Abstract
Red blood cells (RBCs) are innate carriers that can also be engineered to improve the pharmacokinetics and pharmacodynamics of many drugs, particularly bio-therapeutics. Successful loading of drugs, both internally and on the external surface of RBCs, has been demonstrated for many drugs including anti-inflammatory, anti-microbial, and anti-thrombotic agents. Methods for internal loading of drugs within RBCs are now entering clinical use. While internal loading can result in membrane disruption that may compromise biocompatibility, surface loading using either affinity or chemical ligands offers a diverse set of approaches for the production of RBC drug carriers. A wide range of surface determinants is potentially available for this approach, although there remains a need to characterize the effects of coupling agents to these surface proteins. Somewhat surprisingly, recent data also suggest that red cell mediated delivery may confer tolerogenic immune effects. Questions remaining before widespread application of these technologies include determining the optimal loading protocol, source of RBCs, and production logistics, as well as addressing regulatory hurdles. RBC drug carriers, after many decades of progress, are now poised to enter the clinic and broaden the potential application of RBCs in blood transfusion.
Keywords: red blood cells, drug delivery, pharmacokinetics, biocompatibility, immunogenicity
Introduction
Inadequate pharmacokinetics and bio-distribution hinder the delivery and efficacy of many drugs. Conjugation with polymers (e.g., polyethylene glycol, PEG)[1], loading within synthetic carriers (liposomes, polymers, nanoparticles, etc)[2], coupling with natural carrier molecules[3], and other approaches have been devised to reduce elimination and optimize delivery of chemical drugs, biotherapeutics, and imaging probes. In addition to these “artificial” delivery systems, blood elements (red blood cells[4], white blood cells[5], platelets[6]) have special biological features that might enable them to serve as “smart” natural carriers for vascular drug delivery.
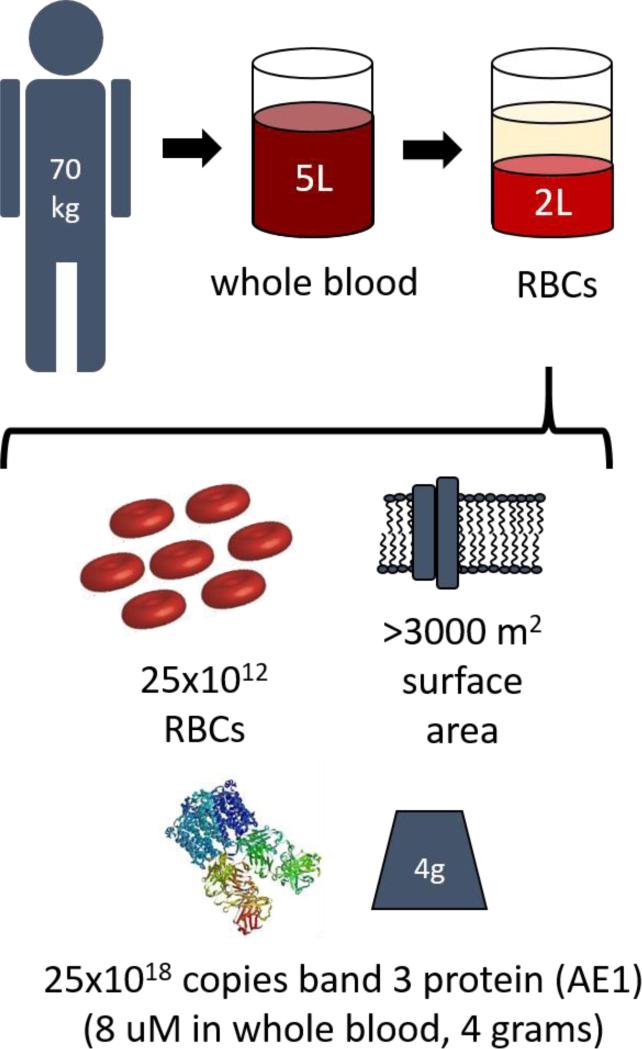
Red blood cells (RBCs) are biocompatible carriers that circulate for three months, encounter diverse intravascular cells and select tissues, and exert numerous vital functions[4, 7, 8], including important roles in homeostasis. The collective surface of the plasma membrane of RBCs is the most extended cellular surface accessible to blood (Figure 1) which provides a dynamic, multifunctional interface that regulates cascades of coagulation[9], and complement[10, 11], among others. RBCs also participate in host defense via carriage of antigens to immune cells and clearance of pathogens from the blood.
Figure 1.
Dimensional analysis of RBCs within a typical adult.
RBC-carriage of drugs often greatly prolongs their circulation and can act upon a variety of vascular targets to correct hemostasis[12], eliminate pathogens and microorganisms[13-15], mitigate toxins[16], and clear other pathological mediators[17, 18] and malignant cells[19]. Some extravascular sites including hepatic sinuses and splenic follicles are also normally accessible to RBCs. RBCs can also be exploited to deliver drugs to extravascular targets that become accessible in the context of the pathological process, for example at sites of vascular damage and bleeding.
Recent findings imply that RBC can serve as “super-carriers” for synthetic nanocarriers of drugs and imaging probes by altering their pharmacokinetics through prolonging their circulation, or engineering their bio-distribution via transfer from the surface of carrier RBC to the pulmonary endothelium[20] and other cell surfaces. These features of RBC carriage provide an impetus for understanding mechanisms underlying redistribution of agents coupled initially to their cell membranes[21]. In addition to altering the pharmacokinetics and biodistribution of a drug cargo, RBC carriage may also alter its functionality. For example, the RBC glycocalyx may protect attached biotherapeutics from plasma inhibitors and mask attached synthetic nanocarriers from clearance mechanisms[22].
Pioneering studies in the nineteen-seventies first explored the idea of RBC drug delivery[23]. However, in the subsequent decades, few groups persistently worked in this area; RBC delivery was generally viewed pessimistically in a drug delivery community focused on artificial carriers. Nonetheless, efforts to understand and utilize RBC drug delivery have evolved into a flourishing direction of biomedical research. Currently, a growing number of academic and industrial groups are actively pursuing use of RBCs for vascular drug delivery, both experimentally and translationally[24].
The RBC drug delivery literature has grown extensively in the last decade, and a number of reviews on the subject has been recently published[4, 7, 24-26]. Here, we focus on aspects of RBC drug delivery most pertinent in the context of transfusion: drug loading, manufacture and delivery regimens, immunological aspects of RBC drug delivery, and the effect of cargoes on the RBC itself.
Drug loading of carrier RBCs
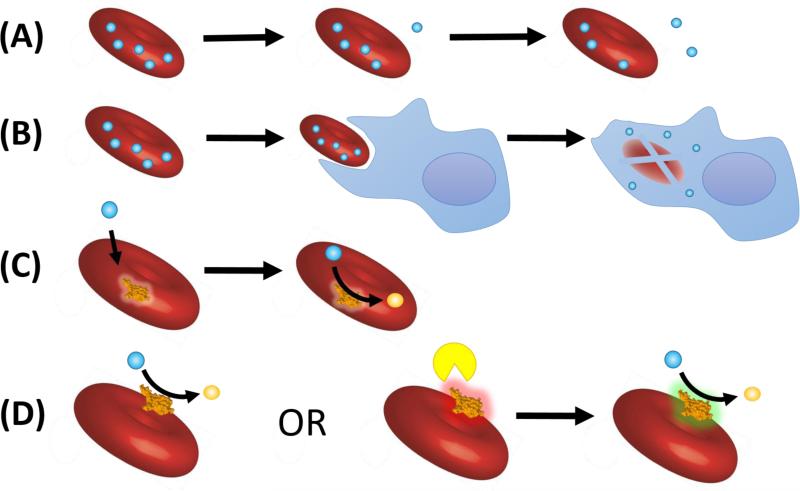
Drugs can either be encapsulated within RBCs or coupled to their surface. Both approaches have been studied for several decades and evolved from early experimental, but clinically unsuitable prototypes, into translationally feasible protocols that yield clinically useful drug carriers. These approaches (Figure 2) are summarized herein, and sources and methods of loading are summarized in Tables 1 and 2, respectively.
Figure 2. General approaches to RBC drug carriers.
(A) Slow drug release from drugs encapsulated within RBCs provides sustained delivery (B) Drug-loaded RBCs are phagocytosed and deliver drugs to cells of the reticuloendothelial system (such as macrophages) (C) Encapsulated enzymes act upon diffusible substrates (D) Surface loaded enzymes act on extracellular targets either constitutively or after activation by other proteins/enzymes to an active state (green), which may provide enhanced specificity.
Table 1.
Pros and cons of the various sources of RBCs for drug-loading
| RBC source | Pros | Cons |
|---|---|---|
| Autologous | • Lessened infectious disease risk • No allo-immunization |
• Donor RBC may be pathologically altered • Larger volume phlebotomy may limit donor eligibility in disease states |
| Allogeneic | • Does not necessitate phlebotomy of patients • Facilitates central manufacture • Existing infrastructure for unit collection and distribution |
• Allo-immunization potential • Infectious disease risks |
| Fresh | • Better circulation/survival • No accumulation of ‘storage lesion’ byproducts |
• Necessitates ‘on-demand’ production • Logistical hurdles to deliver units/drugs to some centers |
| Stored | • Ease of central manufacture • Facilitates production timing |
• ‘Storage lesion’ byproducts and cellular changes may compromise efficacy • May alter interaction with drug cargo or appended ligands |
Table 2.
