Abstract
Purpose
Pancreatic ductal adenocarcinoma (PDAC) is refractory to available treatments. Delineating critical pathways, responsible for disease aggressiveness and therapeutic resistance, may identify effective therapeutic targets. We aimed to identify key pathways contributing to disease aggressiveness by comparing gene expression profiles of tumors from early stage PDAC cases with extremely poor survival (≤ 7 months) and those surviving 2 years or more following surgical resection.
Experimental Design
Gene-expression profiling was performed in tumors in a test cohort of PDAC (N=50), which included short (≤7 months, N=11) and long surviving (≥2 years, N=14) patients, using affymetrix GeneChip Human 1.0 ST array. Key genes associated with disease aggressiveness were identified, using Cox regression, Kaplan Meier and pathway analyses with validations in independent cohorts for mechanistic and functional analyses.
Results
Gene-expression profiling identified 1,820 differentially expressed genes between short and long survival groups with inflammatory gene network ranking 1st. Lower expression of Endothelial Nitric Oxide Synthase Traffic Inducer (NOSTRIN) was associated with worst survival indicating its potential inhibitory role in disease progression. NOSTRIN overexpression suppressed migration and invasion of pancreatic cancer cells and enhanced sensitivity to chemotherapeutic drug gemcitabine. NOSTRIN inhibited production of nitric oxide (NO) by suppressing the activation of endothelial nitric oxide synthase (eNOS). Furthermore, miR-221, bound to the 3′UTR of NOSTRIN and suppressed its expression, and an increased miR-221 expression associated with poor survival in PDAC.
Conclusion
Our findings showed that NOSTRIN is a potential negative regulator of disease aggressiveness, which may be targeted for designing improved treatment strategy in PDAC.
Keywords: Pancreatic cancer, NOSTRIN, miR-221, therapeutic target, prognosis
Introduction
Pancreatic Cancer is the 4th leading cause of death due to cancer with a median patient survival of 6 months and a 5-year survival of a mere 8% (1). Alarmingly, a consistent rise in incidence and death in pancreatic cancer is estimated to make it the second leading cause of cancer-related death by 2030 (2). Among many types, pancreatic ductal adenocarcinoma (PDAC) is the most common (85-90%) and deadly form of pancreatic cancer. The dismal prognosis in PDAC patients is due to the fact that it is highly refractory to available treatments and is usually diagnosed at an advanced stage of the disease (3, 4). A small number (15-20%) of PDAC patients are diagnosed at localized, early stages, and qualify for surgical resection with curative intent (5). However, a majority of these surgically resected patients show recurrence within 2 years following surgery and adjuvant therapy, and only 27% survive up to 5 years (seer.cancer.gov). Several clinical prognostic factors, including stage, grade and resection margin status, are attributed to the outcome in resected patients, and yet variable outcomes exist among resected cases with similar stage, grade or resection margin status (6-8). For example, many stage I/II patients with technically perfect resection and negative resection margin (R0) survive less than 6 months, as compared to other resected patients with similar disease status, who survive 5 years or longer. These facts suggest that investigating the potential molecular differences between tumors from early stage, resected patients with long and short survival may provide insights into the molecular mechanism of disease aggressiveness.
To understand the underlying mechanism of disease aggressiveness and identify potential molecular targets, we tested the hypothesis that a distinct gene expression profile contributes to the enhanced disease progression in short-survival as compared to long-survival groups of resected patients with PDAC. To test this hypothesis, we compared gene expression profile of stage I/II, resected patients in short (≤7 months) and long survival (≥2 years) groups. Our findings showed that NOSTRIN is a negative regulator of disease aggressiveness and is regulated by miR-221 in PDAC, and may be targeted for improved disease outcome.
NOSTRIN was identified as a protein, which binds to endothelial nitric oxide synthase (eNOS) and translocates it to the interior compartment of the cell in a vesicular structure, which results in the inhibition of NO production (9, 10). eNOS is a member of the nitric oxide synthase (NOS) family of enzyme, which catalyzes the conversion of arginine to citrulline with the production of nitric oxide (NO) like inducible NOS (iNOS) and neuronal NOS (nNOS), other members of the family (11, 12). Although, NO performs several critical physiological functions, it is associated with the development and progression of cancer (13-15).
Materials and methods
PDAC Samples
Pancreatic tumor tissue from resected PDAC cases were collected at University of Maryland Medical System (UMMS), Baltimore, MD, through NCI-UMD resource contract and the Department of General, Visceral and Pediatric Surgery, University Medicine Gottingen, Gottingen, Germany with the approval of Institutional Review Board. Clinical and demographic information, including age, sex, clinical staging, differentiation grade, resection margin status and survival from the time of diagnosis were also available with patients consent. Tumor histopathology was classified according to the World Health Organization Classification of Tumors by a Board certified pathologist. The characteristics of the patients in test cohort are shown in Table S1 and S2. Use of the clinical specimens was reviewed and permitted by the NCI-Office of Human Subject Research (OHSR, Exempt# 4678) at the National Institutes of Health, Bethesda, MD.
