Abstract
Aberrant expression of the kinase IKKε in pancreatic ductal adenocarcinoma (PDAC) has been associated with poor prognosis. In this study, we define a pathobiologic function for IKKε in reprogramming glucose metabolism and driving progression in PDAC. Silencing IKKε in PDAC cells, which overexpressed it endogenously, was sufficient to reduce malignant cell growth, clonogenic potential, glucose consumption, lactate secretion and expression of genes involved in glucose metabolism, without impacting the basal oxygen-consumption rate. IKKε silencing also attenuated c-Myc in a manner associated with diminished signaling through an AKT/GSK3β/c-MYC phosphorylation cascade that promoted MYC nuclear accumulation. In an orthotopic mouse model, IKKε-silenced PDAC exhibited a relative reduction in glucose uptake, tumorigenicity and metastasis. Overall, our findings offer a preclinical mechanistic rationale to target IKKε to improve the therapeutic management of PDAC in patients.
Keywords: IKKε, pancreatic cancer, c-MYC, glycolytic metabolism
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies with a 5-year survival rate of about 8% after initial diagnosis (1). It is expected to overtake breast malignancy this year as the third-leading cause of cancer-related deaths in the United States with an estimated 53,070 new diagnoses and 41,780 deaths, and may actually become the second by 2020 if similar trends continue (1, 2). Over the years, significant progress has been made in our understanding of the genetics of PDAC (3); however, these seminal advancements have not helped much in the development of an effective treatment strategy for this lethal malignancy. As a consequence, search for novel, functionally-relevant molecular targets continues, so that effective, mechanism-based approaches for its therapy and management can be formulated.
Inhibitor of kappa kinase subunit-epsilon (IKKε) is an important member of the IKK family along with four other, distinct yet closely-related, members (IKKα, IKKβ, IKKγ and NAK). IKKε plays a central role in innate immunity by inducing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)- and interferon regulatory factor (IRF)-dependent gene transcription of pro-inflammatory cytokines and interferons (4). It is expressed at basal levels in a subset of tissues involved in immune function, and can be readily induced in a variety of cell- and tissue-types upon external stimuli (5). Interestingly, IKKε has also been shown to regulate energy-balance in high fat diet-induced obesity (6) and recognized to possess oncogenic properties in breast cancer (7) with some later reports in other cancers as well (8, 9). In many cancer cases, including PDAC, an upregulation of IKKε, even in the absence of gene-amplification, has been reported and associated with poor clinical outcome (7, 9, 10). However, we lack direct evidence for its oncogenic activity in PDAC along with complete lack of an in-depth understanding of involved molecular pathways.
Cancer cells remain under constant demand for energy and building-blocks to ensure their continued, rapidly-proliferative development. As a result, they adapt to glycolytic metabolism, even when oxygen is not a limiting factor, to meet their demands for quick energy (ATPs) and metabolic intermediates that serve as building-blocks for rapidly dividing cancer cells (11). This shift provides added advantage to the tumor cells i.e. the ability to thrive independently of oxygen diffusion that would otherwise be a limiting factor for rapidly growing tumors (12). Indeed, mounting evidence continues to associate enhanced aerobic-glycolysis to the etiology and malignant progression of several cancers, including pancreatic malignancy (13). This metabolic-shift, in general, is mediated through aberrant activation of oncogenic transcription factors leading to altered expression of genes involved in glucose-import and –metabolism (14). c-MYC serves as a ‘master-regulator’ of growth and cellular metabolism pathways, and its aberrant activation is facilitated at multiple levels (15–17). Emerging clinical and experimental data also support its role in PDAC pathobiology (10).
The present study provides first evidence for a link between IKKε and c-MYC oncoprotein. We demonstrate that IKKε regulates nuclear-retention and stabilization of c-MYC through a cascade of signaling events. We further identify a novel role of IKKε in regulating glucose-metabolism in PDAC, at least in part, through its c-MYC-mediated regulation of metabolic gene expression. IKKε overexpression is also shown to promote growth and metastasis of PDAC cells, thus establishing it as an important molecular target for clinical management.
Materials and Methods
Cell lines and tissue samples
The human pancreatic cell lines were obtained and maintained as previously described (18). All the cell lines were tested intermittently and determined to be free from mycoplasma, and authenticated by either in-house or commercial (Genetica DNA Laboratories, Burlington, NC) short-tandem repeats genotyping. Normal and tumor pancreatic tissue specimens were obtained through the Southern Division of Cooperative Human Tissue Network (CHTN) under an Institutional Review Board-approved protocol.
Antibodies
Antibodies used were: anti-IKKε, -c-MYC (rabbit-monoclonal), -phospho-c-MYCS62, -phospho-c-MYCT58 (rabbit-polyclonal), -ubiquitin (mouse-monoclonal) (Abcam, Cambridge, MA); -phospho-AktT308 (rabbit-monoclonal), -phospho-AktSer473 (rabbit-polyclonal) (Cell Signaling Technologies, Beverly, MA); -GSK3β, -phospho-GSK3βSer9, -Akt (rabbit-monoclonal) (Epitomics, Burlingame, CA); -LaminA, (mouse-monoclonal), -α-tubulin (rabbit-polyclonal) (Santa-Cruz Biotechnology, Dallas, TX). Anti-β-actin-HRP-conjugated (mouse-monoclonal) antibody was from Sigma-Aldrich (St. Louis, MO). All secondary antibodies were from Santa-Cruz Biotechnology.
Transfections and treatments
Generation of stable IKKε-knockdown and control cell lines was done in IKKε-overexpressing MiaPaCa and Colo357 cells by transfection of IKBKE-shRNA-pGFP-B-RS or the control-plasmid, Scr-shRNA-pGFP-B-RS (Origene, Rockville, MD), respectively, using X-tremeGENE HP DNA Transfection Reagent (Roche, Indianapolis, IN) as per the manufacturer’s instructions. Transfectants were selected using blasticidin (2µg/ml) and assessed for IKKε-expression using immunoblotting. For transient knockdown, cells were cultured in 6-well plates and transfected with 100 nM of non-target or ON-TARGETplus SMARTpool IKBKE-targeting siRNAs (Dharmacon, Lafayette, CO) using DharmaFECT (Dharmacon) according to the manufacturer’s instruction. Cyclohexamide (50µM; Sigma-Aldrich) and MG132 (10µM; Sigma-Aldrich) were used to inhibit protein-synthesis or proteasome-degradation machinery, respectively. Cells were treated with GSK3β-inhibitor LiCl (40mM; Sigma-Aldrich) for 6h or transiently-transfected with constitutively active-Akt (pcDNA3-HA PKB T308D S473D, plasmid number 14751, Addgene, Cambridge, MA) mutant or control plasmids to dissect the roles of GSK3β and Akt, respectively.
Immunoblot analysis
Total protein from PDAC cells was isolated in NP-40 lysis buffer supplemented with phosphatase and protease inhibitors (Roche), and estimated using DC Protein-Assay Kit (BioRad, Hercules, CA). Protein samples (60–80 µg, unless noted) were resolved on SDS-PAGE and subjected to immunoblot analysis as described (18, 19). Band intensities were quantitated using ImageJ software.
Growth kinetics assay
Growth rate and population-doubling time (PDT) were determined by counting number of viable cells using the trypan blue dye-exclusion on the Countess® Automated Cell-Counter (Life technology, Carlsbad, CA) every day for eight days, as described (18).
Clonogenicity assays
Anchorage-dependent and anchorage-independent clonogenicity assays were carried out as described (20).
Quantitative polymerase chain reaction (qPCR) and Ingenuity pathway analysis
RNA isolation, cDNA-synthesis and qPCR were performed as described (20) using primers listed in Supplementary Table 2. The altered genes (fold-change≥1.5; p-value≤0.05) were subjected to Ingenuity Pathway Analysis® (IPA®) to identify putative upstream regulator.