Pros and cons of the types of loading of RBC drug carriers
| Loading Type | Pros | Cons |
|---|---|---|
| Ex vivo | • Greater control over surface coupling and degree of loading • More complex loading methods can be performed on isolated RBC |
• Potential compromise of RBC viability and circulation • Complex regulatory, manufacture, and distribution concerns • Sterility risks with open system |
| In vivo | • More reproducible manufacture and facile distribution • Single-entity compound possible • Better defined regulatory structure |
• Must reach target before clearance or degradation • Likely limited to surface-loading |
| External/surface | • Less complex to manufacture • Can engineer degree of loading by using different surface determinants • Affinity changes can be engineered to control drug release |
• May alter membrane rigidity or plasticity • Requires identification and characterization of affinity ligand • Drug often not in native free form |
| Internal | • Protects drug from extracellular clearance, inactivation, and immune recognition • Drug may be encapsulated in free form |
• Compromised membrane integrity • Lessened circulatory survival • Usually requires drug release, or diffusion of substrate into RBC |
Encapsulation of biological and chemical agents into RBCs has been explored for almost half a century[27]. Agents tested for encapsulation in RBC include enzyme replacement therapies[23, 28], antimicrobial and anti-viral agents[29], antigens to modulate immune response[30, 31], and anti-inflammatory drugs[32, 33]. The activity of drugs encapsulated within RBC can be altered by binding to hemoglobin, oxidation catalyzed by iron, interaction with NO-donors, or other catalytic reactions. Enzymes encapsulated inside carrier RBCs can be used to detoxify or neutralize substrates that diffuse through the cell membrane[34]. Multiple studies using a drug encapsulation approach have affirmed the feasibility and promise of infusing drug-loaded RBCs[25, 26, 32, 35-42].
Loss of RBC biocompatibility is a challenging aspect of drug encapsulation[43, 44]. Protocols based on transient osmotic shock and membrane resealing can provide clinically acceptable biocompatible encapsulation of drugs partially freed of hemoglobin [23, 29, 45, 46]. RBCs loaded with small molecules and therapeutic proteins, such as encapsulated dexamethasone and enzymes, have been successfully tested in animal models[46, 47] and in clinical trials[28, 48-51].
Methods to couple therapeutics to the RBC surface have also been explored, including covalent conjugation via amino acids[52, 53], thiol groups[54, 55], sugars[56] and lipids[19, 57]. Examples of RBC surface loading include coupling of antigens and cytokines to stimulate the immune response[58] and antibodies that target RBC-loaded cargoes to their therapeutic sites[55, 59, 60]. Specific examples involving conjugating anti-thrombotic agents, ligands to capture pathological agents and inhibitors of complement are described below. Recently, insertion of lipid-modified biomolecules into the RBC membrane has been used for targeting to phagocytes[19].
As in the case of encapsulation, early methods of surface loading compromised the biocompatibility of RBCs, provoking lysis and elimination via activation of complement and other pathways[17, 61, 62]. Development of methods for RBC surface loading that avoided these adverse effects allowed effective surface loading of RBC and permitted injection in animals without overt shortening of their circulation or other toxicities[60, 63, 64].
Surface loading on RBCs is well suited to deliver agents that modulate hemostasis, including ligands of fibrin[12], heparin[65], and fibrinolytics[66]. For example, RBC carriage prolongs circulation of plasminogen activators (PAs) by orders of magnitude[67], restricts their diffusion into the CNS and pre-existing hemostatic clots, abrogates undesirable interactions with vascular cells[68], and delivers drug to the interior of nascent thrombi[69]. These features accelerate thrombus dissolution and enable thromboprophylaxis in settings where the risk of thrombosis and bleeding are both high[70]. In addition to improving the pharmacokinetics, RBC carriage favorably alters several important properties of PAs: i) switches them from activating pro-inflammatory receptors in the CNS parenchyma to protective signaling via intravascular receptors; ii) accelerates dissolution of cerebral thrombi in animals providing reperfusion, protection of brain tissue and improved survival; iii) attenuates brain damage in rats with intracranial hemorrhage and blunt trauma; and iv) protects brain function in pigs with cerebral thrombosis[68, 71-75]. In stark contrast, unconjugated PAs cause CNS bleeding, neurotoxicity, and lethality in these settings.
Targeting drugs to RBCs
Drug encapsulation within allogeneic or autologous RBCs and conjugation to the RBC surface typically require multi-step manipulations involving cell isolation, numerous incubations, washings, and repacking. However, they are technically challenging procedures that may limit translational prospects. Further, multistep extracorporeal drug loading hinders the utility of RBC drug delivery in some relevant clinical settings, such as emergency medicine.
This problem might be resolved with the advent of drugs or drug carriers endowed with specific affinity to RBCs. Such non-covalent surface RBC loading offers the potential advantage of not only one-step extracorporeal loading, but even in vivo loading of circulating RBCs, bypassing the need for extracorporeal manipulations. To achieve this, drugs or nanocarriers have been conjugated chemically or genetically to ligands that bind safely to RBCs. For example, biotherapeutics including fibrinolytics conjugated with antibody to complement receptor type 1 (CR1, Knops antigen) bind safely to circulating RBCs in mice[76, 77]. This approach capitalizes on the immune-privileged nature of CR1 ligands, both physiological such as C3b-containing immune complexes involved in immune clearance, and artificial, such as anti-CR1 conjugated agents capturing pathogens.
A more general approach involves fusing biotherapeutics to a single chain variable region fragment (scFv) of antibodies that bind to RBCs[78]. In contrast to antibody-based conjugates, monovalent scFv-fusions do not cause cross-linking of RBC acceptor molecules or Fc-mediated side effects[79]. Recombinant technology enables large-scale, GMP-quality production of homogeneous monovalent scFv fusion proteins. A fusion of soluble CR1 with scFv directed to Rh(D) blood group antigens has been used to restore RBC CR1 function in genetically deficient mice[80].
Several groups successfully devised recombinant biotherapeutics fused with the scFv fragment of TER-119, a monoclonal antibody to an epitope associated with the mouse analogue of human glycophorin A (GPA)[81]. TER-119 scFv fused to complement-regulating proteins including DAF and CR1 enhanced RBC resistance to lysis by complement[78, 80, 82]. Neonatal gene transfer of TER-119 scFv/CR1 using a retroviral gene transfer vector prolonged synthesis, sustained coupling to RBCs, and restored protection against complement mediated lysis in genetically deficient mice[83]. TER-119 scFv fused with fibrinolytics provided thromboprophylaxis in mice[84, 85]. TER119 scFv fused with the recombinant endothelial anticoagulant and anti-inflammatory glycoprotein thrombomodulin (TM), had a half-life in the bloodstream ~100-fold longer than soluble TM and proved to be more effective in preventing arterial and venous thrombosis[86].
As illustrated by TER119 scFv fusions, the modular molecular format enables diversification of therapeutic cargoes. In theory, therapeutic features can also be diversified by regulating binding and on- and off-rates using in vitro affinity maturation or fusing cargoes with scFv fragments derived from different antibodies directed to the same antigen. This is projected to enhance control over intravascular residence time and the rate of detachment from RBC, offering the potential for either rapid or protracted drug delivery. From a drug delivery standpoint, it is important to take into account factors such as the number of antigens that may be targeted, their distribution on the RBC surface, their physiological functions in the membrane, their role in membrane integrity, and even potential signaling. Both the nature of the RBC surface anchoring epitope and the properties of the drug cargo portion of the RBC-targeted fusions are important in terms of potential adverse effects including immune response, cellular signaling, and adhesion.
The roster of RBC determinants potentially useful for this purpose is fairly extensive and ranges from high copy number targets such as glycophorin A and band 3 protein to low copy number targets such as complement receptor 1 (CR1) (Table 3)[87]. In theory, many RBC membrane proteins may be amenable to safe ligand coupling and some may prove to offer unique advantages for specific therapeutic purposes, e.g. spatial availability for activation and regulation.
Table 3.
Potential target antigens for coupling therapeutics to RBCs, their non-RBC distribution, likely function, and relative numbers per cell
| RBC Antigen (Blood group designation) | Other Tissue Expression | Function | Est. Sites per RBC |
|---|---|---|---|
| Band 3 Protein (Diego) | Distal and collecting tubules of the kidney | Anion exchange, structural integrity | 106 |
| Glycophorin A (MNS) | Renal epithelium and endothelium | Chaperone for band 3 transport to membrane, major sialoglycoprotein contributing to negatively charged glycocalyx | |
| GLUT1 (none) | Endothelial cells of barrier tissues | Glucose transport | |
| Glycophorin B (MNS) | Renal epithelium and endothelium | Minor sialoglycoprotein contributing to negatively charged glycocalyx | |
| RHCE and RHD (Rhesus) | Largely erythroid specific | Membrane structure and integrity, homology with ammonium transporters | 105 |
| CD59/Protectin (undefined) | Widely expressed | GPI-linked complement regulatory protein, inhibits membrane attack complex | |
| Kell (Kell) | Myeloid tissues, marrow, brain, muscle | Endothelin-3 converting enzyme | 104 |
| CD55/Decay accelerating factor (Cromer) | White blood cells, platelets, lung | GPI-linked complement regulatory protein, accelerates decay of C3 and C5 convertases | |
| ICAM-4 (LW) | Relatively erythroid specific | Intercellular adhesion molecule, ligand for integrins | 103 |
| Complement Receptor 1 (Knops) | White blood cells, glomerulus, follicular dendritic cells | Complement regulatory protein, processing of immune complexes |
As one successful example, CR1, which clears C3b-containing immune complexes, has been targeted with bispecific antibody constructs to remove pathogens from the blood in primate models[15, 88] and CR1-targeted agents have been used to protect against diverse pathologic agents including bacteria, cytokines, viruses, and pathogenic autoantibodies[89-91].