Validation cohort from published microarray data
The correlation of gene expression and survival was validated in Collisson cohort (16). Gene expression data and clinical features of patients were downloaded from Oncomine (17). Clinical features of our test and validation cohorts are shown in Table S2.
RNA isolation, cDNA microarray and quantitative RT-PCR (qRT-PCR)
A standard Trizol protocol (Invitrogen, Carlsbad, CA) was used to extract Total RNA from frozen tumor samples. RNA extraction from cultured cells was performed using the Total RNAextraction kit (Norgenbiotek: Thorold, Canada) following the manufacturer's protocol. RNA quality was evaluated using Agilent 2100 Bioanalyzer (Agilent Technologies). mRNA expression profiling was performed using the Affymetrix GeneChip Human 1.0 ST arrays according to the manufacturer's protocol at the microarray core facility of the National Cancer Institute, Frederick, MD. All arrays were RMA (Robust Multi-array Average) normalized and gene expression summaries were created for each gene by using the average of all probe sets for each gene using Partek Genomics Suite 6.5. Further data analysis was performed using the gene-summarized data. The microarray gene expression data has been deposited in the National Center for Biotechnology Information's (NCBIs) as GSE78229. For quantitative RT-PCR, RNA was first reverse-transcribed using Multi Scribe reverse transcriptase (Life Technology, Foster City, CA). Gene-expression levels were determined by Taqman assay with probes from Applied Biosystems: NOSTRIN (Hs00976555_m1), IGF2BP3 (Hs00559907_g1), AQP9 (Hs00175573_m1), SLC16A3 (Hs00358829_m1), and 18S rRNA (Hs99999901_s1).
Cell Lines and Culture Condition
Human pancreatic cancer cell lines were purchased from the American Type Culture Collection, ATCC (Rockville, MD) and were authenticated by short tandem repeat (STR) analysis. CFPAC-1 cells were grown in Iscove's Modified Dulbecco's Medium. Panc-1 and Mia-paca 2 cells were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, penicillin-streptomycin (50 IU/ml and 50 mg/ml, respectively) and 2 mM L-glutamine in a humidified incubator containing 5% CO2 at 37°C. All products for cell culture were purchased from Gibco (Grand Island, NY).
Generation of stable NOSTRIN-overexpressing cells using lentiviral vectors
Lentiviral NOSTRIN constructs (EX-E1789-Lv105) was purchased from Genecopoeia (Rockville, MD). The details are provided in supplementary methods.
Western blotting
Cells were washed in PBS, lysed with RIPA buffer (Invitrogen, Carlsbad, CA) and standard immunoblotting protocol was used as described in detail is in supplementary method.
Measurement of Nitric Oxide production
Nitric Oxide production was measured using Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer's instruction. Additional detail is provided in the supplementary method.
Drug sensitivity assay
Gemcitabine was purchased from Tocris Bioscience (Ellisville, MO). Mia-paca 2 cells with or without NOSTRIN overexpression were seeded in 96-well plates at a concentration of 2000 cells/well. Gemcitabine at the indicated concentration was added into each well 24 hours after cell seeding and incubated in 37°C incubator with 5% CO2 for 72 hours. The cytotoxicity was evaluated by WST-8 colorimetric assay (Dojindo, Kumamoto, Japan). Mean values were calculated from three different wells in triplicates. For colony formation assay, cells were seeded into 6-well plate at 1000 cells/well, and gemcitabine treatment started 24 hours later until colonies were formed. Colonies were then fixed with methanol and stained with 0.5% Crystal Violate solution to be visualized.
Luciferase Reporter Assay
Cells were cultured in 96-well plates and co-transfected with 50nM of Pri-miR-221 or 50nM negative control), 100ng of luciferase reporter NOSTRIN-3′UTR-WT (1532-1541) or NOSTRIN-3′UTR-MUT (1532-1541 Mutant), and 1ng of pRL-CMV Renilla luciferase reporter. Cells were incubated for 48 hr then washed twice in PBS and harvested for firefly/Renilla luciferase assay using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Additional details are provided in supplementary method.
Caspase activity
Caspase 3/7 activity was measured using Apo-one kit (Promega, Madison, WI) according to manufacturer's instructions. Briefly, 2000 cells were seeded into 96-well plate in triplicates. Chemotherapeutic drugs were added 24 hours after seeding and incubated for 18 or 60 hours. Apo-one reagent was added into each well at a 1:1 ratio, and absorbance at 520nm was measured an hour later using Fluostar microplate reader (Omega, Ortenberg, Germany). Caspase 3/7 activity induced by chemotherapeutic drugs was calculated relative to untreated group. Results were obtained from 3 independent experiments.
Xcelligence real-time migration
Real-time cell migration was measured in CIM E-plates (ACEA, San Diego CA), and cell index was recorded by Xcelligence system (ACEA, San Diego CA) as indicator of cell migration ability. The detail is provided in the supplementary method.
