Abstract
Oesophagostomum sp. is a parasitic nematode that frequently infects wild chimpanzees. Although nodular lesions are commonly associated with infection, some wild chimpanzee populations seem to tolerate Oesophagostomum nodular lesions while those at Gombe and other sites suffer from associated morbidity and mortality. From August 2004 to December 2013, we examined demographic (i.e., age, sex) and individual correlates (i.e., fecal consistency, Oesophagostomum egg production) to Oesophagostomum-associated pathology in 14 individually recognized chimpanzees at Gombe Stream National Park, Tanzania. In addition, we characterized Oesophagostomum-associated pathology in 14 individual sympatric primates including baboons, colobus, and cercopithecid monkeys. In five chimpanzees, there was no evidence of any significant underlying disease aside from oesophagostomiasis to explain the thin condition or diarrhea. All five of these chimpanzees had moderate to numerous parasitic nodules. In general, nodules were more numerous in older chimpanzees. Three of four chimpanzees with the highest average Oesophagostomum egg counts in feces collected during the year prior to their death had numerous parasitic nodules at necropsy. In contrast, the four chimpanzees with the lowest egg counts had only moderate numbers of nodules. No association (P =0.74) was noted between frequency of diarrhea in the year prior to death and the number of nodules noted at necropsy. Nodules were also present in all baboons examined documenting pathology associated with Oesophagostomum infection in wild baboons. In contrast, no lesions were noted in colobus or cercopithecid monkeys, although it is uncertain if they are infected as no fecal studies have been completed in these species to date at Gombe. Sequence of DNA isolated from nodules in chimpanzees matched (99%) Oesophagostomum stephanostomum. Further research is needed to identify the types of Oesophagostomum causing lesions in baboons and to determine if baboons suffer from these infections.
Keywords: Oesophagostomum stephanostomum, chimpanzee, baboon, red colobus, blue monkey
INTRODUCTION
Parasitic infections are common in wild animals, and non-human primates are no exception. Many health studies of wild primates have utilized coprological (fecal) analysis for parasitological research, including the current health-monitoring program in Gombe National Park, Tanzania [Lonsdorf et al., 2006; Travis et al., 2008]. Several parasitological surveys have been conducted over the past 40 years at Gombe and all have identified a variety of parasitic infections within chimpanzees [Bakuza & Nkwengulila, 2009; File et al., 1976; Gillespie et al., 2010; McGrew et al., 1989; Murray et al., 2000]. In all of these studies, potentially pathogenic Oesophagostomum sp. infections were identified in chimpanzees. Oesophagostomum sp. infections have also been identified via coprological evaluation in chimpanzee populations living in Mahale Mountains National Park, Tanzania [Huffman et al., 1997; Kooriyama et al., 2012], Kibale National Park, Uganda [Ashford et al., 2000; Krief et al., 2010; Muehlenbein, 2005], Budongo Forest Reserve, Uganda [Zommers et al., 2013], Nyungwe National Park, Rwanda [Martz et al., unpublished data], and Taï National Park, Cote d’Ivoire [Metzger et al., unpublished data]. At the Mahale, Kibale, and Taï study sites, Oesophagostomum spp. have been identified via coprological evaluation in sympatric primates including several cercopithecid monkey species (Cercopithecus spp.), red colobus (Procolobus badius), black and white colobus (Colobus guereza, C. angolensis, C. polykomos), mangabeys (Lophocebus albigena, Cercocebus atys), yellow baboons (Papio cynocephalus), and olive baboons (Papio anubis) [Bezjian et al., 2008; Ghai et al., 2014; Gillespie et al., 2004, 2005; Kooriyama et al., 2010, 2012; Kouassi et al., 2015]. At Gombe, Oesophagostomum have previously been identified in olive baboons [Murray et al., 2000].