The success of targeting of diverse TER-119 fusions in mice without detectable adverse effects implies a potential utility for high-copy loading using this glycophorin A associated epitope[81], but direct translation from mouse to human RBCs is hindered by lack of TER-119 cross-reactivity with human RBC. Coupling of proteins and nanoparticles to mouse and rat erythrocytes on glycophorin A has also been achieved with a 12-mer peptide ligand known as ERY1, although this ligand also lacks cross-reactivity with human RBC[92, 93]. While ligands to glycophorin A and band 3 on human RBCs have been characterized to some extent, these may significantly alter membrane deformability[94], and little is known about the coupling of biomolecules to most human RBC membrane proteins.
Effects of drugs on RBCs
Adverse effects of drug loading on RBC behavior in the circulation may mirror pathological changes in the plasma membrane and cytoskeleton as occurs in hereditary spherocytosis and elliptocytosis. Alterations associated with RBC aging, disease, and drug loading typically hinder RBC elasticity, durability and biocompatibility, leading to accelerated elimination and potentially lysis[95].
All drug loading approaches - encapsulation, surface conjugation and targeting - can harm RBCs. Although it seems logical that RBC damage would be proportional to the dose as well as the composition of the drug load, there is a lack of systematic side-by-side comparative studies of these issues. Non-covalent surface coupling using affinity ligands would be expected to be more benign than invasive protocols such as numerous washings, hypotonic opening, and chemical modification, but again there is a need for detailed investigation.
Usually, the initial phase of RBC clearance is most profoundly affected by drug loading due to rapid hepatic and splenic elimination of the cells most severely altered. Increased uptake does not necessarily compromise the objectives of drug delivery and if often not associated with evidence of organ dysfunction or marked biochemical changes. In contrast, even a modest increase in pulmonary uptake is a reason for concern, as it usually reflects RBC aggregation and retention in the microvasculature, which can impair oxygen exchange. Similarly, intravascular hemolysis with the risk of renal and other organ damage is a potentially serious dose-limiting toxicity.
Encapsulation is arguably more invasive than surface anchoring. The need to generate even transient pores in the RBC membrane for gradient-driven import of drugs inevitably challenges its integrity. This can lead to at least partial loss of essential mechanical properties of the cell membrane that maintain reversible deformability and resistance to damage by hemodynamic stress and squeezing through the microvasculature. However, even surface loading may alter the cytoskeleton and expose phosphatidyl serine (PS), which serves as a cofactor for assembly and activation of coagulation and complement reactions. Alterations of cell shape, loss of glycocalyx, depletion of energy sources, and loss of ions or water can lead to frank loss of RBCs during drug encapsulation (which may exceed 50% in protocols currently undergoing clinical testing) and shorten the intravascular survival of the remaining drug-loaded RBCs.
Non-covalent affinity targeting using monovalent scFv-fusions is less damaging in principal, but unguided anchoring of drugs to the RBC's surface may nonetheless impede biocompatibility and alter behavior in vivo through reorganization or blocking of protective components (e.g. DAF, CD59, CR1) or inhibition of CD47 that confers RBCs with “self” or “do not eat me” signals to phagocytes[96]. Extensive coverage of the RBC membrane may lead to alterations in membrane plasticity and other disruptive side effects commonly seen after encapsulation as discussed below.
Cross-linking of membrane components can cause potentially harmful side effects, especially when multivalent ligands are employed. However, even monovalent ligands can alter RBCs, for example, by reducing deformability[94] through conformational changes in the anchoring protein[97]. These effects are target protein dependent as ligands appended to some targets can increase deformability, as has been observed with CR1[98], while several others decrease deformability, as seen with glycophorin A and band 3[97, 99, 100]. Ligand binding to glycophorin A was also shown to result in increased intracellular reactive oxygen species[100]. The extent to which such effects are dependent on multi-valency is uncertain. Ligands binding to different epitopes on the same anchoring molecule differ in their effects depending on their size, affinity, charge and proximity of the targeted epitope to RBC surface. Ligand-induced changes may cause overt hemolysis and anemia (as in some antibody-mediated transfusion reactions), or more subtle (but more important in the context of drug delivery) changes, such as abnormal flow dynamics and cellular distribution in the blood due to changes in membrane rigidity[101, 102].
Binding of ligands may also induce RBC membrane vesiculation[103] and release of microparticles causing inflammation and thrombosis, as seen in hemolytic RBC disorders[104-106]. Exposure of PS, a typical “symptom” of RBC damage, transforms the RBC surface into pro-coagulant interface favoring assembly of and activation of clotting factors, as mentioned above[9, 107] as well as promoting adhesion to the endothelium[108]. Hemoglobin and other internal RBC components released through “leaky” membranes can cause inflammation and toxic reactions such as acute renal damage[109]. The potential for deleterious effects of RBC modification will likely depend on the pathophysiologic context. For example, although a reduction in RBC deformability might be deleterious in some settings, it has also been shown to inhibit malarial parasite invasion[110].
Modification of RBCs may even enhance biocompatibility in some cases. For example, ‘universal’ RBCs generated by enzymatic treatment to convert them to type O have been transfused successfully into healthy recipients[111]. Also, targeting of certain drugs such as scFv-TER-119 fusions carrying complement inhibitors have been demonstrated to alleviate damage to RBCs deficient in endogenous complement inhibitors, as described above.
Immunological considerations
Drug loading to RBCs also may alter immunological features of the resultant complex. One might expect that anchoring foreign biological objects (molecules, their fragments and complexes, microorganisms) to the RBC surface would induce immune responses, as evidenced by drug-related autoimmune RBC antibodies. In some cases these antibodies can be neutralized by free drug but frequently they only bind to the drug/membrane complex[112, 113]. Whether induced or preformed, such antibodies can cause severe, life-threatening hemolytic anemia.
Enzymes released by certain pathogens, particularly during severe infection, can alter the RBC surface causing normally cryptic structures to be exposed. This is generally referred to as “T-activation” wherein various T antigen carbohydrates are expressed during the time of infection[114]. Naturally occurring antibodies to these normally cryptic structures that are present in all normal serum (except newborns) makes T-activated cells “polyagglutinable” and can causes the RBCs to lyse when patients are transfused with plasma.
T-activation and drug-induced antibodies point to the sensitivity of the immune system to seemingly minor perturbations of surface structures on RBCs. Indeed, transfusion of incompatible RBCs based on a single amino acid difference in a single membrane protein (e.g. Jk(a) vs. Jk(b) antigens) can induce polyclonal IgG and life-threatening hemolysis or hemolytic disease of the newborn. However, despite the relatively high degree of polymorphism in RBC surface proteins, only a subset of transfusion recipients ultimately develop alloantibodies[115]. The mechanisms by which these antibodies are generated is a subject of extensive investigation given their relevance to allogeneic transfusion[116, 117]. Recently developed models[118] have demonstrated some unique aspects of how RBCs interface with the host immune system, including “antigen modulation” by C3 on the RBC surface[119], and resistance of a subset of allogeneic RBCs to antibody mediated clearance[120]. Importantly, erythroid-specific expression of otherwise strongly immunogenic antigens, was able to induce humoral tolerance in mouse models[121].
In this context, in an intriguing and somewhat counterintuitive finding, coupling antigens to RBCs by fusion to Ter-119 induced T cell deletion and conferred immunological tolerance, including in clinically relevant models of autoimmune type 1 diabetes[122]. The authors speculated that surface-loaded carrier RBCs undergo apoptosis to yield non-immunogenic remnants conferring stealth immunological features onto the appended cargoes. Similarly, tolerance to L-asparaginase, a therapeutic for leukemia which is, in part, limited by drug-neutralizing antibodies, can be induced by RBC-mediated delivery[123]. RBC-mediated delivery may induce tolerance through the induction of regulatory T cells[124]. Reduced immunogenicity of therapeutic enzymes by encapsulation within RBCs[49, 125] has also been attributed to separation of the cargo from immune system by the RBC plasma membrane. Nevertheless, the consequences of exposing RBC components normally hidden from the immune system caused by loading and potential immunogenicity of a fraction of the drug cargo retained on the RBC surface must be carefully examined for each intended use.