Statistical Analysis
Kaplan-Meier analysis was performed, using Graphpad Prism 6.0, to evaluate the differences in survival probability in resected pancreatic cancer cases. Univariable and multivariable Cox Proportional-hazards regression analysis was performed using STATA 11 (StataCorp LP, College Station, TX) to investigate the association of NOSTRIN expression level in tumors and other clinical factors to determine their association with survival in PDAC cases. Final multivariable models were based on stepwise addition and removal of clinical prognostic factors found to be associated with survival in univariable analysis (P<0.05). These models did not violate proportional hazards assumptions based on Schoenfeld residuals. For these analysis, resection margin status was dichotomized as positive (R1) versus negative (R0); staging as stage I/IIA versus stage IIB, and differentiation grade as G1-G2 versus G3-G4.
Results
Gene-expression profiling of tumors from short and long survival groups of resected patients identified NOSTRIN as a prognostic biomarker in PDAC
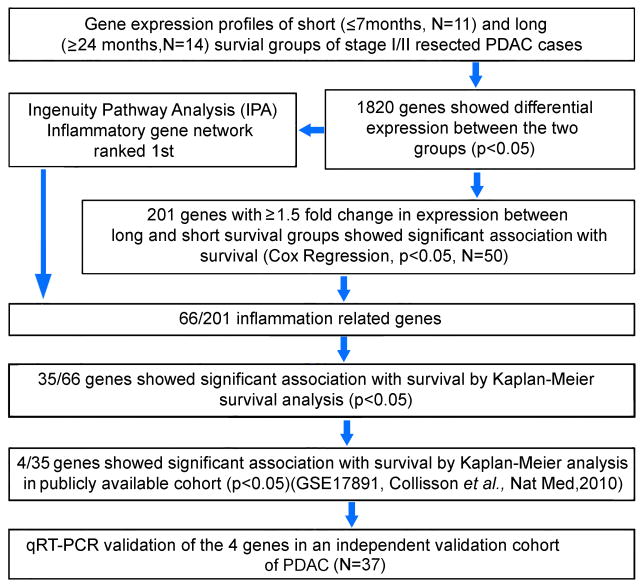
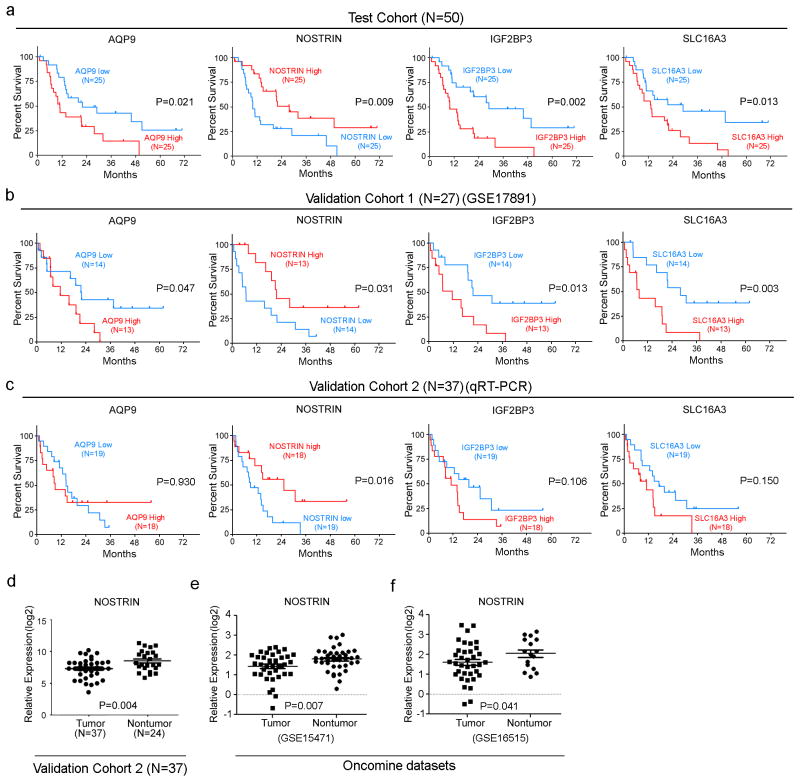
We compared gene expression profile of tumors from patients with short (≤7 months, N=11) and long survival (≥2 years, N=14) in our test cohort of a total of 50 resected PDAC patients, using affymetrix GeneChip Human 1.0 ST arrays (GSE78229) (Figure 1). The clinical characteristics of these patients are described in Supplementary Tables S1 and S2. Comparing gene expression, using ANOVA, identified 1,820 differentially expressed genes between short and long survival groups (Supplementary Table S3). Ingenuity Pathway Analysis (Ingenuity Systems, www.ingenuity.com) using 1,820 genes ranked inflammatory genes as the top gene network. Next, we assessed the prognostic significance of these 1,820 genes first by Cox Regression analysis in the test cohort (N=50), which resulted in the identification of 201 genes that were associated with survival (p<0.05) (Supplementary Table S4). Gene ontology analysis classified 66 out of 201, as inflammatory genes (Supplementary Table S5). The fact that inflammatory mediators play a significant role in the development and progression of pancreatic cancer (18), and that the inflammatory genes are the top ranking gene network in our data set, we pursued further investigation on the 66 inflammatory genes. Kaplan-Meier analyses showed that 35 of 66 inflammatory genes were associated with survival (Supplementary Table S6). Four out of 35 potentially prognostic genes AQP9, NOSTRIN, IGF2BP3, SLC16A3, could be validated in publically available gene expression data set (GSE 17891) (16) (Figure 2a,b). We then used qRT-PCR to further validate 4 genes in an additional validation cohort-2 (N=37), which showed that a decreased expression of NOSTRIN was associated with poorer survival (Figure 2c). Additionally, multivariable analysis showed that NOSTRIN is an independent predictor of prognosis in PDAC (Supplementary Table S7). Furthermore, a lower expression of NOSTRIN was found in tumors as compared to adjacent nontumor pancreas (Figure 2d), which could be validated in two additional publically available datasets [(GSE 15471; GSE 16515)(19, 20)] (Figure 2e, f). Additionally, immunohistochemical staining revealed that NOSTRIN largely expressed in tumor cells (Supplementary Figure S1). These finding showed that a lower expression of NOSTRIN is associated with poor survival in resected PDAC patients and may negatively regulate disease progression.