Luciferase assay
Control or IKKε-silenced PDAC cells were transfected with either a negative control or c-MYC-responsive luciferase-promoter-reporter plasmid (Cignal MYC Reporter Assay Kit, SABiosciences), and assayed as per manufacturer’s protocol.
Nuclear and cytoplasmic fractionation
Cytoplasmic- and nuclear-extracts were prepared using Nuclear-Extract Kit (Active Motif, Carlsbad, CA) following the manufacturer’s instructions.
Co-immunoprecipitation analysis
Co-immunoprecipitation was performed using c-MYC-specific antibody as described (21).
Glucose uptake and lactate production assays
Glucose and lactate concentration in the culture-media was determined using the glucose- and lactate-assay kit (Biovision, Milpitas, CA) as per manufacturer’s instructions. To measure glucose-uptake, cells were first incubated in glucose-free, FBS-free media for 6h followed by incubation with a glucose free DMEM media supplemented with 100µM of fluorescent D-glucose derivative, 2-NBDG (Invitrogen) for 3h and analyzed by fluorescence imaging or flow cytometry using FACS AriaII™ (BD Bioscience, San Jose, CA).
Measurement of ECAR and OCR
Basal rate of glycolysis and oxidative phosphorylation was determined by measuring the extracellular-acidification (ECAR) and the oxygen-consumption rates (OCR) using the Seahorse-XF24 Analyzer (Seahorse Bioscience, Billerica, MA) as per manufacturer’s instructions. Briefly, 4×104 cells/well were seeded in XF24 cell-culture microplates in a two-step process and incubated at 37°C for 24h. Subsequently, culture-media was changed to XF Assay Medium (supplemented with 5mM Glucose), and plates loaded into the XF24 analyzer to record the data.
Assessment of tumorigenicity and glucose uptake in vivo
Animal studies were conducted under protocol approved by Institutional Animal Care and Use Committee. Luciferase-tagged IKKε-knockdown or control cells (1×106cells/50µL) were injected into the pancreas of immunocompromised mice (Harlan Laboratories, Prattville, AL, USA) (10 mice/group). After 1 week, tumor growth was monitored every third day by palpation and weekly by non-invasive in vivo imaging. For glucose-uptake, mice were injected i.p. with 100µL of XenoLight RediJect 2-DeoxyGlucosone (2-DG) (Perkin Elmer, Waltham, MA) 24h prior to final imaging (28 days post-implantation) and epi-fluorescence recorded using Xenogen-IVIS-cooled CCD optical system (IVIS Spectrum) next day. To image tumors, mice were given i.p. injections of D-Luciferin (150 mg kg−1 body-weight) and bioluminescence recorded using the Xenogen-IVIS-cooled CCD optical system. Following euthanasia, primary tumors were resected and mice imaged for detection of metastases. Final measurements of tumor-weight and volumes were also made from resected xenografts.
WST-1 proliferation assay
Cells (3×103/well) were seeded in 96-well plates and growth examined using WST-1 assay kit (Roche) and analyzed as described (19).
Statistical analysis
The experiments were conducted in triplicates and repeated at least three times. Wherever appropriate, the data were also subjected to unpaired two-tailed Student’s t-test. p≤0.05 was considered significant.
Results
IKKε is overexpressed and associated with increased growth and clonogenicity of PDAC cells
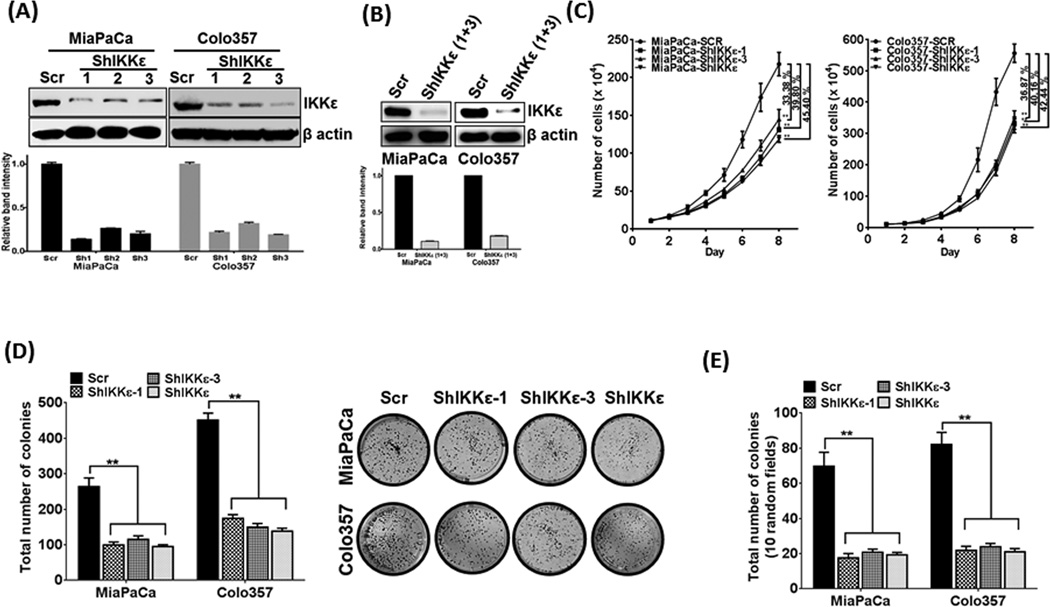
We first analyzed IKKε expression in a set of malignant (n=21) and non-neoplastic (n=7) pancreas. Similar to a published report (10), we observed an overexpression of IKKε in 81% cases of malignant pancreas, while no (n=5) or very low (n=2) expression was detected in non-neoplastic cases (Supplementary figure 1A). In search of model cell lines for functional studies, we analyzed IKKε expression in a panel of 14 PDAC cell lines, of which, 12 expressed high to moderate levels, while 2 had low/null expression (Supplementary figure 1B). Considering that IKKε-overexpressing cells would likely have IKKε-dependent growth mechanisms, we selected MiaPaCa and Colo357 cells that carry most common PDAC genetic aberrations (K-Rasmut, TP53mut, CDKN2Adel/inactive) and have been well characterized for their aggressiveness and metastatic potential (22, 23) for functional studies. IKKε expression was silenced by stable transfection of three different IKBKE-targeted shRNA-expression constructs (ShIKKε#1–3). Control cells were generated by stable transfections with non-targeted scrambled sequence (Scr)-expression construct. Stable silencing of IKKε was analyzed by immunoblotting in polyclonal populations from individual constructs (Figure 1A). As ShIKKε plasmid #1 and #3-transfectants exhibited most potent reduction in IKKε, compared to control, they were combined to generate pooled population (ShIKKε) (Figure 1B). IKKε inhibition resulted in significant growth reduction in MiaPaCa (~45.4%) and Colo357 (~42.4%) cells on the eight day of growth kinetics (Figure 1C) due to increase in their PDT calculated during exponential growth-phase (96–144 h). Silencing of IKKε prolonged PDT from ~36.46h and ~21.14h to ~45.71h and ~30.37h in MiaPaCa-ShIKKε and Colo357-ShIKKε cells, respectively, relative to their controls (Supplementary table 2). Moreover, when seeded at low density, we observed a significant decrease (p<0.05) in plating-efficiency of MiaPaCa-ShIKKε (~2.8 fold) and Colo357-ShIKKε (~3.2 fold) cells as compared to their controls (Figure 1D). A significant (p<0.05) decrease (~3.5 and ~3.9 fold, respectively) was also recorded in anchorage-independent clonogenic potential (an in-vitro measure of tumorigenecity) of MiaPaCa-ShIKKε and Colo357-ShIKKε cells relative to their controls (Figure 1E). To further confirm the role of IKKε in pancreatic cancer cell growth, we also ectopically overexpressed IKKε in BxPC3 cells that has its low endogenous expression (Supplementary Figure 2A). IKKε-overexpression led to increase in growth of BxPC3 cells by ~37.94% on eighth day of growth kinetics due to decrease in doubling time (Supplementary figure 2B).