A patient's immune and disease status may also modulate response to drug-RBC therapeutics. Not all transfusion recipients make detectable alloantibodies to foreign RBC antigens (“non-responders”). In the absence of inflammation, mice transfused with RBCs expressing transgenic human glycophorin A (hGPA) antigen did not produce detectable anti-hGPA immunoglobulins in contrast to the same protocol in the setting of inflammation[126], perhaps due to changes in the subset of antigen presenting cells that phagocytose RBCs[127]. In fact, these mice demonstrated evidence of true immunologic tolerance in that later challenge with hGPA in the context of inflammation still did not result in an immune response. These data suggest that transfusion of RBCs (potentially including some RBC drug-carriers) might induce tolerance rather than provoking a harmful host response in the absence of inflammation. It remains to be demonstrated how anti-inflammatory agents bound to RBC might alter this balance of tolerigenicity and immunogenicity. Overall, this area of RBC drug delivery research and its effect on the immune response is in further need of systematic and detailed mechanistic studies.
Regimens and dosing of drug delivery by RBC
Drug encapsulation and surface loading ex vivo and targeting to circulating RBC in vivo each have pros and cons that vary in part with the therapeutic goal (Figure 2).
Vascular infusion provides the logical route of administering RBCs loaded ex vivo using drug encapsulation or surface binding, and portable devices suitable for sterile loading of autologous or donor RBCs using a standard automated procedure have been devised and clinically tested. Loading via encapsulation demonstrated efficacy in the delivery of dexamethasone[33], asaparaginase[35], and enzyme replacements[37], among others. As a prototypical example, a dose of dexamethasone-loaded RBCs (Erydex) is prepared from 50 mL of blood, which is approximately 1% of the total blood volume of a 70 kg adult. After infusion of a suspension of loaded RBCs, the injected dose of the drug is carried by a small fraction of total circulating RBC.
Ex vivo RBC loading regimens involving a small fraction of RBCs theoretically pose less danger of generalized adverse effects and enable “focal” drug depots. The potential value of such “focal” modes of action has been demonstrated in studies of spatiotemporal parameters of dissolution of fibrin clots by RBC-bound fibrinolytics. RBC-bound fibrinolytics quickly formed focal zones of expedited fibrinolysis (“cavities”) within clots, which expand due to directional force of flow, coalescing into patent channels that facilitate reperfusion prior to clot dissolution. In contrast, fibrinolytics evenly distributed throughout the clot provided reperfusion only after substantial dissolution of the clot[128, 129]. The RBC surface also provides a favorable microenvironment for therapeutic activity by way of a protective glycocalyx.
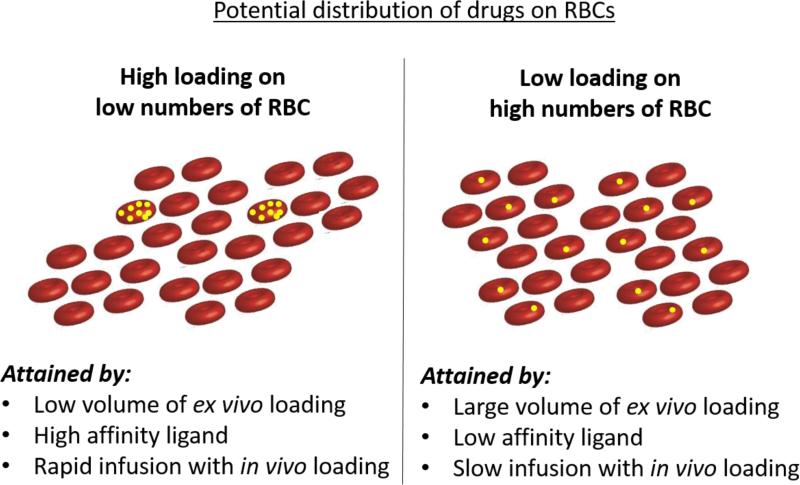
Therapeutic goals may differ in terms of the optimal distribution of drug throughout circulating RBCs (Figure 3). In some situations, a more even distribution of a drug in the bloodstream might be desirable. In theory, loading drug onto or within a larger proportion of autologous RBCs might be possible but would require larger volume phlebotomy or continuous loading via a closed extracorporeal circuit, such as an apheresis machine. A more even distribution may be attained when fusion proteins are targeted to circulating RBCs. This approach expands the versatility of the approach because the proportion of RBCs carrying the drug can be modulated by changes in dose and rate of infusion. In theory, bolus injection of a small dose of a high-affinity scFv fusion may create a situation similar to infusion of pre-loaded RBCs with few RBCs carrying the large majority of the drug, whereas slow infusion may result in more even “painting” of many circulating RBCs with drugs. In theory, the dose and duration of drug in the circulation can also be modulated by using fusions that bind to different epitopes on a target and with different affinities.
Figure 3.
Potential distribution of drugs on or within RBCs and the approaches by which they may be attained.
Although greatly sustained circulation is generally a favorable feature in drug design, control of this feature is an important consideration for RBC carriers particularly with respect to potential toxicities. Routes of clearance will involve either intrinsic clearance of the RBC itself (for example, via the spleen), or clearance of the appended or loaded agent as it detaches from the surface, is released from within, or is otherwise degraded. In the former case, we must consider factors that govern the rate of RBC clearance. One's own red cells are thought to circulate for approximately 120 days, and transfused units, after initial rapid clearance of a subset of RBCs (usually <10% within 24 hours, and required by the FDA to be <25% for transfused units), are thought to have similar lifespan[130]. The degree to which a portion of red cells are cleared rapidly appears to depend on several factors such as prior storage[131], and potentially even host factors related to ex vivo survival[132]. Strategies to facilitate clearance if reversal is needed could theoretically include red-cell exchange by apheresis, which would provide immediate removal from circulation. However, this approach necessitates high exposure to allogeneic units and potentially invasive procedures for adequate intravascular access, so its use may be limited to acute toxicities or with agents of very narrow therapeutic indices. Alternatively, control of release or detachment of the appended or loaded agent can also modulate delivery parameters. Incorporation of cleavable or activatable linkers has been described for many biotherapeutics, especially antibody-drug conjugates for cancer therapy[133], and activatable zymogens have also been applied to RBC-coupled agents[85]. Such technologies offer an important handle for potential control of delivery. For internally loaded agents, rate of diffusion out of RBCs is an important parameter and can be expected to be highly dependent on loading technique and the biochemical properties of the desired agent.
Although parenteral administration (in vivo loading) of a recombinant fusion protein offers a more controllable and straightforward path toward the fabrication and regulatory approval of RBC surface-coupled therapeutics, one can also envision scenarios in which ex vivo surface loading of RBCs for subsequent transfusion of the drug-loaded RBCs is favorable. As one example, any agent that may be rapidly sequestered and cleared when soluble may be less amenable to in vivo loading. Alteration of donor RBC units in the clinical realm is already widely practiced in the form of red cell additive solutions, RBC washing, irradiation, and leukoreduction[134]. Another example of RBCs modified ex vivo for clinical application (although not for therapeutic delivery) is the tagged red blood cell scan in which RBC are loaded with isotopes for imaging to identify the source of occult lower gastrointestinal bleeding[135]. In this example, RBC manipulation is typically performed by nuclear medicine specialists using specialized, FDA-approved commercial kits.
Transfusion aspects of RBC drug delivery
In the transfusion medicine or blood banking realm, modification of RBCs results in the designation of blood banks as manufacturers of drugs and thus many hospital blood banks are regulated as such by the FDA and are subject to strict inspection and accreditation requirements[136]. A common characteristic of RBC manipulations in the clinical setting is the preferred maintenance of closed, sterile connection systems whenever possible to limit contamination. Access of RBC units in an ‘open’ system is possible, although it typically results in products that must be administered within 24 hours. Such a limitation may be tolerable for particular RBC carriers.
The stability of RBC-drug complexes for in vitro storage requires characterization for each drug and method of loading. With present loading technologies, RBCs have no shelf-life after drug encapsulation: i.e. they must be injected in immediately after loading. Trauma inherent in cell isolation, washing, and drug loading is more likely to damage senescent or otherwise impaired RBCs. In this sense, ex vivo drug loading may fortuitously eliminate weak carriers and select for RBCs that enhance delivery, albeit at the cost of reduced yield.
If transfusable, drug-loaded RBCs are to become a clinical reality, the source of the RBCs must be carefully considered. Autologous donation mitigates the need for immunological matching with recipients as well as many concerns about infection, but this approach may be limited by health requirements for autologous donation[134], depending on the volume of red cells that will be needed to manufacture a particular therapeutic and potential for anemia. For example, although dexamethasone-loaded RBCs currently in clinical trial require only about 50 mL of whole blood for manufacture, a typical whole blood collection is 400-500 mL, a volume that may be problematic for treatment of diseases in which recipients are not likely to be eligible for autologous collection. Conversely, allogeneic RBC units represent a large, well-established, and safe potential pool for manufacture of surface loaded RBC carriers and have already been used in trials of asparaginase-loaded RBCs[35]. However, issues of alloimmunization, infectious diseases, and transfusion reactions (allergic, febrile, etc) cannot be definitively eliminated. Finally, ex vivo culture of mature RBCs that can be surface modified[137], while demonstrating promise in very early pre-clinical settings (and unmodified cells in healthy human recipients[138]) remains far from widespread clinical availability and questions of allogeneic versus autologous sources would remain.
