal Operculum | 169 |
| Postcentral Gyrus | 86 | ||||||
| Supramarginal Gyrus | 33 | ||||||
| Planum Temporale | 20 | ||||||
| Heschl’s Gyrus | 6 | ||||||
| Right | 58 −18 26 | 363 | 14.69 | .000 | Parietal Operculum | 117 | |
| Supramarginal Gyrus | 127 | ||||||
| Postcentral Gyrus | 76 | ||||||
| Planum Temporale | 23 | ||||||
| Left | −30 2 −4 | 315 | 15.64 | .000 | Posterior Insula | 124 | |
| Putamen | 74 | ||||||
| Heschl’s Gyrus | 39 | ||||||
| Central Opercular Cortex | 21 | ||||||
| Planum Polare | 10 | ||||||
| Midline | 4 28 24 | 128 | 12.36 | .05 | ACC | 128 | |
| Left | −38 −42 −36 | 125 | 8.77 | .05 | Cerebellum | 67 | |
| Fusiform Gyrus | 25 | ||||||
| Inferior Temporal Gyrus | 24 | ||||||
| Left | −24 52 34 | 124 | 13.17 | .05 | Frontal Pole | 124 | |
| Right | 34 4 0 | 119 | 12.55 | .05 | Posterior Insula | 50 | |
| Putamen | 15 | ||||||
|
| |||||||
| CLBP > HC, happiness > baseline | Right | 28 −82 −40 | 208 | 24.18 | .013 | Cerebellum | 208 |
Acknowledgments
Supported by: Grants from the National Center for Complementary and Integrative Health (NCCIH) to MER (R01AT001424-05A2) and JEL (F31AT007898-03), as well as the National Institute of Nursing Research (1R01NR015314-01A1).
Footnotes
Disclosure
This research was funded by the National Center for Complementary and Integrative Health through grants to MER (R01AT001424-05A2) and JEL (F31AT007898-03), as well as the National Institute of Nursing Research (1R01NR015314-01A1). There is no conflict of interest among authors.
References
- 1.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 2.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balthazar MLF, de Campos BM, Franco AR, Damasceno BP, Cendes F. Whole cortical and default mode network mean functional connectivity as potential biomarkers for mild Alzheimer’s disease. Psychiatry Res Neuroimaging. 2014;221:37–42. doi: 10.1016/j.pscychresns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Behzadi Y, Restom K, Liau J, Liu TT. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- 6.Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Bijsterbosch J, Smith S, Forster S, John OP, Bishop SJ. Resting state correlates of subdimensions of anxious affect. J Cogn Neurosci. 2014;26:914–926. doi: 10.1162/jocn_a_00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: how, where, and what to look for using functional imaging. Discov Med. 2011;11:209. [PubMed] [Google Scholar]
- 9.Buckner RL. The brain’s default network. Ann New …. 2008;1124:1–38. doi: 10.1196/annals.1440.011. Available: http://onlinelibrary.wiley.com/doi/10.1196/annals.1440.011/full. [DOI] [PubMed] [Google Scholar]
- 10.Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 11.Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell P, Wynne-Jones G, Muller S, Dunn KM. The influence of employment social support for risk and prognosis in nonspecific back pain: a systematic review and critical synthesis. Int Arch Occup Environ Health. 2013;86:119–137. doi: 10.1007/s00420-012-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai XJ, Ofen N, Gabrieli JDE, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12:S43–S48. doi: 10.1111/j.1526-4637.2011.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark DM, Teasdale JD. Constraints on the effects of mood on memory. J Pers Soc Psychol. 1985;48:1595. [Google Scholar]
- 17.Clyde Z, de C, Williams AC. New Ave Prev chronic Musculoskelet pain Disabil Pain Res Clin Manag. Amsterdam, NL: Elsevier; 2002. Depression and mood; pp. 67–82. [Google Scholar]
- 18.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive–affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb cortex. 2006;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD, Naliboff BD. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proc Natl Acad Sci. 2011;108:11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairhurst M, Fairhurst K, Berna C, Tracey I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. 2012 doi: 10.1371/journal.pone.0048711. [DOI] [PMC free article] [PubMed]