Figure 1. IKKε supports the growth and clonogenicity of PDAC cells.
(A) Total protein isolated from stably transfected PDAC cells was examined for IKKε expression by immunoblotting. (B) Clonal population of transfectants emerging from IKKε-targeting ShRNA constructs #1 and #3, which consistently produced maximum silencing of IKKε, were pooled (ShIKKε), propagated and monitored for IKKε expression by immunoblot assay. β-actin was used as loading control. (C) Growth kinetics of IKKε-silenced clonal and pooled population was studied relative to their controls for eight days using the Trypan-blue exclusion assay. (D) Plating-efficiency was measured by seeding the PDAC cells at low density. (E) Anchorage-independent colony formation was measured as an in vitro measure of tumorigenic ability of control and IKKε-silenced PDAC cells. Data is presented as mean ± S.D., n=3; **p≤0.05.
Silencing of IKKε inhibits glycolytic metabolism in PC
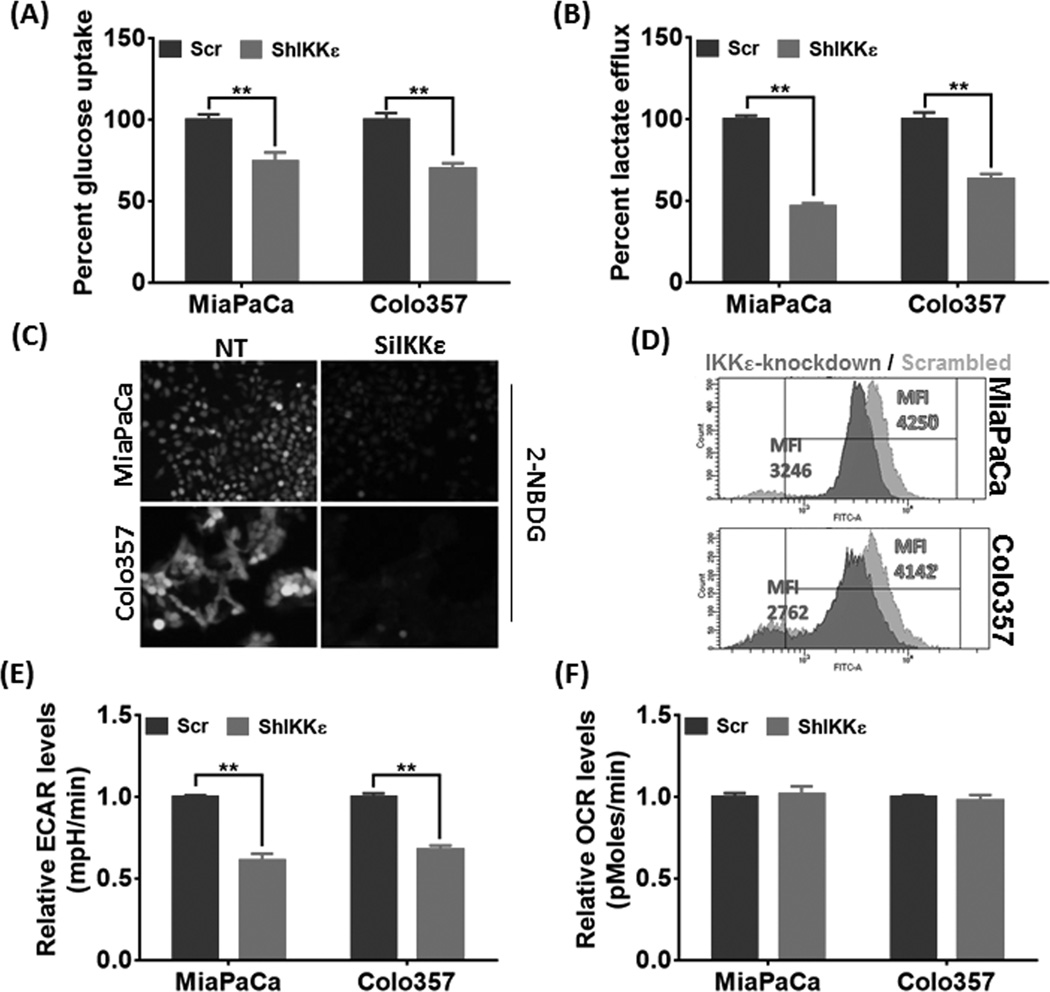
As a defined hallmark, tumor cells sustain their rapid growth by shifting to glycolytic metabolism to ensure quick and sufficient supply of energy and macromolecular precursors (11, 14). Therefore, we evaluated the influence of IKKε-silencing on glucose metabolism in PDAC cells. First, consumption of glucose and the lactate release was measured from the used culture media of control and IKKε-silenced PDAC cells, which demonstrated substantial attenuation of glucose-consumption (Figure 2A) and lactate-secretion (Figure 2B) upon IKKε-silencing. The influence on glucose-uptake in a cell-autonomous manner in control and IKKε-silenced cells was further investigated using the fluorescent glucose analog 2-NBDG, which was imaged by florescence microscopy or quantified using flow-cytometry upon incorporation into the cells. These experiments were carried out after transient silencing of IKKε in MiaPaCa and Colo357 cell lines using ON-TARGETplus SMARTpool IKBKE-targeting siRNAs or non-targeting control siRNAs, since stables lines expressed green fluorescent protein which could hinder 2-NBDG signal detection. IKKε silencing by siRNA was determined at 72h by immunoblotting (Supplementary Figure 3). In line with above observation, cellular accumulation of 2-NBDG was significantly less in IKKε-silenced PDAC cells compared to their control cells (Figure 2C) as quantitative analysis by flow-cytometry revealed a reduction in the mean fluorescence intensity by 23.62% and 33.32% in MiaPaCa-SiIKKε and Colo357-SiIKKε cells, respectively, relative to their controls (Figure 2D). To get further insight into altered metabolic phenotype, we monitored basal glycolytic metabolism and oxidative phosphorylation by ECAR and OCR using Seahorse-XF Extracellular-Flux Analyzer. ECAR is a surrogate measure of glycolysis and an alternate measure for lactate secretion, while OCR measures the changes in dissolved-oxygen concentration. We observed a significant decrease in the glycolytic activity of IKKε-silenced cells relative to control cells; however, no substantial change in oxygen-consumption was recorded (Figures 2E and F). In concordance, overexpression of IKKε in BxPC3 cells enhanced glucose-uptake and lactate-efflux, which was also reflected in elevated ECAR (Supplementary figure 4). This suggests that IKKε silencing impacts glycolytic phenotype only without having noticeable effect on the electron-transport system machinery.
Figure 2. IKKε-silencing suppresses glucose-uptake and consumption in PDAC cells.
(A) Glucose-uptake and (B) lactate-efflux was measured in the used culture-media and normalized to cell counts. The data is depicted as percent change in IKKε-silenced cells relative to their respective controls. (C & D) PDAC cells were transfected with non-targeting control (NT) or IKBKE-targeting (SiIKKε) siRNAs. After 72 hours, cells were cultured in glucose-free FBS-free media for 6 hours and further incubated with glucose-free media supplemented with 100µM 2-NBDG for 3 hours. Thereafter, either the cells were visualized under fluorescent microscope and photographed (C), or subjected to flow cytometry analysis (D). Representative images are from independent experiments. (E & F) To examine basal ECAR and OCR, 4×104 cells/well were seeded in XF24 cell-culture microplates and incubated at 37°C overnight. Next day, culture-media was replaced with XF Assay Medium (supplemented with 5mM Glucose) and plates loaded into the XF24 analyzer. Data is presented as mean ± S.D., n=3; **p≤0.05.