The schedule for production and administration of ex vivo RBC-loaded or RBC-coupled drugs must also be taken into consideration. Most commonly used additive solutions result in RBC products that can be stored up to 42 days[132]. However, once a RBC unit is modified (for example, following irradiation), this ‘shelf-life’ can be significantly reduced, going down to at most 24 hours when an ‘open’ system must be used. Possibilities for carrier RBCs may include loading of drugs into fresh RBCs for immediate administration (as is currently practiced for RBC-loaded drugs in trial and tagged RBC scans), but also loading or coupling of drugs into/onto stored units (both autologous and allogeneic). In addition, further storage of drug-loaded RBCs may be possible post-loading or post-coupling (for example, to simplify central manufacture and subsequent distribution), but concerns about the potential adverse effects of storage, such as changes in membrane properties and accumulation of ‘storage lesion’ byproducts, would be introduced with such an approach[139-142], While the results of recent trials suggest that stored RBCs are not inferior in a variety of patient populations[143-146], the relevant endpoints and risk/benefit profiles are likely to be significantly different in the design of RBC-coupled/loaded drugs for prophylaxis or for treatment of chronic diseases.
Conclusion
Three general approaches to drug delivery – natural, synthetic, and hybrid carriers– have distinct potential utilities, benefit/risk ratios, and investment/cost ratios that vary among pathological conditions, clinical settings, and even among individual patients. RBC carriers incorporate facets of each of these approaches, and, from this perspective, conditions that typically involve blood transfusion or cellular therapy represent a very attractive area for their application. Technical, organizational, financial, and safety issues inherent to RBC carriers are manageable as illustrated by early successes in drug-loaded RBCs and engineered cellular immunotherapies. Thus, loading RBCs with drugs seems a natural extension of treatment of diseases that currently include or rely upon transfusion. Surgical and other types of trauma, sickle cell disease, anemia and other cytopenias, and acute lung injury are scenarios in which combining the effects of transfusion with pharmacotherapy delivered by RBCs may provide added benefit.
On the other hand, the roster of pathophysiology that might benefit from RBC-mediated drug delivery broadens the potential application of red cell transfusion and cellular therapies to include oncological, neurological, cardiovascular, metabolic, infectious, and other diseases, as well as intoxications with endogenous and exogenous noxious agents. RBC-carried imaging and diagnostic agents may find additional utilities in these and other conditions. One can hope that decisive improvement of therapeutic or diagnostic outcomes provided by RBC-mediated delivery in such disorders will eventually expand the general utility of blood transfusion.
Highlights.
Red blood cells (RBCs) are promising carriers for bio-therapeutics to enhance their PK and control their site and mechanism of action.
RBC drug carriers include those loaded internally or externally and may be prepared ex vivo or in vivo from allogeneic or autologous units.
Surface coupling onto membrane antigens offers a biocompatible, flexible platform for loading of drugs.
The optimal epitopes for coupling onto RBCs remain to be definitively characterized.
Drug loading onto RBCs may alter immune responses, including potential induction of tolerance.
Acknowledgments
Funding
R01 HL121134, R01 HL125462.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J Pharm Sci. 2016;105:460–475. doi: 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Jhaveri A, Torchilin V. Intracellular delivery of nanocarriers and targeting to subcellular organelles. Expert Opin Drug Deliv. 2016;13:49–70. doi: 10.1517/17425247.2015.1086745. [DOI] [PubMed] [Google Scholar]
- 3.Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliv Rev. 2015;91:109–124. doi: 10.1016/j.addr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang B, Abraham WD, Zheng Y, Bustamante Lopez SC, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7:291ra294. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickens B, Mao Y, Li D, Siegel DL, Poncz M, Cines DB, Zheng XL. Platelet-delivered ADAMTS13 inhibits arterial thrombosis and prevents thrombotic thrombocytopenic purpura in murine models. Blood. 2015;125:3326–3334. doi: 10.1182/blood-2014-07-587139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villa CH, Pan DC, Zaitsev S, Cines DB, Siegel DL, Muzykantov VR. Delivery of drugs bound to erythrocytes: new avenues for an old intravascular carrier. Ther Deliv. 2015;6:795–826. doi: 10.4155/tde.15.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprandel U, Way JL. Erythrocytes as Drug Carriers in Medicine. Springer Science & Business Media; 2013. [Google Scholar]
- 9.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–3845. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odera M, Otieno W, Adhiambo C, Stoute JA. Dual role of erythrocyte complement receptor type 1 in immune complex-mediated macrophage stimulation: implications for the pathogenesis of Plasmodium falciparum malaria. Clin Exp Immunol. 2011;166:201–207. doi: 10.1111/j.1365-2249.2011.04459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies KA, Hird V, Stewart S, Sivolapenko GB, Jose P, Epenetos AA, Walport MJ. A study of in vivo immune complex formation and clearance in man. J Immunol. 1990;144:4613–4620. [PubMed] [Google Scholar]
- 12.Coller BS, Springer KT, Beer JH, Mohandas N, Scudder LE, Norton KJ, West SM. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J Clin Invest. 1992;89:546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn SE, Nardin A, Klebba PE, Taylor RP. Escherichia coli bound to the primate erythrocyte complement receptor via bispecific monoclonal antibodies are transferred to and phagocytosed by human monocytes in an in vitro model. J Immunol. 1998;160:5088–5097. [PubMed] [Google Scholar]
- 14.Gyimesi E, Bankovich AJ, Schuman TA, Goldberg JB, Lindorfer MA, Taylor RP. Staphylococcus aureus bound to complement receptor 1 on human erythrocytes by bispecific monoclonal antibodies is phagocytosed by acceptor macrophages. Immunol Lett. 2004;95:185–192. doi: 10.1016/j.imlet.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Hahn CS, French OG, Foley P, Martin EN, Taylor RP. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia. J Immunol. 2001;166:1057–1065. doi: 10.4049/jimmunol.166.2.1057. [DOI] [PubMed] [Google Scholar]
- 16.Sharma R, Zhao H, Al-Saleem FH, Ubaid AS, Puligedda RD, Segan AT, Lindorfer MA, Bermudez R, Elias M, Adekar SP, Simpson LL, Taylor RP, Dessain SK. Mechanisms of enhanced neutralization of botulinum neurotoxin by monoclonal antibodies conjugated to antibodies specific for the erythrocyte complement receptor. Mol Immunol. 2014;57:247–254. doi: 10.1016/j.molimm.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzykantov VR, Taylor RP. Attachment of biotinylated antibody to red blood cells: antigen-binding capacity of immunoerythrocytes and their susceptibility to lysis by complement. Anal Biochem. 1994;223:142–148. doi: 10.1006/abio.1994.1559. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson PJ, Reist CJ, Martin EN, Johnson C, Greene KL, Kuhn S, Niebur J, Emlen W, Taylor RP. Antigen-based heteropolymers. A potential therapy for binding and clearing autoantibodies via erythrocyte CR1. Arthritis Rheum. 1995;38:190–200. doi: 10.1002/art.1780380207. [DOI] [PubMed] [Google Scholar]
- 19.Mukthavaram R, Shi G, Kesari S, Simberg D. Targeting and depletion of circulating leukocytes and cancer cells by lipophilic antibody-modified erythrocytes. J Control Release. 2014;183:146–153. doi: 10.1016/j.jconrel.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anselmo AC, Gupta V, Zern BJ, Pan D, Zakrewsky M, Muzykantov V, Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7:11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooyman DL, Byrne GW, McClellan S, Nielsen D, Tone M, Waldmann H, Coffman TM, McCurry KR, Platt JL, Logan JS. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269:89–92. doi: 10.1126/science.7541557. [DOI] [PubMed] [Google Scholar]
- 22.Atukorale PU, Yang YS, Bekdemir A, Carney RP, Silva PJ, Watson N, Stellacci F, Irvine DJ. Influence of the glycocalyx and plasma membrane composition on amphiphilic gold nanoparticle association with erythrocytes. Nanoscale. 2015;7:11420–11432. doi: 10.1039/c5nr01355k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dale GL, Kuhl W, Beutler E. Incorporation of glucocerebrosidase into Gaucher's disease monocytes in vitro. Proc Natl Acad Sci U S A. 1979;76:473–475. doi: 10.1073/pnas.76.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnani M. Erythrocytes as carriers for drugs: the transition from the laboratory to the clinic is approaching. Expert Opin Biol Ther. 2012;12:137–138. doi: 10.1517/14712598.2012.650163. [DOI] [PubMed] [Google Scholar]