Figure 1.
Schematic representation of the strategy to distinguish genes associated with disease aggressiveness in long vs. short survival groups of resected PDAC patients.
Figure 2. Identification and validation of candidate genes with prognostic significance in PDAC.
a) Kaplan-Meier analysis showing that an increased expression of AQP9, IGF2BP3 and SLC16A3 is associated with poor survival, however, a lower expression of NOSTRIN is associated with poor survival in test cohort. (b) Validation in publically available data set. (c) Validation by qRT-PCR analysis in a second validation cohort showing that a lower expression of NOSTRIN is associated with poor survival. Association of AQP9, IGFBP3 and SLC16A3 with survival could not be validated by qRT-PCR in the second validation cohort. (d) A lower expression of NOSTRIN was found by qRT-PCR in tumors as compared to adjacent nontumor pancreas, which could be further validated in publically available datasets (e,f).
NOSTRIN suppresses invasiveness of pancreatic cancer cells by inhibiting the activation of eNOS and NO production
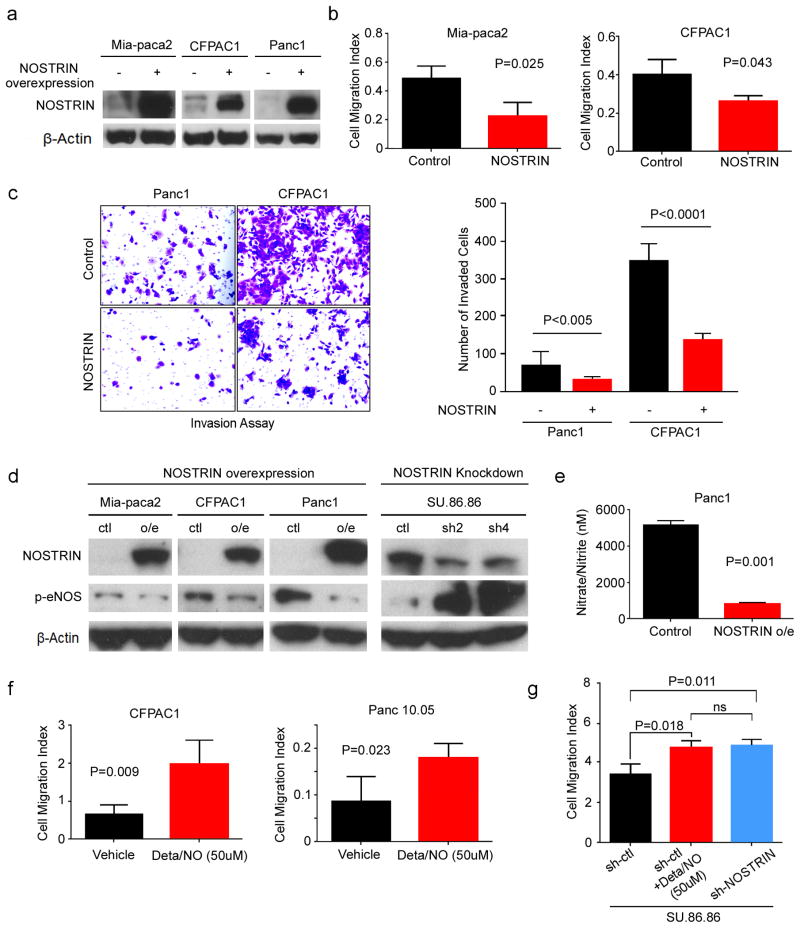
To determine the potential role of NOSTRIN in the progression of pancreatic cancer, we generated pancreatic cancer cell lines with lenti-viral mediated stable expression of NOSTRIN (Figure 3a). NOSTRIN overexpressing Mia-paca2 and CFPAC1 pancreatic cancer cell lines showed significantly reduced mobility and invasion (Figure 3b,c). As mentioned earlier, NOSTRIN has been described to bind eNOS and modulates its intracellular distribution and reduces the release of NO. Additionally, eNOS-generated NO may contribute to the development and progression of pancreatic cancer (21). Therefore, we tested the hypothesis that NOSTRIN-induced suppression of pancreatic cancer cell migration and invasion is mediated through the inhibition of eNOS and subsequent reduction in NO level. NOSTRIN overexpressing cells showed a reduced expression of phosphorylated (activated) eNOS (p-eNOS) (Figure 3d). Consistently, shRNA-mediated knockdown of NOSTRIN enhanced the expression of p-eNOS (Figure 3d). Phosphorylation of eNOS is required for its activation and subsequent generation of NO (22). We then examined, if NOSTRIN-mediated inhibition of p-eNOS affects the NO production in the pancreatic cancer cells. NOSTRIN overexpressing cells generated a significantly lower amount of NO, as determined by the level of nitrate/nitrite, stable end products of NO in culture medium (Figure 3e). Furthermore, treatment of pancreatic cancer cells with NO-donor drug, Deta/NO, enhanced cell migration (Figure 3f). Consistently, the increase in cell migration, following shRNA-mediated knockdown of NOSTRIN was comparable to the increased cell migration observed following the treatment with NO-donor drug (Figure 3g). These findings showed that NOSTRIN suppresses pancreatic cancer cell mobility and invasiveness by inhibiting eNOS activation and NO production.