IKKε alters the expression of genes of glucose metabolic pathway that converge at c-MYC
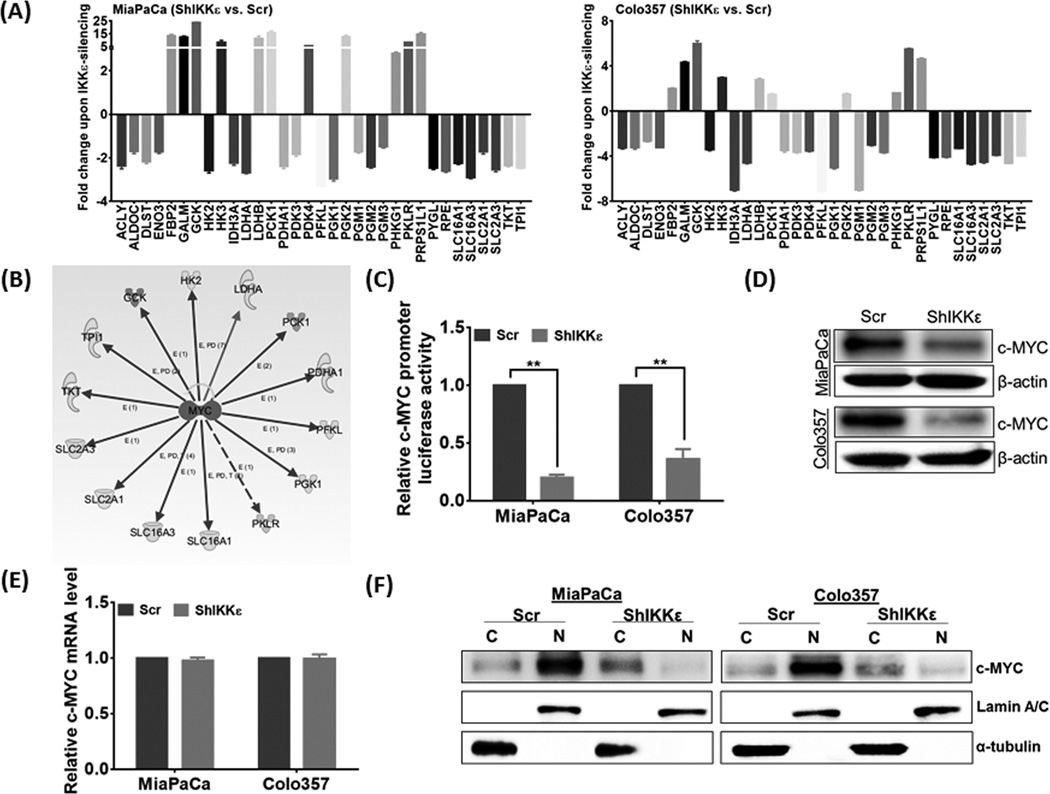
To identify the altered gene expression associated with shift in glucose-metabolism, we employed a qPCR-based custom array that included 80 genes involved in glucose metabolism. A total of 33 genes were found to be dysregulated upon IKKε-silencing including those involved in glucose-transport, glycolysis, gluconeogenesis, TCA cycle, pentose-phosphate pathway, and lactate-transport (Figure 3A). Gene-expression dataset was subjected to the Ingenuity Pathway Analysis (IPA) software in search for a putative upstream transcriptional regulator mediating the effect of IKKε-silencing. In-silico analysis suggested c-MYC to be a candidate upstream regulator of altered gene expression (Figure 3B). To confirm the predicted inhibition of c-MYC, we examined its transcriptional activity using a c-MYC-responsive promoter-reporter system and observed significant abrogation (79% and 63%, p<0.05) in luciferase activity of IKKε-silenced MiaPaCa and Colo357 cells, respectively, relative to their controls (Figure 3C). This correlated with reduction in total c-MYC protein (Figure 3D) without any significant change in its transcripts levels (Figure 3E). Interestingly, subcellular fractionation demonstrated greater nuclear accumulation of c-MYC in IKKε-expressing control cells, while relatively greater cytoplasmic levels of c-MYC were detected in IKKε-silenced cells (Figure 3F). In line with these observations, ectopic expression of IKKε in BxPC3 cells also elevated c-MYC protein level along with greater nuclear accumulation (Supplementary figure 5). These cells; however, sustained significant c-MYC in the cytoplasm, thus suggesting the involvement of additional cell-type and/or context-dependent mechanism(s) in regulation of c-MYC by IKKε.
Figure 3. IKKε alters the expression of genes encoding glucose-metabolizing enzymes and regulates c-MYC expression and sub-cellular localization.
(A) Expression of genes involved in glucose-metabolism was measured by qRT-PCR. Data shown as fold change in IKKε-silenced cells relative to control. (B) Differential gene-expression dataset was subjected to Ingenuity Pathway Analysis that predicted c-MYC as a potential upstream-regulator. (C) Transcriptional activity of c-MYC was measured using luciferase promoter-reporter assay. Data (mean ± S.D, n=3) are presented as fold-change in normalized luciferase activity, **p≤0.05. Expression of c-MYC in IKKε-knockdown and control cells was examined at (D) protein and (E) transcript levels by immunoblot and qRT-PCR assays, respectively. ACTB (for mRNA) and β-actin (for protein) were used as internal controls. (F) Expression of c-MYC in cytoplasmic- and nuclear-fractions was examined by immunoblot analysis. Lamin A and α-tubulin were used as loading controls for nuclear and cytoplasmic fractions, respectively.
c-MYC downregulation upon IKKε-silencing is caused by its nuclear export and subsequent proteasomal degradation
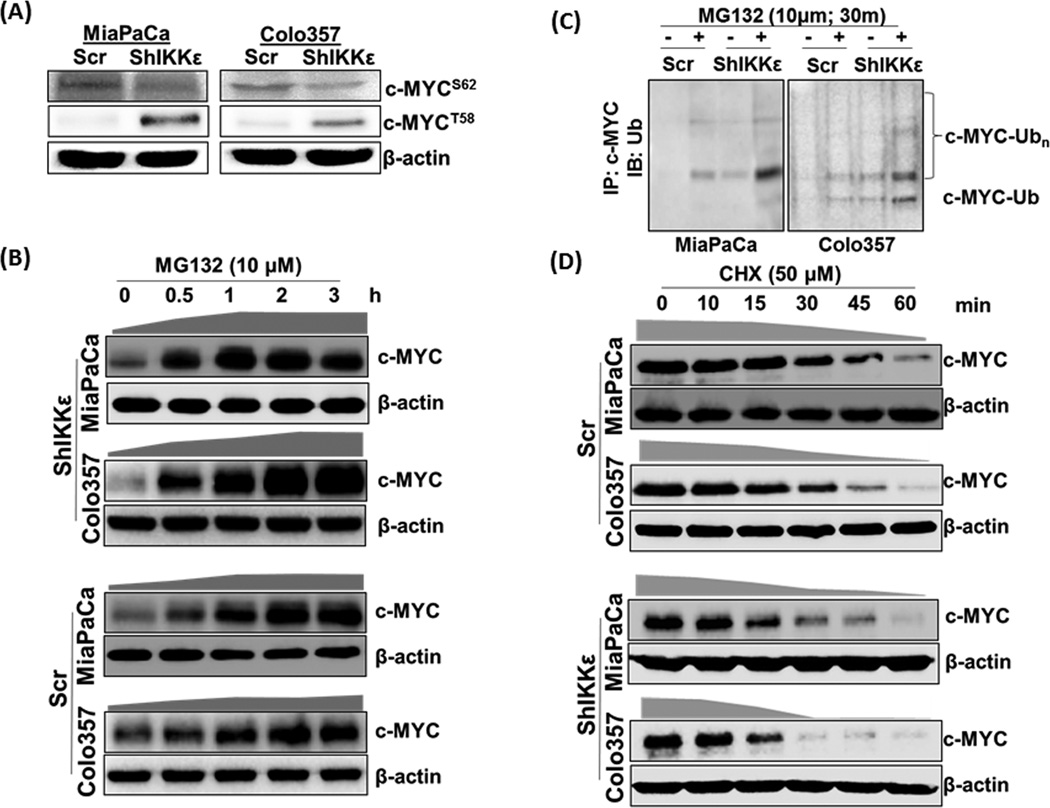
Differential subcellular distribution of c-MYC besides its overall repression at the protein level prompted us to investigate underlying molecular mechanism(s). Phosphorylation at conserved serine-62 (S62) residue of c-MYC governs the activation and nuclear import of c-MYC and subsequent phosphorylation of threonine-58 (T58) residue leads to its nuclear export followed by degradation (15–17). Therefore, we examined their levels in IKKε-overexpressing and –silenced PDAC cells, which demonstrated a decrease in phospho-c-MYCS62 in IKKε-silenced cells (Figure 4A) likely due to diminished total C-MYC protein content. However, despite reduced levels of total c-MYC, IKKε-silenced PDAC cells had significantly increased levels of phospho-c-MYCT58, while negligible c-MYCT58 phosphorylation was detected in control cells of both PDAC lines (Figure 4A). A similar correlation between differential c-MYC-phosphorylation and IKKε was also observed upon forced IKKε expression in BxPC3 cells (Supplementary figure 5). Since c-MYCT58 phosphorylation is known to promote its nuclear export followed by ubiquitin (Ub)-mediated proteasomal degradation (15–17), we next examined the effect of MG-132 (proteasome inhibitor)-treatment on c-MYC levels. To correct the differences in initial protein levels, we adjusted protein loading as mentioned in the figure legend. IKKε-silenced PDAC cells exhibited a time-dependent increase in total c-MYC levels upon MG-132 treatment (Figure 4B, upper panel). Similar observation was also recorded in MG-132-treated control cells (Figure 4B, lower panel); however, rate of c-MYC accumulation in these cells was relatively slower compared to IKKε-silenced cells. To confirm that c-MYC stabilization was a result of decreased degradation of ubiquitinated-c-MYC, we performed immunoprecipitation using anti-c-MYC antibody followed by immunoprobing with anti-Ub antibody. An accumulation of Ub-c-MYC upon MG132 treatment was observed in both control and IKKε-silenced cells; however, latter exhibited elevated levels (Figure 4C). To measure overall impact of IKKε-silencing on the stability of c-MYC, we measured its turn-over after blocking protein synthesis by cyclohexamide (CHX) (Figure 4D). Differences in initial protein levels were corrected by adjusting protein loading as mentioned in the figure legend. Subsequent analyses based on densitometry measurements of c-MYC signals predicted half-life of c-MYC to be about 41 min and 30 min in MiaPaCa and Colo357 cells, respectively, whereas it was decreased to ~21 min and 16 min in IKKε-silenced MiaPaCa and Colo357 cells, respectively (Supplementary figure 6).