- 25.Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118:145–160. doi: 10.1016/j.jconrel.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Rossi L, Serafini S, Pierige F, Antonelli A, Cerasi A, Fraternale A, Chiarantini L, Magnani M. Erythrocyte-based drug delivery. Expert Opin Drug Deliv. 2005;2:311–322. doi: 10.1517/17425247.2.2.311. [DOI] [PubMed] [Google Scholar]
- 27.Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973;70:2663–2666. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bax BE, Bain MD, Fairbanks LD, Webster AD, Chalmers RA. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase. Br J Haematol. 2000;109:549–554. doi: 10.1046/j.1365-2141.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 29.Rossi L, Serafini S, Cappellacci L, Balestra E, Brandi G, Schiavano GF, Franchetti P, Grifantini M, Perno CF, Magnani M. Erythrocyte-mediated delivery of a new homodinucleotide active against human immunodeficiency virus and herpes simplex virus. J Antimicrob Chemother. 2001;47:819–827. doi: 10.1093/jac/47.6.819. [DOI] [PubMed] [Google Scholar]
- 30.Banz A, Cremel M, Rembert A, Godfrin Y. In situ targeting of dendritic cells by antigen-loaded red blood cells: A novel approach to cancer immunotherapy. Vaccine. 2010;28:2965–2972. doi: 10.1016/j.vaccine.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Magnani M, Chiarantini L, Mancini U, Rossi L, Vittoria E, Fazi A. Red blood cells as an antigen-delivery system. Biotechnol Appl Biochem. 1992;16:188–94. [PubMed] [Google Scholar]
- 32.Bossa F, Latiano A, Rossi L, Magnani M, Palmieri O, Dallapiccola B, Serafini S, Damonte G, De Santo E, Andriulli A, Annese V. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol. 2008;103:2509–2516. doi: 10.1111/j.1572-0241.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 33.Rossi L, Serafini S, Cenerini L, Picardi F, Bigi L, Panzani I, Magnani M. Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease. Biotechnol Appl Biochem. 2001;33:85–89. doi: 10.1042/ba20000087. [DOI] [PubMed] [Google Scholar]
- 34.Godfrin Y, Bax B. Enzyme bioreactors as drugs. Drugs Future. 2012;37:263–272. [Google Scholar]
- 35.Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, Auvrignon A, Corm S, Dombret H, Chevallier P, Galambrun C, Huguet F, Legrand F, Mechinaud F, Vey N, Philip I, Liens D, Godfrin Y, Rigal D, Bertrand Y. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol. 2011;153:58–65. doi: 10.1111/j.1365-2141.2011.08588.x. [DOI] [PubMed] [Google Scholar]
- 36.Godfrin Y, Horand F, Franco R, Dufour E, Kosenko E, Bax BE, Banz A, Skorokhod OA, Lanao JM, Vitvitsky V, Sinauridze E, Bourgeaux V, Gunter KC. International seminar on the red blood cells as vehicles for drugs. Expert Opin Biol Ther. 2012;12:127–133. doi: 10.1517/14712598.2012.631909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bax BE, Bain MD, Fairbanks LD, Webster AD, Ind PW, Hershfield MS, Chalmers RA. A 9-yr evaluation of carrier erythrocyte encapsulated adenosine deaminase (ADA) therapy in a patient with adult-type ADA deficiency. Eur J Haematol. 2007;79:338–348. doi: 10.1111/j.1600-0609.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 38.Pierige F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Bax BE, Bain MD, Scarpelli M, Filosto M, Tonin P, Moran N. Clinical and biochemical improvements in a patient with MNGIE following enzyme replacement. Neurology. 2013;81:1269–1271. doi: 10.1212/WNL.0b013e3182a6cb4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krantz A. Red cell-mediated therapy: opportunities and challenges. Blood Cells Mol Dis. 1997;23:58–68. doi: 10.1006/bcmd.1997.0119. [DOI] [PubMed] [Google Scholar]
- 41.Patel PD, Dand N, Hirlekar RS, Kadam VJ. Drug loaded erythrocytes: as novel drug delivery system. Curr Pharm Des. 2008;14:63–70. doi: 10.2174/138161208783330772. [DOI] [PubMed] [Google Scholar]
- 42.Rossi L, Serafini S, Pierige F, Castro M, Ambrosini MI, Knafelz D, Damonte G, Annese V, Latiano A, Bossa F, Magnani M. Erythrocytes as a controlled drug delivery system: clinical evidences. J Control Release. 2006;116:e43–45. doi: 10.1016/j.jconrel.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Pérez MT, Alvarez FJ, García-Pérez AI, Lucas L, Tejedor MC, Sancho P. Heterogeneity of hypotonically loaded rat erythrocyte populations as detected by counter-current distribution in aqueous polymer two-phase systems. J Chromatogr B Biomed Appl. 1996;677:45–51. doi: 10.1016/0378-4347(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez FJ, Herraez A, Murciano JC, Jordan JA, Diez JC, Tejedor MC. In vivo survival and organ uptake of loaded carrier rat erythrocytes. J Biochem. 1996;120:286–291. doi: 10.1093/oxfordjournals.jbchem.a021411. [DOI] [PubMed] [Google Scholar]
- 45.Ktavtzoff R, Desbois I, Doinel C, Colombat P, Lamagnere JP, Chassaigne M, Ropars C. Immunological response to L-asparaginase loaded into red blood cells. Adv Exp Med Biol. 1992;326:175–182. doi: 10.1007/978-1-4615-3030-5_21. [DOI] [PubMed] [Google Scholar]
- 46.Tonetti M, Astroff B, Satterfield W, De Flora A, Benatti U, DeLoach JR. Construction and characterization of adriamycin-loaded canine red blood cells as a potential slow delivery system. Biotechnol Appl Biochem. 1990;12:621–629. [PubMed] [Google Scholar]
- 47.Teisseire B, Ropars C, Villereal MC, Nicolau C. Long-term physiological effects of enhanced O2 release by inositol hexaphosphate-loaded erythrocytes. Proc Natl Acad Sci U S A. 1987;84:6894–6898. doi: 10.1073/pnas.84.19.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hambye AS, Verbeke KA, Vandermeiren RP, Joosens EJ, Verbruggen AM, De Roo MJ. Comparison of modified technetium-99m albumin and technetium-99m red blood cells for equilibrium ventriculography. J Nucl Med. 1997;38:1521–1528. [PubMed] [Google Scholar]
- 49.Kravtzoff R, Colombat PH, Desbois I, Linassier C, Muh JP, Philip T, Blay JY, Gardenbas M, Poumier-Gaschard P, Lamagnere JP, Chassaigne M, Ropars C. Tolerance evaluation of L-asparaginase loaded in red blood cells. Eur J Clin Pharmacol. 1996;51:221–225. doi: 10.1007/s002280050187. [DOI] [PubMed] [Google Scholar]
- 50.Kravtzoff R, Desbois I, Lamagnere JP, Muh JP, Valat C, Chassaigne M, Colombat P, Ropars C. Improved pharmacodynamics of L-asparaginase-loaded in human red blood cells. Eur J Clin Pharmacol. 1996;49:465–470. doi: 10.1007/BF00195932. [DOI] [PubMed] [Google Scholar]
- 51.Chessa L, Leuzzi V, Plebani A, Soresina A, Micheli R, D'Agnano D, Venturi T, Molinaro A, Fazzi E, Marini M, Ferremi Leali P, Quinti I, Cavaliere FM, Girelli G, Pietrogrande MC, Finocchi A, Tabolli S, Abeni D, Magnani M. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial. Orphanet J Rare Dis. 2014;9:5. doi: 10.1186/1750-1172-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muzykantov VR, Zaltsman AB, Smirnov MD, Samokhin GP, Morgan BP. Target-sensitive immunoerythrocytes: interaction of biotinylated red blood cells with immobilized avidin induces their lysis by complement. Biochim Biophys Acta. 1996;1279:137–143. doi: 10.1016/0005-2736(95)00260-x. [DOI] [PubMed] [Google Scholar]
- 53.Magnani M, Mancini U, Bianchi M, Fazi A. Comparison of uricase-bound and uricase-loaded erythrocytes as bioreactors for uric acid degradation. Adv Exp Med Biol. 1992;326:189–194. doi: 10.1007/978-1-4615-3030-5_23. [DOI] [PubMed] [Google Scholar]
- 54.Bayer EA, Safars M, Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem. 1987;161:262–271. doi: 10.1016/0003-2697(87)90450-7. [DOI] [PubMed] [Google Scholar]
- 55.Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am J Pathol. 1987;128:276–285. [PMC free article] [PubMed] [Google Scholar]
- 56.Wilchek M, Ben-Hur H, Bayer EA. p-Diazobenzoyl biocytin--a new biotinylating reagent for the labeling of tyrosines and histidines in proteins. Biochem Biophys Res Commun. 1986;138:872–879. doi: 10.1016/s0006-291x(86)80577-0. [DOI] [PubMed] [Google Scholar]
- 57.Shi G, Mukthavaram R, Kesari S, Simberg D. Distearoyl anchor-painted erythrocytes with prolonged ligand retention and circulation properties in vivo. Adv Healthc Mater. 2014;3:142–148. doi: 10.1002/adhm.201300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiarantini L, Droleskey R, Magnani M, DeLoach JR. In vitro targeting of erythrocytes to cytotoxic T-cells by coupling of Thy-1.2 monoclonal antibody. Biotechnol Appl Biochem. 1992;15:171–184. [PubMed] [Google Scholar]
- 59.Smirnov VN, Domogatsky SP, Dolgov VV, Hvatov VB, Klibanov AL, Koteliansky VE, Muzykantov VR, Repin VS, Samokhin GP, Shekhonin BV, et al. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc Natl Acad Sci U S A. 1986;83:6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin acylation prevents the complement-dependent lysis of avidin-carrying erythrocytes. Biochem J. 1991;273(Pt 2):393–397. doi: 10.1042/bj2730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muzykantov VR, Seregina N, Smirnov MD. Fast lysis by complement and uptake by liver of avidin-carrying biotinylated erythrocytes. Int J Artif Organs. 1992;15:622–627. [PubMed] [Google Scholar]