Figure 3. NOSTRIN suppresses invasiveness of pancreatic cancer cells by inhibiting eNOS-derived NO production.
(a) Western blot showing NOSTRIN overexpression in 3 PDAC cell lines. (b) Overexpression of NOSTRIN reduced cell migration in Mia-paca 2 and CFPAC-1 pancreatic cancer cells. Cell migration index was recorded by Xcelligence system for up to 24 hr after cells were seeded in the upper wells. Histogram represents the migration index at 24 hr. Cell migration index was calculated by averaging the values in quadruplicate wells. Curves were compared using student t-test. P values less than 0.05 were considered significant. (c) Panc1 and CFPAC1 cells with NOSTRIN overexpression showed reduced invasion as determined by BD invasion assay. Representative picture showing cell invasion at 24 hr. Average cell number in 5 randomly selected fields for each cell lines is shown in the histogram. Results are compared using student t-test. (d) Western blot showing that overexpression of NOSTRIN reduced p-eNOS, while the knockdown of NOSTRIN by shRNA increased p-eNOS level. (e) NOSTRIN overexpression inhibited the production of nitric oxide in PANC1 cells. (f) Exposure to nitric oxide by treatment with NO-donor drug Deta/NO, at indicated concentration, increased cell migration in CFPAC and Panc10.05 cell lines. (g) shRNA-mediated knockdown of NOSTRIN and exposure to NO-donor increased cell migration to comparable levels. Experiments were repeated 3 times. Error bars indicate standard deviation (SD).
NOSTRIN promotes Gemcitabine-induced apoptotic cell death of PDAC cell lines
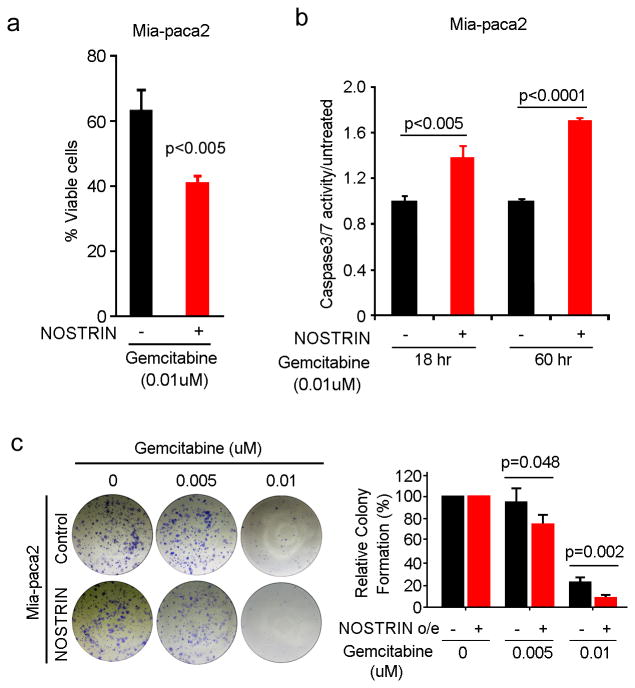
PDAC is highly resistant to available chemotherapeutic drugs. Gemcitabine is the standard of care drug both in early stage resected and advanced cases. We examined, if NOSTRIN modulates the sensitivity of pancreatic cancer cells to gemcitabine. NOSTRIN overexpression significantly reduced the viability and colony-forming ability of pancreatic cancer cells following gemcitabine treatment (Figure 4a,b and Supplementary Figure S2). We then examined, if increased sensitivity is due to enhanced apoptosis by determining caspase3/7 activity. NOSTRIN-overexpressing cells showed significantly enhanced caspase3/7 activity following treatment with gemcitabine (Figure 4c). These findings showed that enhanced expression of NOSTRIN sensitizes tumor cells to chemotherapeutic drug gemcitabine.