Oesophagostomum spp. are zoonotic intestinal nematode worms within the family Strongyloidae. In humans, Oesophagostomum spp. infection can result in uninodular or multinodular disease (oesophagostomiasis). Uninodular disease presents as a single, painful, large abdominal mass, whereas in multi-nodular disease there are numerous granulomas within the colonic wall and mesentery that are associated with weight loss, diarrhea, and abdominal pain [Storey et al., 2000]. Adult Oesophagostomum live within the intestines and eggs are excreted into the feces with development to the infective third-stage larvae (L3) outside of the host, generally in 4–7 days (see Gasser et al. [2006] for review). Infection is thought to occur via ingestion of L3 within contaminated water, soil, or food. Studies of Oesophagostomum dentatum have demonstrated survival of infective larvae within the environment for up to or greater than 1 year depending on humidity, temperature, and foliage [Rose & Small, 1980; Waruiru et al., 1998]. After ingestion, L3 burrow into the intestinal wall and form nodules within which they develop to fourth-stage larvae (L4). L4 can either remain within nodules or return to the intestinal lumen to develop into adults.
Pathologic lesions associated with Oesophagostomum sp. infection have been described in wild chimpanzees from Kibale National Park in Uganda (Pan troglodytes schweinfurthii), Taï National Park in Cote d’Ivoire (Pan troglodytes verus), and Gombe National Park, Tanzania (Pan troglodytes schweinfurthii) [Krief et al., 2008; Terio et al., 2011]. At all of these sites, lesions were consistent with the multi-nodular form. While chimpanzees in the Kibale and Taï populations had died of other causes and lacked observable clinical signs associated with the disease [Krief et al., 2008], at Gombe some of the individual chimpanzees had concurrent weight loss and clinical signs suggestive of significant infections [Terio et al., 2011]. Similarly, chimpanzees at Mahale also appear to suffer from oesophagostomiasis [Huffman et al., 1997]. Why chimpanzees at some sites seem to tolerate Oesophagostomum nodular lesions while those at Gombe and other sites suffer from associated morbidity and mortality is uncertain.
The goal of this study was to better understand Oesophagostomum infections in non-human primates at Gombe. We utilized data collected over the past 11 years as part of the comprehensive health monitoring program at Gombe [Gillespie et al., 2010; Lonsdorf et al., 2006; Terio et al., 2011; Travis et al., 2008] to test associations between ante-mortem fecal parameters and post-mortem lesions in chimpanzees. As some chimpanzees have been noted clinically to be in thin condition with no other significant underlying disease [Terio et al., 2011], we hypothesized that chimpanzees with more severe infections, defined as increased numbers of intestinal and mesenteric granulomas, would have a higher frequency of non-solid feces. Oocyst numbers (egg counts) do not always correspond to severity of infection as they can be impacted by season, sample quality and host factors [Gillespie, 2006; Gillespie et al., 2010]. However, patterns in the prevalence of diarrhea correspond to patterns in gastrointestinal parasitism [Lonsdorf et al., 2016], so we hypothesized that average fecal egg counts during the year prior to death in animals with more moderate to severe oesophagostomiasis would be higher than in those with mild disease at necropsy. To better understand the ecology of Oesophagostomum infections within the Gombe ecosystem, given the widespread prevalence among sympatric primates in other populations, we hypothesized that nodular lesions associated with Oesophagostomum sp. larvae would be present within the intestines and mesentery of sympatric baboons, colobus, and cercopithecid monkeys. Finally, to better characterize the infections, we predicted that the Oesophagostomum sp. in chimpanzees at Gombe would be similar to those characterized in other wild chimpanzee populations. The results of this study will, thus, provide preliminary data upon which we can build subsequent, more comprehensive surveys of Oesophagostomum sp. infections in order to further elucidate the pathogenesis and conservation impact of this infection on non-human primates.
METHODS
Permission and support to carry out research at Gombe were granted by the Government of Tanzania, Tanzania National Parks, Tanzania Commission for Science and Technology, and the Tanzania Wildlife Research Institute. All samples and data were collected non-invasively and in compliance with the IUCN Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations [Gilardi et al., 2015]. Fixed tissues were imported in accordance with CITES and USFWS regulations. The study adhered to the American Society of Primatologists Principles for the Ethical Treatment of NonHuman Primates.