- 62.Muzykantov VR, Smirnov MD, Samokhin GP. Streptavidin-induced lysis of homologous biotinylated erythrocytes. Evidence against the key role of the avidin charge in complement activation via the alternative pathway. FEBS Lett. 1991;280:112–114. doi: 10.1016/0014-5793(91)80216-p. [DOI] [PubMed] [Google Scholar]
- 63.Muzykantov VR, Murciano JC. Attachment of antibody to biotinylated red blood cells: immuno-red blood cells display high affinity to immobilized antigen and normal biodistribution in rats. Biotechnol Appl Biochem. 1996;24:41–45. [PubMed] [Google Scholar]
- 64.Muzykantov VR, Smirnov MD, Klibanov AL. Avidin attachment to red blood cells via a phospholipid derivative of biotin provides complement-resistant immunoerythrocytes. J Immunol Methods. 1993;158:183–190. doi: 10.1016/0022-1759(93)90212-p. [DOI] [PubMed] [Google Scholar]
- 65.Müller M, Büchi L, Woodtli K, Haeberli A, Beer JH. Preparation and characterization of ‘heparinocytes’: erythrocytes with covalently bound low molecular weight heparin. FEBS Lett. 2000;468:115–119. doi: 10.1016/s0014-5793(00)01204-7. [DOI] [PubMed] [Google Scholar]
- 66.Muzykantov VR, Sakharov DV, Smirnov MD, Samokhin GP, Smirnov VN. Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot. Biochim Biophys Acta. 1986;884:355–362. doi: 10.1016/0304-4165(86)90184-4. [DOI] [PubMed] [Google Scholar]
- 67.Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther. 2005;312:1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 68.Murciano JC, Higazi AA, Cines DB, Muzykantov VR. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J Control Release. 2009;139:190–196. doi: 10.1016/j.jconrel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21:891–896. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 70.Villa CH, Muzykantov VR, Cines DB. The emerging role for red blood cells in haemostasis: opportunity for intervention. ISBT Science Series. 2016;11:158–164. [Google Scholar]
- 71.Armstead WM, Christine AJ, Higazi AA, Cines DB. Urokinase plasminogen activator impairs SNP and PGE2 cerebrovasodilation after brain injury through activation of LRP and ERK MAPK. J Neurotrauma. 2008;25:1375–1381. doi: 10.1089/neu.2008.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005;36:2265–2269. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- 73.Armstead WM, Ganguly K, Riley J, Kiessling JW, Cines DB, Higazi AA, Zaitsev S, Muzykantov VR. Red blood cell-coupled tissue plasminogen activator prevents impairment of cerebral vasodilatory responses through inhibition of c-Jun-N-terminal kinase and potentiation of p38 mitogen-activated protein kinase after cerebral photothrombosis in the newborn pig. Pediatr Crit Care Med. 2011;12:e369–375. doi: 10.1097/PCC.0b013e3181fe40a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Danielyan K, Ganguly K, Ding BS, Atochin D, Zaitsev S, Murciano JC, Huang PL, Kasner SE, Cines DB, Muzykantov VR. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118:1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29:1463–1474. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor RP, Sutherland WM, Reist CJ, Webb DJ, Wright EL, Labuguen RH. Use of heteropolymeric monoclonal antibodies to attach antigens to the C3b receptor of human erythrocytes: a potential therapeutic treatment. Proc Natl Acad Sci U S A. 1991;88:3305–3309. doi: 10.1073/pnas.88.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaitsev S, Danielyan K, Murciano JC, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108:1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spitzer D, Unsinger J, Bessler M, Atkinson JP. ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement. Mol Immunol. 2004;40:911–9. doi: 10.1016/j.molimm.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 79.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106:4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oudin S, Libyh MT, Goossens D, Dervillez X, Philbert F, Reveil B, Bougy F, Tabary T, Rouger P, Klatzmann D, Cohen JH. A soluble recombinant multimeric anti-Rh(D) single-chain Fv/CR1 molecule restores the immune complex binding ability of CR1-deficient erythrocytes. J Immunol. 2000;164:1505–1513. doi: 10.4049/jimmunol.164.3.1505. [DOI] [PubMed] [Google Scholar]
- 81.Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 82.Spitzer D, Unsinger J, Mao D, Atkinson JP, Spitzer D, Molina H, Wu X. In vivo correction of complement regulatory protein deficiency with an inhibitor targeting the red blood cell membrane. J Immunol. 2005;175:7763–70. doi: 10.4049/jimmunol.175.11.7763. [DOI] [PubMed] [Google Scholar]
- 83.Spitzer D, Wu X, Ma X, Xu L, Ponder KP, Atkinson JP. Cutting edge: treatment of complement regulatory protein deficiency by retroviral in vivo gene therapy. J Immunol. 2006;177:4953–6. doi: 10.4049/jimmunol.177.8.4953. [DOI] [PubMed] [Google Scholar]
- 84.Zaitsev S, Spitzer D, Murciano JC, Ding BS, Tliba S, Kowalska MA, Bdeir K, Kuo A, Stepanova V, Atkinson JP, Poncz M, Cines DB, Muzykantov VR. Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fibrinolysis. J Pharmacol Exp Ther. 2010;332:1022–1031. doi: 10.1124/jpet.109.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zaitsev S, Spitzer D, Murciano JC, Ding BS, Tliba S, Kowalska MA, Marcos-Contreras OA, Kuo A, Stepanova V, Atkinson JP, Poncz M, Cines DB, Muzykantov VR. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood. 2010;115:5241–5248. doi: 10.1182/blood-2010-01-261610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zaitsev S, Kowalska MA, Neyman M, Carnemolla R, Tliba S, Ding BS, Stonestrom A, Spitzer D, Atkinson JP, Poncz M, Cines DB, Esmon CT, Muzykantov VR. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood. 2012;119:4779–4785. doi: 10.1182/blood-2011-12-398149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burton NM, Bruce LJ. Modelling the structure of the red cell membrane. Biochem Cell Biol. 2011;89:200–215. doi: 10.1139/o10-154. [DOI] [PubMed] [Google Scholar]
- 88.Taylor RP, Sutherland WM, Martin EN, Ferguson PJ, Reinagel ML, Gilbert E, Lopez K, Incardona NL, Ochs HD. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J Immunol. 1997;158:842–850. [PubMed] [Google Scholar]
- 89.Repik A, Pincus SE, Ghiran I, Nicholson-Weller A, Asher DR, Cerny AM, Casey LS, Jones SM, Jones SN, Mohamed N, Klickstein LB, Spitalny G, Finberg RW. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1) Clin Exp Immunol. 2005;140:230–240. doi: 10.1111/j.1365-2249.2005.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reist CJ, Liang HY, Denny D, Martin EN, Scheld WM, Taylor RP. Cross-linked bispecific monoclonal antibody heteropolymers facilitate the clearance of human IgM from the circulation of squirrel monkeys. Eur J Immunol. 1994;24:2018–2025. doi: 10.1002/eji.1830240913. [DOI] [PubMed] [Google Scholar]
- 91.Taylor RP, Martin EN, Reinagel ML, Nardin A, Craig M, Choice Q, Schlimgen R, Greenbaum S, Incardona NL, Ochs HD. Bispecific monoclonal antibody complexes facilitate erythrocyte binding and liver clearance of a prototype particulate pathogen in a monkey model. J Immunol. 1997;159:4035–4044. [PubMed] [Google Scholar]
- 92.Kontos S, Hubbell JA. Improving protein pharmacokinetics by engineering erythrocyte affinity. Mol Pharm. 2010;7:2141–2147. doi: 10.1021/mp1001697. [DOI] [PubMed] [Google Scholar]
- 93.Sahoo K, Koralege RS, Flynn N, Koteeswaran S, Clark P, Hartson S, Liu J, Ramsey JD, Pope C, Ranjan A. Nanoparticle Attachment to Erythrocyte Via the Glycophorin A Targeted ERY1 Ligand Enhances Binding without Impacting Cellular Function. Pharm Res. 2016;33:1191–1203. doi: 10.1007/s11095-016-1864-x. [DOI] [PubMed] [Google Scholar]
- 94.Paulitschke M, Nash GB, Anstee DJ, Tanner MJ, Gratzer WB. Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood. 1995;86:342–348. [PubMed] [Google Scholar]
- 95.Gallagher PG, Chang SH, Rettig MP, Neely JE, Hillery CA, Smith BD, Low PS. Altered erythrocyte endothelial adherence and membrane phospholipid asymmetry in hereditary hydrocytosis. Blood. 2003;101:4625–4627. doi: 10.1182/blood-2001-12-0329. [DOI] [PubMed] [Google Scholar]
- 96.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 97.Chasis JA, Mohandas N, Shohet SB. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J Clin Invest. 1985;75:1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glodek AM, Mirchev R, Golan DE, Khoory JA, Burns JM, Shevkoplyas SS, Nicholson-Weller A, Ghiran IC. Ligation of complement receptor 1 increases erythrocyte membrane deformability. Blood. 2010;116:6063–6071. doi: 10.1182/blood-2010-04-273904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sticher B, Giger U, Naef R, Burger R, Lutz HU. Naturally occurring human anti-band 3 autoantibodies accelerate clearance of erythrocytes in guinea pigs. Blood. 1995;85 [PubMed] [Google Scholar]
- 100.Khoory J, Estanislau J, Elkhal A, Lazaar A, Melhorn MI, Brodsky A, Illigens B, Hamachi I, Kurishita Y, Ivanov AR, Shevkoplyas S, Shapiro NI, Ghiran IC. Ligation of Glycophorin A Generates Reactive Oxygen Species Leading to Decreased Red Blood Cell Function. PLoS One. 2016;11:e0141206. doi: 10.1371/journal.pone.0141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aleman MM, Walton BL, Byrnes JR, Wolberg AS. Fibrinogen and red blood cells in venous thrombosis. Thromb Res. 2014;133(Suppl 1):S38–40. doi: 10.1016/j.thromres.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aarts PA, Heethaar RM, Sixma JJ. Red blood cell deformability influences platelets--vessel wall interaction in flowing blood. Blood. 1984;64:1228–1233. [PubMed] [Google Scholar]