- 23.Fardo F, Allen M, Jegindø E-ME, Angrilli A, Roepstorff A. Neurocognitive evidence for mental imagery-driven hypoalgesic and hyperalgesic pain regulation. Neuroimage. 2015;120:350–361. doi: 10.1016/j.neuroimage.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol. 1999;67:776. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- 25.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13:163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Ramirez J, Wu J. Network-based biomarkers in Alzheimer’s disease: review and future directions. Front Aging Neurosci. 2014:6. doi: 10.3389/fnagi.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green aL, Wang S, Stein JF, Pereira EaC, Kringelbach ML, Liu X, Brittain JS, Aziz TZ. Neural signatures in patients with neuropathic pain. Neurology. 2009;72:569–571. doi: 10.1212/01.wnl.0000342122.25498.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guenot M, Isnard J, Sindou M. Advances and technical standards in neurosurgery. Springer; 2004. Surgical anatomy of the insula; pp. 265–288. [DOI] [PubMed] [Google Scholar]
- 31.Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3:e1794–e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haythornthwaite JA, Sieber WJ, Kerns RD. Depression and the chronic pain experience. Pain. 1991;46:177–184. doi: 10.1016/0304-3959(91)90073-7. [DOI] [PubMed] [Google Scholar]
- 33.Ichesco E, Puiu T, Hampson JP, Kairys AE, Clauw DJ, Harte SE, Peltier SJ, Harris RE, Schmidt-Wilcke T. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain. 2016 doi: 10.1002/ejp.832. [DOI] [PubMed] [Google Scholar]
- 34.Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T. Altered functional connectivity between the insula and the cingulate cortex in patients with TMD – a pilot study. Headache. 2012;52:441–454. doi: 10.1111/j.1526-4610.2011.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanda M, Nagamine T, Ikeda A, Ohara S, Kunieda T, Fujiwara N, Yazawa S, Sawamoto N, Matsumoto R, Taki W. Primary somatosensory cortex is actively involved in pain processing in human. Brain Res. 2000;853:282–289. doi: 10.1016/s0006-8993(99)02274-x. [DOI] [PubMed] [Google Scholar]
- 36.Kelly S, Lloyd D, Nurmikko T, Roberts N. Retrieving autobiographical memories of painful events activates the anterior cingulate cortex and inferior frontal gyrus. J Pain. 2007;8:307–314. doi: 10.1016/j.jpain.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Kesler SR. Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol Aging. 2014;35:S11–S19. doi: 10.1016/j.neurobiolaging.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krämer HH, Stenner C, Seddigh S, Bauermann T, Birklein F, Maihöfner C. Illusion of pain: pre-existing knowledge determines brain activation of “imagined allodynia”. J Pain. 2008;9:543–551. doi: 10.1016/j.jpain.2008.01.340. [DOI] [PubMed] [Google Scholar]
- 41.Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34:3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letzen JE, Sevel LS, Gay CW, O’Shea AM, Craggs JG, Price DD, Robinson ME. Test-Retest Reliability of Pain-Related Brain Activity in Healthy Controls Undergoing Experimental Thermal Pain. J Pain. 2014;15:1008–1014. doi: 10.1016/j.jpain.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 2005;25:1214–1223. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 46.Lin C-S. Brain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One. 2014;9:e94300. doi: 10.1371/journal.pone.0094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loggia ML, Berna C, Kim J, Cahalan CM, Martel M-O, Gollub RL, Wasan AD, Napadow V, Edwards RR. The lateral prefrontal cortex mediates the hyperalgesic effects of negative cognitions in chronic pain patients. J Pain. 2015 doi: 10.1016/j.jpain.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. PAIN®. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y, Klein GT, Wang MY. Can pain be measured objectively? Neurosurgery. 2013;73:24–25. doi: 10.1227/01.neu.0000432627.18847.8e. [DOI] [PubMed] [Google Scholar]
- 50.Martin M. On the induction of mood. Clin Psychol Rev. 1990;10:669–697. [Google Scholar]
- 51.Mayer JD, Allen IP, Beauregard K. Mood inductions for four specific moods: A procedure employing guided imagery. J Ment Imag. 1995;19:133–150. [Google Scholar]
- 52.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merskey H, Bogduk N. IASP Task Force on Taxonomy Part III: Pain Terms, A Current List with Definitions and Notes on Usage. IASP Task Force Taxon. 1994:209–214. Available: http://www.iasp-pain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/default.htm#Pain.