Figure 4. IKKε-induced c-MYC expression and localization is controlled through its inhibition of phosphorylation-mediated nuclear-export and subsequent degradation.
(A) Phosphorylated c-MYCS62 and c-MYCT58 were analyzed by immunoblot using specific-antibodies. (B) Cells were treated with the proteasome inhibitor (MG132, 10µM) for indicated time period, total protein isolated and effect on c-MYC expression determined by immunoblot analysis. We used 60 and 25µg protein from IKKε-silenced and control cells, respectively, to correct for differences in initial protein levels. β-actin was used as loading control. (C) Equal amount (500µg) of protein from Scr and ShIKKε PDAC cells untreated or treated with MG132 (10µM, 30 min) was subjected to immunoprecipitation with anti-c-MYC antibody followed by immunoblot with anti-Ub antibody. (D) To monitor the turnover of c-MYC protein, cells were treated with cycloheximide (50µM), neo-protein-synthesis inhibitor, for indicated time-intervals. Thereafter, total protein was isolated and changes in c-MYC expression monitored by immunoblotting. Considering differences in c-MYC levels between control and IKKε-silenced cells, we used different amounts (60µg and 150µg, respectively) to keep initial signal at near-similar intensity.  - represents the rate of total c-MYC accumulation, and
- represents the rate of total c-MYC accumulation, and  - represents the rate of c-MYC degradation based on densitometry of the data presented.
- represents the rate of c-MYC degradation based on densitometry of the data presented.
c-MYC destabilization upon IKKε-silencing is mediated through Akt-repression-induced GSK3β activation
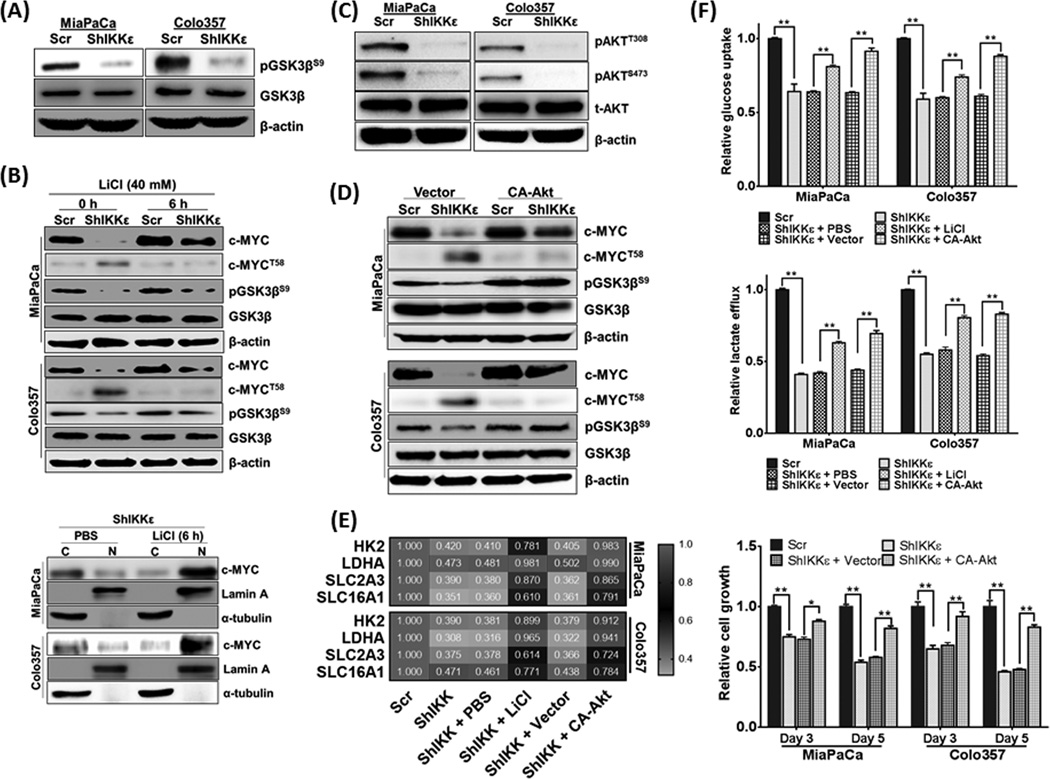
Considering a role of GSK3β in c-MYCT58 phosphorylation (15), we examined its activation status in IKKε-expressing and –silenced PDAC cells. GSK3βS9 phosphorylation, which is known to cause its inactivation (24), was decreased in both IKKε-silenced PDAC cell lines, without any appreciable changes in total GSK3β (Figure 5A). When IKKε-silenced PDAC cells were treated with LiCl, an inhibitor of GSK3β (25), it restored total c-MYC levels to significant extent, which correlated with decreased phospo-c-MYCT58 and enhanced phospo-GSK3βS9 levels (Figure 5B). Furthermore, LiCl treatment also enhanced the nuclear retention of c-MYC in IKKε-silenced PDAC cells (Figure 5B). We next investigated if IKKε was directly able to phosphorylate GSK3β through its Ser/Thr kinase activity as reported for GSK3α (26). However, no interaction between IKKε and GSK3β was observed in reciprocal co-immunoprecipitation assays (data not shown). Hence, we focused our attention to Akt, which is known to inactivate GSK3β through its S9 phosphorylation (27). Moreover, IKKε can also directly phosphorylate Akt at both T308 and S473, independent of PI3K and mTORC2 activities, respectively (28, 29). A significant reduction in AktT308/S473 levels was observed in immunoblot analyses in IKKε-silenced PDAC cells (Figure 5C). Similarly, ectopic expression of IKKε in BxPC3 cells promoted Akt activation as evidenced by phosphorylation at Thr-308 and Ser-473 sites, and thus resulting in Ser-9 phosphorylation of GSK3β (Supplementary figure 7). Thus, to further explore the role of activated Akt, we expressed its constitutively active mutant-form (T308D-S473D; CA-Akt) in IKKε-silenced cells through transient transfection. Upon ectopic activation of Akt, significant upregulation of c-MYC protein was detected that correlated with phospho-GSK3βS9 and reduced c-MYCT58 levels (Figure 5D). Thereafter, we ascertained a role of IKKε/Akt/GSK3β signaling axis in glucose-metabolism and expression of involved gene targets. First, the effect of LiCl and CA-Akt on relative mRNA expression of some randomly selected genes (HK2, LDHA, SLC2A3 and SLC16A1) was examined followed by glucose-uptake measurements. LiCl and CA-Akt activity rescued the gene expression either partially (SLC2A3 and SLC16A1) or almost completely (HK2 and LDHA) in IKKε-silenced cells relative to that in control cells (Figure 5E). Similarly, a significant increase in glucose-uptake and lactate-efflux was also observed upon LiCl treatment and CA-Akt expression in IKKε-silenced cells, which correlated with their enhanced growth (Figure 5F).