Figure 4. Overexpression of NOSTRIN sensitizes PDAC cell lines to chemotherapeutic drug, gemcitabine, by enhancing apoptosis.
(a) NOSTRIN overexpression sensitized Mia-paca2 cells to Gemcitabine. Histogram showing the percentage of viable Mia-paca2 cells with or without NOSTRIN overexpression after 72 hours exposure to gemcitabine. (b) NOSTRIN overexpressing cells showed an enhanced apoptosis, as determined by determined by Capsase3/7 activity, following treatment with gemcitabine. (c) Representative pictures of colony formation assay in Mia-paca2 cells with or without NOSTRIN overexpression, following treatment with gemcitabine at 0.01 and 0.005uM concentration. All experiments were repeated 3 times. Error bars indicate standard deviation (SD).
NOSTRIN is regulated by miR-221
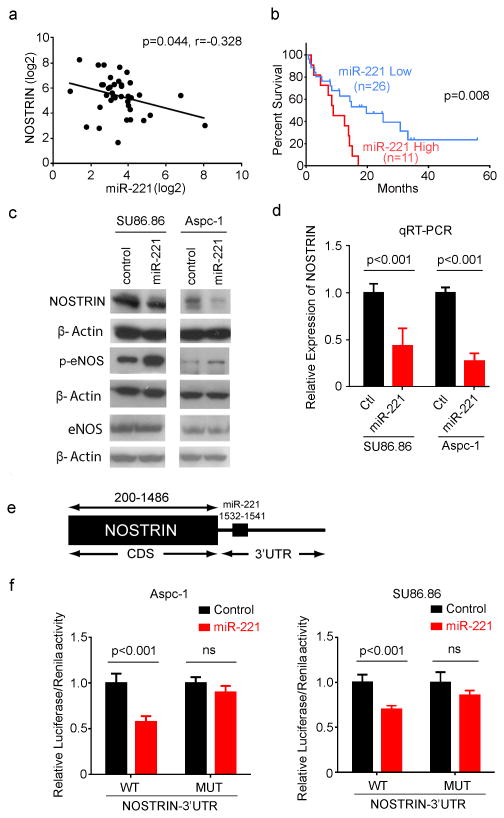
To further delineate the role of NOSTRIN in PDAC, we next examined the regulation of NOSTRIN. miRNAs are important regulators of gene expression and are implicated in the development and progression of pancreatic cancer (23, 24). Examination of 3′UTR of NOSTRIN, using Target Scan and MiR-Walk target prediction module identified potential miR-221/222 binding sites. miR-221 is highly expressed in pancreatic cancer (23) and a higher plasma level of miR-221 is positively correlated with distant metastasis (25). We found a negative correlation between NOSTRIN and miR-221/222 expression, as determined by qRT-PCR in tumors from PDAC cases (N=37) (Figure 5a and Supplementary Figure S3a). Furthermore, an increased expression of miR-221 associated with poorer prognosis in PDAC patients (Figure 5b). However, miR-222 expression didn't show any association with survival in our sample set (Supplementary Figure S3b). Therefore, we pursued our further investigation with miR-221. Overexpression of miR-221 resulted in a decrease in the expression of NOSTRIN both at mRNA and protein level along with an increase in the level of phosphorylated eNOS (p-eNOS) (Figure 5c,d). Furthermore, a potential miR-221 binding site was identified in 3′UTR of NOSTRIN, and luciferase reporter-based activity assay showed that miR-221 binds to the 3′UTR and down regulates NOSTRIN-luciferase reporter activity. However, this effect was abolished with mutations in the miR-221 binding site (Figure 5e,f). These data showed that NOSTRIN is down regulated by miR-221 resulting in an increased level of p-eNOS.
Figure 5. NOSTRIN is regulated by miR-221.
(a) The expression of NOSTRIN and miR-221 showed positive correlation in PDAC (N=37). (b) A higher expression of miR-221 (upper tertile, as determined by qRT-PCR) associated with poor survival in patients with PDAC. (c) Overexpression of miR-221 down regulated NOSTRIN and increased the expression of p-eNOS in ASPC-1 and SU86.86 cell lines. (d). miR-221 mediated decrease in NOSTRIN mRNA expression as determined by qRT-PCR. (e) Schema showing the predicted miR-221 binding site on 3′UTR of NOSTRIN gene. (f) NOSTRIN-Luciferase reporter activity assay showing reduced reporter activity in miR-221 expressing cells. miR-221 induced reduction in reporter activity was abolished when the miR-221 binding site was mutated in NOSTRIN 3′UTR. All experiments were repeated 3 times. Error bars indicate standard deviation (SD).