- 54.Murphy K, Birn RM, Bandettini PA. Resting-state FMRI confounds and cleanup. Neuroimage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napadow V, Kim J, Clauw DJ, Harris RE. Brief Report: Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64:2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V, Birn RM. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peltz E, Seifert F, DeCol R, Dörfler A, Schwab S, Maihöfner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. doi: http://dx.doi.org/10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 60.Preminger S, Harmelech T, Malach R. Stimulus-free thoughts induce differential activation in the human default network. Neuroimage. 2011;54:1692–1702. doi: 10.1016/j.neuroimage.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Qi R, Zhang LJ, Luo S, Ke J, Kong X, Xu Q, Liu C, Lu H, Lu GM. Default Mode Network Functional Connectivity: A Promising Biomarker for Diagnosing Minimal Hepatic Encephalopathy: CONSORT-Compliant Article. Medicine (Baltimore) 2014;93:e227. doi: 10.1097/MD.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 63.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 64.Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119–157. doi: 10.1016/j.cortex.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- 66.Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmid J, Bingel U, Ritter C, Benson S, Schedlowski M, Gramsch C, Forsting M, Elsenbruch S. Neural underpinnings of nocebo hyperalgesia in visceral pain: A fMRI study in healthy volunteers. Neuroimage. 2015;120:114–122. doi: 10.1016/j.neuroimage.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 68.Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;63:749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- 69.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spitzer WO, LeBlanc FE. Scientific approach to the assessment and management of activity-related spinal disorders: a monograph for clinicians: report of the Quebec Task Force on Spinal Disorders. Harper & Row; 1987. [PubMed] [Google Scholar]
- 72.Tang NKY, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138:392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 73.Tessitore A, Russo A, Giordano A, Conte F, Corbo D, De Stefano M, Cirillo S, Cirillo M, Esposito F, Tedeschi G. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;14:89. doi: 10.1186/1129-2377-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tracey I, Mantyh PW. The Cerebral Signature for Pain Perception and Its Modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Tung K-C, Uh J, Mao D, Xu F, Xiao G, Lu H. Alterations in resting functional connectivity due to recent motor task. Neuroimage. 2013;78:316–324. doi: 10.1016/j.neuroimage.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate Classification of Structural MRI Data Detects Chronic Low Back Pain. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Upadhyay J, Lemme J, Anderson J, Bleakman D, Large T, Evelhoch JL, Hargreaves R, Borsook D, Becerra L. Test–retest reliability of evoked heat stimulation BOLD fMRI. J Neurosci Methods. 2015:1–9. doi: 10.1016/j.jneumeth.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volkow ND, Baler RD. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA psychiatry. 2013;70:661–663. doi: 10.1001/jamapsychiatry.2013.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wager TD, Atlas LY, Lindquist Ma, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–97. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (80- ) 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 82.Wagner G, Koschke M, Leuf T, Schlösser R, Bär K-J. Reduced heat pain thresholds after sad-mood induction are associated with changes in thalamic activity. Neuropsychologia. 2009;47:980–987. doi: 10.1016/j.neuropsychologia.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whitfield-Gabrieli S, Nieto-Castanon A. A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 85.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Wiech K, Vandekerckhove J, Zaman J, Tuerlinckx F, Vlaeyen JWS, Tracey I. Influence of prior information on pain involves biased perceptual decision-making. Curr Biol. 2014;24:R679–R681. doi: 10.1016/j.cub.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wiederhold BK, Gao K, Sulea C, Wiederhold MD. Virtual reality as a distraction technique in chronic pain patients. Cyberpsychology, Behav Soc Netw. 2014;17:346–352. doi: 10.1089/cyber.2014.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woo C-W, Wager TD. Neuroimaging-based biomarker discovery and validation. Pain. 2015:156. doi: 10.1097/j.pain.0000000000000223. Available: http://journals.lww.com/pain/Fulltext/2015/08000/Neuroimaging_based_biomarker_discovery_and.5.aspx. [DOI] [PMC free article] [PubMed]
- 89.Woo C-W, Wager TD. What reliability can and cannot tell us about pain report and pain neuroimaging. Pain. 2016 doi: 10.1097/j.pain.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 90.Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Long X, Zang Y. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One. 2009;4:e5743. doi: 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang CH, Mundahl J, Case M, Datta Y, Nelson S, Gupta K, He B. Resting State Neural Network Properties in Sickle Cell Disease Patients. Blood. 2014;124:2701. [Google Scholar]
- 92.Zhu DC, Covassin T, Nogle S, Doyle S, Russell D, Pearson RL, Monroe J, Liszewski CM, DeMarco JK, Kaufman DI. A Potential Biomarker in Sports-Related Concussion: Brain Functional Connectivity Alteration of the Default-Mode Network Measured with Longitudinal Resting-State fMRI over Thirty Days. J Neurotrauma. 2015;32:327–341. doi: 10.1089/neu.2014.3413. [DOI] [PubMed] [Google Scholar]