Figure 5. Subcellular localization and stabilization of c-MYC is governed through IKKε/Akt/GSK3β axis.
(A) GSK3βS9 phosphoryation was determined by immunoblotting using specific antibodies. (B) PDAC cells were treated with GSK3β inhibitor, LiCl (40 µM, 6 h) followed by total protein isolation and immunoblotting (upper panel). Subsequently, cytoplasmic and nuclear levels of c-MYC were also measured following LiCl treatment (lower panel). (C) Determination of phospho-AktT308/S473 by immunoblotting suggested their reduced expression in IKKε-silenced PDAC cells. (D) Cells were transfected with constitutively active-Akt (CA-Akt) or control plasmid followed by measurement of c-MYC, c-MYCT58, pGSK3βS9 and total GSK3β by immunoblotting. In parallel experiments, effect of LiCl treatment and CA-Akt transfection was investigated on (E) mRNA levels of HK2, LDHA, SLC2A3 and SLC16A1 by qRT-PCR, (F) glucose-uptake (upper panel) and lactate-efflux (middle panel), and growth recovery on day 3 and 5 by WST1 assay (lower panel). Bars represent the mean ± S.D. (n=3); **p≤0.05. β-actin was used as loading control for total protein, and Lamin A and α-tubulin used as controls for nuclear- and cytoplasmic-fractions, respectively. qPCR data was normalized with ACTB expression.
IKKε promotes glucose-uptake and tumorigenicity of PDAC cells in an orthotopic mouse model
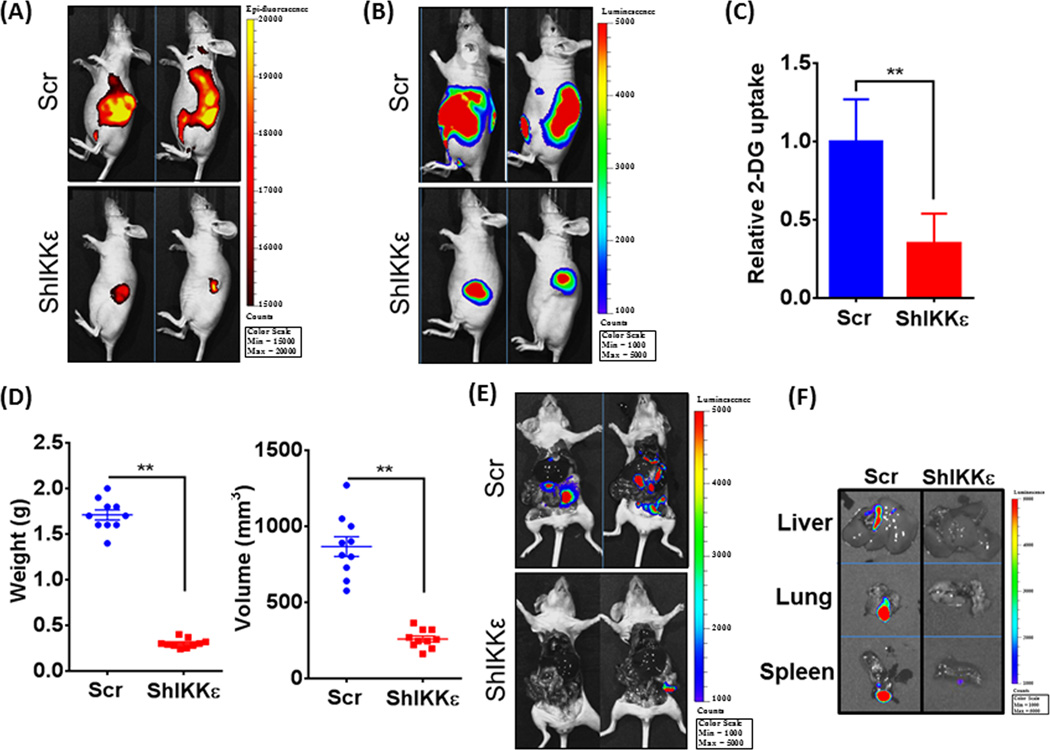
To assess the functional relevance of IKKε in vivo, we injected luciferase-tagged MiaPaCa-Scr/MiaPaCa-ShIKKε cells directly into the pancreas of mice, and monitored tumor growth every alternate day by palpation and weekly by non-invasive bioluminescence imaging. A significantly greater fluorescent signal was detected in all mice from MiaPaCa-Scr group as compared to that from MiaPaCa-ShIKKε group indicating higher glucose-uptake by the tumors (Figure 6A). To normalize the glucose-uptake with tumor size, we also performed bioluminescent imaging of tumors following D-luciferin injection (Figure 6B). Normalized fluorescence signal also suggested significantly greater glucose-uptake in IKKε-overexpressing MiaPaCa-Scr cells compared to IKKε-silenced MiaPaCa-ShIKKε cells (Figure 6C). End-point measurement also confirmed significant decrease in tumor growth in IKKε-silenced group. Average weight and volume of the tumors developed in control group were recorded to be 1.71 ± 0.05 g and 866 ± 64.86 mm3 as compared to 0.303 ± 0.01 g; and 259.9 ± 19.42 mm3, respectively, in IKKε-silenced group (Figure 6D). Imaging of mice after resection of primary tumors exhibited strong bioluminescence signals in various organs of control group mice only suggesting distant metastasis (Figure 6E), which was further confirmed by ex-vivo imaging of resected organs (spleen, liver and lung) (Figure 6F).
Figure 6. IKKε-downregulation suppresses glucose-uptake, tumor growth and metastasis in orthotopic PDAC xenografts.
Luciferase-tagged control or IKKε-silenced MiaPaCa cells were implanted into the pancreas of athymic nude mice (n=10 per group). (A) A day prior to end-point, mice were injected with fluorescent analog of glucose, 2-DG (100µL) intraperitoneally and epiflourescence measured after 24h using the IVIS imaging system. (B) Prior to sacrificing the mice, D-luciferin (150 mg kg−1 body weight) was injected intraperitoneally and bio-luminescence imaging data recorded using the IVIS imaging system. (C) In vivo glucose-uptake normalized with bioluminescent tumor measurements at the end time point and shown as relative 2-DG uptake. (D) After sacrifice, tumors were resected and measured for weight and volume, and (E) mice imaged to visualize metastases. (F) To further confirm metastases, livers, lungs and spleens were carefully removed from the mice and imaged separately. All data is representative. Bars represent the mean ± S.D. (n = 10 mice); **p ≤ 0.05.