- 103.Willekens FL, Werre JM, Groenen-Dopp YA, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJ. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 104.Koshiar RL, Somajo S, Norstrom E, Dahlback B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS One. 2014;9:e104200. doi: 10.1371/journal.pone.0104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol. 2014;34:313–320. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]
- 106.Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F, Loufrani L, Grimaud L, Lambry JC, Charue D, Kiger L, Renard JM, Larroque C, Le Clesiau H, Tedgui A, Bruneval P, Barja-Fidalgo C, Alexandrou A, Tharaux PL, Boulanger CM, Blanc-Brude OP. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125:3805–3814. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peyrou V, Lormeau JC, Herault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost. 1999;81:400–406. [PubMed] [Google Scholar]
- 108.Closse C, Dachary-Prigent J, Boisseau MR. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br J Haematol. 1999;107:300–302. doi: 10.1046/j.1365-2141.1999.01718.x. [DOI] [PubMed] [Google Scholar]
- 109.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 110.Pasvol G, Chasis JA, Mohandas N, Anstee DJ, Tanner MJ, Merry AH. Inhibition of malarial parasite invasion by monoclonal antibodies against glycophorin A correlates with reduction in red cell membrane deformability. Blood. 1989;74:1836–1843. [PubMed] [Google Scholar]
- 111.Olsson ML, Clausen H. Modifying the red cell surface: towards an ABO-universal blood supply. Br J Haematol. 2008;140:3–12. doi: 10.1111/j.1365-2141.2007.06839.x. [DOI] [PubMed] [Google Scholar]
- 112.Garratty G. Immune hemolytic anemia caused by drugs. Expert Opin Drug Saf. 2012;11:635–642. doi: 10.1517/14740338.2012.678832. [DOI] [PubMed] [Google Scholar]
- 113.Arndt PA, Leger RM, Garratty G. Serologic characteristics of ceftriaxone antibodies in 25 patients with drug-induced immune hemolytic anemia. Transfusion. 2012;52:602–612. doi: 10.1111/j.1537-2995.2011.03321.x. [DOI] [PubMed] [Google Scholar]
- 114.Crookston KP, Reiner AP, Cooper LJ, Sacher RA, Blajchman MA, Heddle NM. RBC T activation and hemolysis: implications for pediatric transfusion management. Transfusion. 2000;40:801–812. doi: 10.1046/j.1537-2995.2000.40070801.x. [DOI] [PubMed] [Google Scholar]
- 115.Heddle NM, Soutar RL, O'Hoski PL, Singer J, McBride JA, Ali MA, Kelton JG. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–1005. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 116.Zimring JC, Stowell SR, Johnsen JM, Hendrickson JE. Effects of genetic, epigenetic, and environmental factors on alloimmunization to transfused antigens: Current paradigms and future considerations. Transfus Clin Biol. 2012;19:125–131. doi: 10.1016/j.tracli.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Ryder AB, Zimring JC, Hendrickson JE. Factors Influencing RBC Alloimmunization: Lessons Learned from Murine Models. Transfus Med Hemother. 2014;41:406–419. doi: 10.1159/000368995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith NH, Henry KL, Cadwell CM, Bennett A, Hendrickson JE, Frame T, Zimring JC. Generation of transgenic mice with antithetical KEL1 and KEL2 human blood group antigens on red blood cells. Transfusion. 2012;52:2620–2630. doi: 10.1111/j.1537-2995.2012.03641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Girard-Pierce KR, Stowell SR, Smith NH, Arthur CM, Sullivan HC, Hendrickson JE, Zimring JC. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. Blood. 2013;122:1793–1801. doi: 10.1182/blood-2013-06-508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liepkalns JS, Cadwell CM, Stowell SR, Hod EA, Spitalnik SL, Zimring JC. Resistance of a subset of red blood cells to clearance by antibodies in a mouse model of incompatible transfusion. Transfusion. 2013;53:1319–1327. doi: 10.1111/j.1537-2995.2012.03910.x. [DOI] [PubMed] [Google Scholar]
- 121.Hudson KE, Hendrickson JE, Cadwell CM, Iwakoshi NN, Zimring JC. Partial tolerance of autoreactive B and T cells to erythrocyte-specific self-antigens in mice. Haematologica. 2012;97:1836–1844. doi: 10.3324/haematol.2012.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci U S A. 2013;110:E60–68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci Adv. 2015;1:e1500112. doi: 10.1126/sciadv.1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA. Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci Rep. 2015;5:15907. doi: 10.1038/srep15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murray AM, Pearson IF, Fairbanks LD, Chalmers RA, Bain MD, Bax BE. The mouse immune response to carrier erythrocyte entrapped antigens. Vaccine. 2006;24:6129–6139. doi: 10.1016/j.vaccine.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 126.Smith NH, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE. Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs. Blood. 2012;119:1566–1569. doi: 10.1182/blood-2011-09-382655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Richards AL, Hendrickson JE, Zimring JC, Hudson KE. Erythrophagocytosis by plasmacytoid dendritic cells and monocytes is enhanced during inflammation. Transfusion. 2016;56:905–916. doi: 10.1111/trf.13497. [DOI] [PubMed] [Google Scholar]
- 128.Gersh KC, Zaitsev S, Cines DB, Muzykantov V, Weisel JW. Flow-dependent channel formation in clots by an erythrocyte-bound fibrinolytic agent. Blood. 2011;117:4964–4967. doi: 10.1182/blood-2010-10-310409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gersh KC, Zaitsev S, Muzykantov V, Cines DB, Weisel JW. The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics. J Thromb Haemost. 2010;8:1066–1074. doi: 10.1111/j.1538-7836.2010.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. doi: 10.3389/fphys.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–1485. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 132.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10(Suppl 2):s7–11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 134.Aabb . Standards for Perioperative Autologous Blood Collection and Administration. American Association of Blood Banks (AABB); 2012. [Google Scholar]
- 135.Tabibian JH, Wong Kee Song LM, Enders FB, Aguet JC, Tabibian N. Technetium-labeled erythrocyte scintigraphy in acute gastrointestinal bleeding. Int J Colorectal Dis. 2013;28:1099–1105. doi: 10.1007/s00384-013-1658-0. [DOI] [PubMed] [Google Scholar]
- 136.U.S.F.a.D. Administration Blood and Blood Products. 2014 http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/
- 137.Shi J, Kundrat L, Pishesha N, Bilate A, Theile C, Maruyama T, Dougan SK, Ploegh HL, Lodish HF. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc Natl Acad Sci U S A. 2014;111:10131–10136. doi: 10.1073/pnas.1409861111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec PY, Francois S, Trugnan G, Peyrard T, Marie T, Jolly S, Hebert N, Mazurier C, Mario N, Harmand L, Lapillonne H, Devaux JY, Douay L. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Risbano MG, Kanias T, Triulzi D, Donadee C, Barge S, Badlam J, Jain S, Belanger AM, Kim-Shapiro DB, Gladwin MT. Effects of Aged Stored Autologous Red Blood Cells on Human Endothelial Function. Am J Respir Crit Care Med. 2015;192:1223–1233. doi: 10.1164/rccm.201501-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–885. doi: 10.1111/j.1537-2995.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zimring JC. Established and theoretical factors to consider in assessing the red cell storage lesion. Blood. 2015;125:2185–2190. doi: 10.1182/blood-2014-11-567750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cluitmans JC, Hardeman MR, Dinkla S, Brock R, Bosman GJ. Red blood cell deformability during storage: towards functional proteomics and metabolomics in the Blood Bank. Blood Transfus. 2012;10(Suppl 2):s12–18. doi: 10.2450/2012.004S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 144.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, Carson JL, Levy JH, Whitman G, D'Andrea P, Pulkrabek S, Ortel TL, Bornikova L, Raife T, Puca KE, Kaufman RM, Nuttall GA, Young PP, Youssef S, Engelman R, Greilich PE, Miles R, Josephson CD, Bracey A, Cooke R, McCullough J, Hunsaker R, Uhl L, McFarland JG, Park Y, Cushing MM, Klodell CT, Karanam R, Roberts PR, Dyke C, Hod EA, Stowell CP. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, Ddungu H, Kyeyune D, Musisi E, Opoka R, Stowell CP, Dzik WH. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA. 2015;314:2514–2523. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 146.Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, Blajchman MA, Walsh TS, Stanworth SJ, Campbell H, Capellier G, Tiberghien P, Bardiaux L, van de Watering L, van der Meer NJ, Sabri E, Vo D, A. Investigators. G. Canadian Critical Care Trials Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]