Discussion
Prognosis in patients with PDAC continues to be dismal with the lack of effective treatment in advanced disease. Understanding the underlying mechanism of disease aggressiveness is crucial for identification of desperately needed novel and effective therapeutic targets. Early detection and surgical resection do improve survival as compared to advanced disease but the median survival remains less than 2 years even in resected cases. However, a small percentage of resected patients do show prolonged survival of 5 years and in some cases even 10 years. We hypothesized that comparing gene expression profile of tumors from early stage, resected, patients with long and short survival may provide insight into the mechanistic regulation of disease aggressiveness and identification of candidate therapeutic targets. In this study, we compared mRNA expression profile of short (≤7months) and long (≥2 years) survival groups of early stage (I/II), resected patients and validated our findings in multiple independent cohorts of PDAC. Our findings showed that NOSTRIN is a potential negative regulator of disease aggressiveness and is modulated by miR-221 in PDAC.
NOSTRIN was discovered as a protein, which binds to eNOS, regulates its intracellular distribution, and inhibits the generation of NO (9). In addition to NOSTRIN, another protein, called eNOS interacting protein (NOSIP), binds with eNOS resulting in its dislocation from plasma membrane and inhibition of NO production (10, 26). However, in our gene expression analysis of tumors from short and long survival groups of PDAC patient cohort, NOSIP was not differentially expressed. Additionally, platelet-endothelial cell adhesion molecule-1 (PECAM-1) regulates eNOS activity and localization through transcriptionally enhancing the expression of NOSTRIN (27). The functional role of NOSTRIN in pancreatic cancer has not been described. Previously, a meta-analysis of gene expression described NOSTRIN as a member of multi-gene signature associated with pancreatic cancer prognosis (28). The findings in our study additionally show that NOSTRIN is an independent predictor of prognosis in resected PDAC. Our examination of the functional role of NOSTRIN in pancreatic cancer revealed that it suppresses the migratory and invasive properties of pancreatic cancer cell lines and increases their sensitivity to chemotherapeutic drug, gemcitabine by enhancing apoptosis. These findings are consistent with the hypothesis that NOSTRIN is a potential negative regulator of disease aggressiveness.
NO is a free radical with multiple functions including vasodilation, neurotransmission and inflammatory response. Moreover, NO has been implicated in the development and progression of cancer (12). NO is generated during the conversion of arginine to citruline, a reaction catalyzed by a family of nitric oxide synthases (NOS), which includes neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). Both iNOS and eNOS are implicated in the development and progression of pancreatic cancer (21, 29, 30). In a recent study, genetic and pharmacological inhibition of eNOS resulted in reduced tumorigenesis in a genetically engineered mouse model of PDAC (21). eNOS activity is regulated through phosphorylation in addition to the calcium concentration, and phosphorylated eNOS (p-eNOS) can produce a high and sustained level of NO (22, 31). shRNA-mediated inhibition of eNOS reduced tumor growth of pancreatic cancer cell lines containing highly phosphorylated eNOS (32). Suppression of p-eNOS level and NO production by NOSTRIN in our study indicated that tumor inhibitory role of NOSTRIN may be mediated through inhibition of eNOS-activation and subsequent decrease in NO production. Treatment with NO-donor compound Deta/NO enhanced migration of pancreatic cancer cell lines. Additionally, knocking down NOSTRIN resulted in increased migration of pancreatic cancer cells and was comparable to the increase in cell migration following treatment with NO-donor. These findings suggest that NOSTRIN-induced reduction in the p-eNOS and NO production may negatively regulate the metastatic potential of tumor cells.
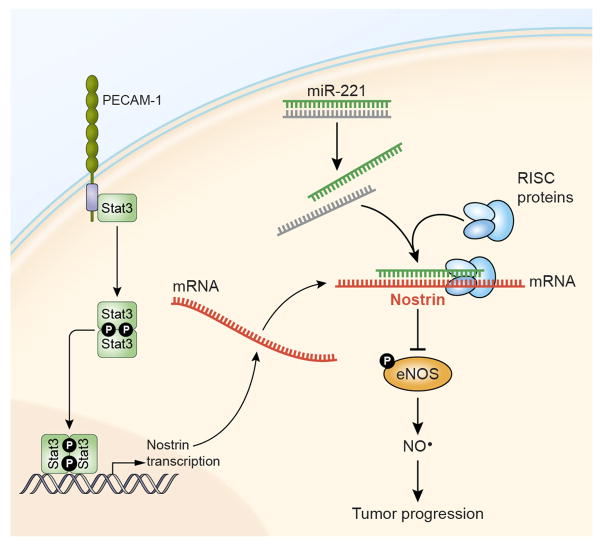
Determining the potential mechanisms for the regulation of NOSTRIN may provide insights into developing strategies for its therapeutic targeting. miRNA-mediated regulation of cancer-related genes has been described as one of the important event in the development and progression of cancer (24). A number of miRNAs are implicated in PDAC and are described as both prognostic biomarkers as well as functional components associated with therapeutic resistance and disease aggressiveness (23, 33, 34). Analysis of the 3′UTR of NOSTRIN revealed potential binding site for miR-221, which is earlier described to be highly expressed in PDAC. Our findings showed that miR-221 targets NOSTRIN and reduces its expression. Furthermore, an increased expression of mir-221 in our cohort was associated with poorer survival in resected cases of PDAC. Whereas, PECAM/STAT3 signaling pathway transcriptionally enhances the expression of NOSTRIN (27), we did not see any difference in the expression of PECAM in tumors between the long and short surviving PDAC patients following resection. Therefore, it is conceivable that inhibition of mir-221 may enhance NOSTRIN leading to the inhibition of eNOS activity, NO production and tumor progression in PDAC (Figure 6).