Discussion
PDACs are highly aggressive, exhibiting rapid growth and metastasis, which is one important reason for their high lethality in patients (2, 30, 31). As a result, efforts have focused on understanding the molecular causes of PDAC aggressiveness as well as on formulating strategies that could target this very feature of pancreatic malignancy. It is now being widely recognized that cancer cells re-wire their metabolic state to fulfill the enhanced requirements of bioenergetics and biomass production, so that they can maintain their rapid proliferation (11, 13). Glucose, a major source of energy, gets metabolized through a combination of anaerobic glycolysis and oxidative phosphorylation in normal cells. However, cancer cells reprogram the glucose-metabolism to support their fast growth by shifting their dependence of ATP production from oxidative phosphorylation to ‘aerobic’ glycolysis- an observation referred to as “Warburg effect” (11, 13, 14). Moreover, altered glucose-metabolism in cancer cells has also been associated with their metastatic potential and therapeutic-resistance (32, 33). In these contexts, our observation of IKKε-mediated regulation of glucose-metabolism is highly significant and suggests an important role of IKKε in molecular pathogenesis of PDAC. This is even more interesting considering a role of deregulated glucose-metabolism in inflammation (34). In fact, chronic inflammation is known to exacerbate glucose-metabolism and also promote cancer progression (35). Thus, these vicious connections of altered glucose-metabolism, inflammation and cancer, and suggested role of IKKε in these phenotypes makes our findings even more noteworthy for future therapeutic- and preventive- intervention points.
From the mechanistic standpoint, we observed dysregulation of several genes involved in glucose-metabolism upon IKKε silencing. This involved downregulation of genes associated with glucose- and lactate-transport (GLUT1, GLUT3, MCT1, and MCT4) and glycolysis (ALDOC, ENO3, GALM, GCK, HK2, HK3, etc.). As per the published data, PDAC cells are known to overexpress GLUT1, while the upregulation of GLUT3 along with GLUT1 has been shown in many other cancer types and correlated with poor prognosis (36–38). Similarly, expression and activity of HK2 has been observed to be upregulated in nearly all types of cancers (32). Notably, enhanced activity of HK2 is required for the initiation and maintenance of K-Ras driven cancers, and its inhibition is shown to reduce tumor growth both in vitro and in vivo (39). There is also evidence to suggest the utility of HK2 as a prognostic marker in PDAC patients (40). The strong reliance towards aerobic glycolysis by cancer cells leads to the production of lactate by the enzyme LDHA, which is then exported to the tumor-microenvironment by lactate-transporters (MCT1-4) to have a multitude of functions in cancer growth and metastasis (41). Further, in a majority of pancreatic tumors that express high levels of MCT4, stromal cells have also been reported to have high MCT4 expression; indicating a putative co-relation between these two events (42). Interestingly, while knockdown of IKKε led to the reduction of lactate-efflux in the culture media, we did not observe any change in basal OCR in our study. This suggests that while IKKε knockdown hampers glycolytic-metabolism, it does not affect oxidative phosphorylation. Furthermore, observed reduction in lactate levels could also be due to the drop in LDHA and lactate-transporters, MCT1 and MCT4. This is significant as the accumulation of lactate within the cell could otherwise alter intracellular pH and subsequently induce metabolic feedback inhibitions, ultimately hampering ATP production by glycolysis (43).
Our findings also identified c-MYC as an important mediator in potentiating the effect of IKKε on glucose-metabolism. This is very significant, as c-MYC is frequently deregulated not only in PDAC, but several other cancers as well (44, 45). c-MYC is shown to integrate cellular metabolism with survival and proliferation of cancer cells through the regulation of a number of genes (46). Under normal conditions, c-MYC transcriptional activity is controlled at several levels involving transcriptional and post-transcriptional regulation, post-translational modification, subcellular distribution and protein turn-over (16). Moreover, interaction of c-MYC with other nuclear proteins has also been suggested to alter its genomic occupancy and transcriptional activation. Our data demonstrates that IKKε does not alter mRNA levels of c-MYC in PDAC cells, but supports its nuclear-retention and total protein stability. Moreover, we show that IKKε stabilizes c-MYC through inactivation of GSK3β, which is known to promote c-MYCT58 phosphorylation (25). Despite a previous report demonstrating direct interaction of IKKε with GSK3α (26), we did not observe its similar interaction with GSK3β; its phosphorylation was rather dependent on IKKε-mediated Akt activation. Activity of GSK3β has been demonstrated to be diminished by extracellular signals upon GSK3β-Ser-9 phosphorylation mediated by Akt and other kinases (47). Initial studies identified that constitutively-activated IKKε could replace Myr-Akt leading to transformation of immortalized human mammary epithelial cells (7). It was later confirmed that IKKε can directly phosphorylate and enhance AktT308/S473 levels even in the absence of external stimuli, and independent of other regulators (48). Our findings also demonstrated significant downregulation of Akt phosphorylation at both Thr-308 and Ser-473 sites in IKKε-silenced cells, which correlated with the decrease in inhibitory GSK3βS9 phosphorylation. Interestingly, despite regained Akt activation and GSK3β inactivation in IKKε-silenced PDAC cells, it did not lead to complete restoration of c-MYC expression, metabolic-shift and tumor growth. This may be due to technical limitation or more likely due to other IKKε-responsive, cross-talking signaling-networks that are involved in potentiating its pathobiological functions. It is possible that IKKε-silencing may modulate the activity of other molecular targets, such as PP2A, which are known to alter c-MYC expression and/or its activation (49, 50).
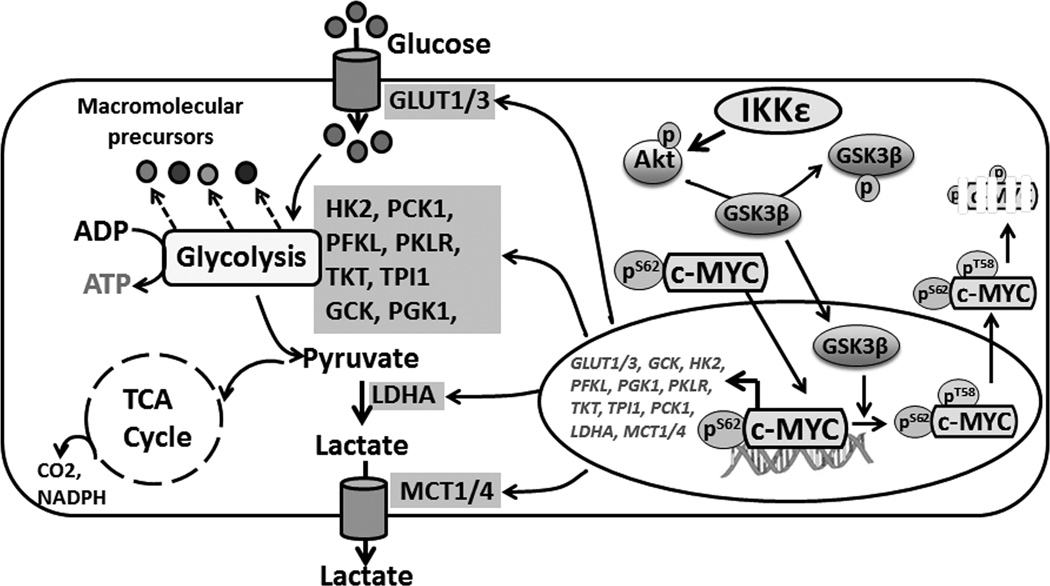
In summary, we have defined a novel role of IKKε in PDAC pathogenesis through its regulation of glycolytic phenotype. We have also demonstrated that IKKε acts as a novel regulator of c-MYC by promoting its nuclear retention and stabilization. c-MYC stability is afforded by IKKε-mediated Akt activation, which leads to phosphorylation-mediated inhibition of GSK3β, and, in turn, promotes nuclear-retention and increase in overall transcriptional activity (Figure 7). Our results, thus, provide strong rationale for further testing of IKKε as a novel molecular target to counter PDAC progression and its therapeutic management.
Figure 7. Schematic representation of IKKε signaling in PDAC.
IKKε promotes glycolytic-metabolism and pancreatic tumor growth through its regulation of Akt/GSK3β/c-MYC axis. c-MYC is an important mediator of IKKε signaling whose nuclear-retention and -stabilization is controlled by IKKε through a chain of events that include Akt activation resulting in inhibitory phosphorylation of GSK3β and the escape of c-MYC from GSK3β-mediated nuclear-efflux and subsequent degradation. Once stabilized, c-MYC activates multiple factors of glycolytic pathway with concomitant increase in glucose-uptake and lactate-efflux, leading to quick and efficient energy production to serve as prelude for synthesis of downstream molecules/factors involved in pancreatic tumor growth and metastasis.
Supplementary Material
Acknowledgments
We would like to thank Mr. Steven McClellan, Manager, Flow cytometry core at the USA Mitchell Cancer Institute for his assistance with flow cytometry. We also thank Ms. Barbara Putnam (USAMCI) for careful reading of the manuscript.
Funding Support: This work was supported in part by NIH grants (R01CA175772 and U01CA185490) to A.P. Singh. The laboratory of S. Singh is supported by NIH grants (R01CA204801 and R03CA186223). Their laboratories also receive funding and resource support from the University of South Alabama Mitchell Cancer Institute.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 5.Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 6.Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 8.Peant B, Forest V, Trudeau V, Latour M, Mes-Masson AM, Saad F. IkappaB-Kinase-epsilon (IKKepsilon/IKKi/IkappaBKepsilon) expression and localization in prostate cancer tissues. Prostate. 2011;71:1131–1138. doi: 10.1002/pros.21329. [DOI] [PubMed] [Google Scholar]
- 9.Guo JP, Shu SK, He L, Lee YC, Kruk PA, Grenman S, et al. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am J Pathol. 2009;175:324–333. doi: 10.2353/ajpath.2009.080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng A, Guo J, Henderson-Jackson E, Kim D, Malafa M, Coppola D. IkappaB Kinase epsilon expression in pancreatic ductal adenocarcinoma. Am J Clin Pathol. 2011;136:60–66. doi: 10.1309/AJCP2JJGYNIUAS2V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 12.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 13.Blum R, Kloog Y. Metabolism addiction in pancreatic cancer. Cell Death Dis. 2014;5:e1065. doi: 10.1038/cddis.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;3:1133–1137. [PubMed] [Google Scholar]
- 16.Farrell AS, Sears RC. MYC degradation. Cold Spring Harb Perspect Med. 2014;4:a014365. doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amati B. Myc degradation: dancing with ubiquitin ligases. Proc Natl Acad Sci U S A. 2004;101:8843–8844. doi: 10.1073/pnas.0403046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT-and ERK-dependent activation of NF-kappaB pathway. Oncotarget. 2014;5:8778–8789. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan MA, Srivastava SK, Bhardwaj A, Singh S, Arora S, Zubair H, et al. Gemcitabine triggers angiogenesis-promoting molecular signals in pancreatic cancer cells: Therapeutic implications. Oncotarget. 2015;6:39140–39150. doi: 10.18632/oncotarget.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava SK, Bhardwaj A, Arora S, Singh S, Azim S, Tyagi N, et al. MYB is a novel regulator of pancreatic tumour growth and metastasis. Br J Cancer. 2015;113:1694–1703. doi: 10.1038/bjc.2015.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, et al. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget. 2014;5:11490–11500. doi: 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, et al. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina M, Wandosell F. Deconstructing GSK-3: The Fine Regulation of Its Activity. Int J Alzheimers Dis. 2011;2011:479249. doi: 10.4061/2011/479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 26.Gulen MF, Bulek K, Xiao H, Yu M, Gao J, Sun L, et al. Inactivation of the enzyme GSK3alpha by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance. Immunity. 2012;37:800–812. doi: 10.1016/j.immuni.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Wang H, Burg MB, Ferraris JD. Inhibitory phosphorylation of GSK-3beta by AKT, PKA, and PI3K contributes to high NaCl-induced activation of the transcription factor NFAT5 (TonEBP/OREBP) Am J Physiol Renal Physiol. 2013;304:F908–F917. doi: 10.1152/ajprenal.00591.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, et al. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci U S A. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo JP, Coppola D, Cheng JQ. IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–37398. doi: 10.1074/jbc.M111.287433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Vaccaro V, Sperduti I, Vari S, Bria E, Melisi D, Garufi C, et al. Metastatic pancreatic cancer: Is there a light at the end of the tunnel? World J Gastroenterol. 2015;21:4788–4801. doi: 10.3748/wjg.v21.i16.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidalgo M, Cascinu S, Kleeff J, Labianca R, Lohr JM, Neoptolemos J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15:8–18. doi: 10.1016/j.pan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 33.Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 36.Ito H, Duxbury M, Zinner MJ, Ashley SW, Whang EE. Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery. 2004;136:548–556. doi: 10.1016/j.surg.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 37.Dai DW, Lu Q, Wang LX, Zhao WY, Cao YQ, Li YN, et al. Decreased miR-106a inhibits glioma cell glucose uptake and proliferation by targeting SLC2A3 in GBM. BMC Cancer. 2013;13:478. doi: 10.1186/1471-2407-13-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayala FR, Rocha RM, Carvalho KC, Carvalho AL, da Cunha IW, Lourenco SV, et al. GLUT1 and GLUT3 as potential prognostic markers for Oral Squamous Cell Carcinoma. Molecules. 2010;15:2374–2387. doi: 10.3390/molecules15042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Wang L, Zhang Y, Wang J, Deng Y, Lin D. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016;16:9. doi: 10.1186/s12935-016-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa H, Nagano H, Konno M, Eguchi H, Koseki J, Kawamoto K, et al. The combination of the expression of hexokinase 2 and pyruvate kinase M2 is a prognostic marker in patients with pancreatic cancer. Mol Clin Oncol. 2015;3:563–571. doi: 10.3892/mco.2015.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baek G, Tse YF, Hu Z, Cox D, Buboltz N, McCue P, et al. MCT4 defines a glycolytic subtype of pancreatic cancer with poor prognosis and unique metabolic dependencies. Cell Rep. 2014;9:2233–2249. doi: 10.1016/j.celrep.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 43.Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 44.Hessmann E, Schneider G, Ellenrieder V, Siveke JT. MYC in pancreatic cancer: novel mechanistic insights and their translation into therapeutic strategies. Oncogene. 2016;35:1609–1618. doi: 10.1038/onc.2015.216. [DOI] [PubMed] [Google Scholar]
- 45.Cai Q, Medeiros LJ, Xu X, Young KH. MYC-driven aggressive B-cell lymphomas: biology, entity, differential diagnosis and clinical management. Oncotarget. 2015;6:38591–38616. doi: 10.18632/oncotarget.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo JP, Coppola D, Cheng JQ. IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–37398. doi: 10.1074/jbc.M111.287433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Farrell AS, Allen-Petersen B, Daniel CJ, Wang X, Wang Z, Rodriguez S, et al. Targeting inhibitors of the tumor suppressor PP2A for the treatment of pancreatic cancer. Mol Cancer Res. 2014;12:924–939. doi: 10.1158/1541-7786.MCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von der LN, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11:1189–1200. doi: 10.1016/s1097-2765(03)00193-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.