Figure 6.
NOSTRIN inhibits disease aggressiveness by suppressing eNOS-mediated NO production and is post-transcriptionally regulated by miR-221 in pancreatic cancer. PECAM/Stat3 signaling is earlier shown to enhance the transcription of NOSTRIN (27). (NO: Nitric Oxide; eNOS: endothelial nitric oxide synthase, PECAM-1: Platelet-endothelial cell adhesion molecule-1).
Taken together, our findings identify NOSTRIN as a potential negative regulator of disease progression, which can be targeted in designing rational treatment strategy in patients with PDAC.
Supplementary Material
Statement of translational relevance.
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy, which is mostly detected at an advanced stage, and is minimally responsive to currently available treatments. Even in patients with early detection, and surgical resection with curative intent, disease recurrence is highly frequent. Identifying critical pathways contributing to disease aggressiveness and novel therapeutic targets is high research priority. Here, we describe that endothelial nitric oxide synthase traffic inducer (NOSTRIN), negatively regulates disease aggressiveness in patients with PDAC and is regulated by miR-221. Additional mechanistic and functional analyses linked NOSTRIN to the inhibition of endothelial nitric oxide synthase (eNOS) activation and the production of nitric oxide, which is implicated in pancreatic cancer. Our findings identified NOSTRIN as a novel candidate target for potential therapeutic intervention in PDAC.
Acknowledgments
Authors thank Ms. Elise Bowman for handling of clinical samples and maintenance of tissue database, and the personnel at University of Maryland Medical System for collection of clinical samples under NCI-UMD resource contract. This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH.
Funding: Intramural Research Program of the Center for Cancer Research, NCI, NIH
Footnotes
Conflict of Interest: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016 doi: 10.1016/S0140-6736(16)00141-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng L, Wolfgang CL. Which patients with resectable pancreatic cancer truly benefit from oncological resection: is it destiny or biology? Cancer Biol Ther. 2015;16:360–2. doi: 10.1080/15384047.2014.1002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–9. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–6. doi: 10.1007/s11605-007-0384-8. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2002;99:17167–72. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oess S, Icking A, Fulton D, Govers R, Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396:401–9. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 13.Tamir S, deRojas-Walker T, Gal A, Weller AH, Li X, Fox JG, et al. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res. 1995;55:4391–7. [PubMed] [Google Scholar]
- 14.Tamir S, Tannenbaum SR. The role of nitric oxide (NO.) in the carcinogenic process. Biochim Biophys Acta. 1996;1288:F31–6. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Shi GG, Yao JC, Gong W, Wei D, Wu TT, et al. Expression of endothelial nitric oxide synthase correlates with the angiogenic phenotype of and predicts poor prognosis in human gastric cancer. Gastric Cancer. 2005;8:18–28. doi: 10.1007/s10120-004-0310-7. [DOI] [PubMed] [Google Scholar]
- 16.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–27. [PubMed] [Google Scholar]
- 20.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–66. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampson BL, Kendall SD, Ancrile BB, Morrison MM, Shealy MJ, Barrientos KS, et al. Targeting eNOS in Pancreatic Cancer. Cancer Res. 2012;72:4472–82. doi: 10.1158/0008-5472.CAN-12-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. The Journal of endocrinology. 2011;210:271–84. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 24.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, et al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108:361–9. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Muller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 27.McCormick ME, Goel R, Fulton D, Oess S, Newman D, Tzima E. Platelet-endothelial cell adhesion molecule-1 regulates endothelial NO synthase activity and localization through signal transducers and activators of transcription 3-dependent NOSTRIN expression. Arterioscler Thromb Vasc Biol. 2011;31:643–9. doi: 10.1161/ATVBAHA.110.216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haider S, Wang J, Nagano A, Desai A, Arumugam P, Dumartin L, et al. A multi-gene signature predicts outcome in patients with pancreatic ductal adenocarcinoma. Genome medicine. 2014;6:105. doi: 10.1186/s13073-014-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Xie K. Nitric oxide and pancreatic cancer pathogenesis, prevention, and treatment. Current pharmaceutical design. 2010;16:421–7. doi: 10.2174/138161210790232194. [DOI] [PubMed] [Google Scholar]
- 30.Vickers SM, MacMillan-Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg. 1999;134:245–51. doi: 10.1001/archsurg.134.3.245. [DOI] [PubMed] [Google Scholar]
- 31.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 32.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–9. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soubani O, Ali AS, Logna F, Ali S, Philip PA, Sarkar FH. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis. 2012;33:1563–71. doi: 10.1093/carcin/bgs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–17. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.