Gombe National Park is a 35 km2 park located at the western border of Tanzania along Lake Tanganyika. This park is home to many different species of primates, including three communities of chimpanzees (Kasekela, Mitumba, and Kalande), olive baboons, blue monkeys, and red colobus monkeys. From August 2004 to December 2013, carcasses suitable for necropsy were available from naturally deceased sympatric primates (N =28) greater than 6 months of age including 14 chimpanzees (4 males, 10 females), five olive baboons (two males, two females, one undetermined gender), four red colobus (one male, one female), and five blue (Cercopithecus mitus) or hybrid (Cercopithecus mitus × ascanius) monkeys (three male and two undetermined gender). Individual information is presented in Table I. All of the chimpanzees were individually recognized with known ages. Four baboons were known individuals of advanced age and two were unknown. None of the colobus or blue monkeys were known individuals, but ages of all were classified as adult based on morphology and lesions. Carcasses were recovered between 6 and 48 hr of death and necropsied immediately or frozen (−20°C) until trained personnel were available. All personnel involved with necropsies were trained in appropriate biosafety precautions and primate necropsy techniques.
TABLE I.
Numbers of Granulomas at the Time of Death in Comparison With Body Condition, Average Fecal Egg Counts, and Ante-Mortem Diarrhea in Non-Human Primates of Gombe National Park
| ID | Sex | Agea | Chimpanzee community or species | Granulomas | Other significant lesions | Conditionb | Ave egg countsc | Diarrheae |
|---|---|---|---|---|---|---|---|---|
| Bb-053a | F | 0.5 | Kasekela | None | Trauma | Good | NDd | ND |
| BB-042a | F | 0.75 | Mitumba | None | Trauma | Good | ND | ND |
| Ch-124 | F | 6 | Mitumba | Numerous | None | Thin | 40.5 (2) | 0.20 |
| Ch-069 | M | 8 | Mitumba | Few | Trauma | Good | ND | ND |
| Ch-061 | F | 13 | Kasekela | Numerous | Acute bacterial sepsis | Emaciated | 29 (2) | 0.60 |
| Ch-019 | F | 15 | Kasekela | Moderate | None | Emaciated | 5.5 (4) | 0.50 |
| Ch-028 | F | 16 | Kasekela | Moderate | None | Good | 19.25 (4) | 0.13 |
| Ch-021 | F | 20 | Kasekela | Few | AIDS-like disease | Emaciated | 4 (5) | 0.33 |
| Ch-036 | F | 24 | Kasekela | Numerous | AIDS-like disease | Emaciated | 234 (2) | 0.50 |
| Ch-045 | M | 28 | Mitumba | Numerous | Trauma | Good | ND | 0.27 |
| Ch-099 | F | 29 | Kasekela | Moderate | Trauma | Thin | 39 (1) | 0.00 |
| Ch-013 | M | 37 | Kasekela | Moderate | Renal disease | Emaciated | 12 (2) | 0.75 |
| Ch-016 | M | 39 | Kasekela | Numerous | None | Emaciated | ND | 0.67 |
| Ch-024 | F | 44 | Kasekela | Numerous | Trauma | Thin | ND | 0.10 |
| 72057 | F | Aged | Baboon | Numerous | Excessive teeth wear | Thin | ND | ND |
| 112056 | F | Adult | Baboon | Moderate | Renal/cardiac disease | Thin | ND | ND |
| 112069 | M | Adult | Baboon | Numerous | None | Good | ND | ND |
| 112070 | Unknown | Adult | Baboon | Few | Renal disease | Good | ND | ND |
| 112071 | M | Aged | Baboon | Numerous | Peritonitis | Emaciated | ND | ND |
| 112055 | M | Adult | Cercopithecus | None | Renal disease | Thin | ND | ND |
| 112067 | Unknown | Adult | Cercopithecus | None | Pneumonia | Good | ND | ND |
| 112057 | M | Adult | Cercopithecus | None | Renal disease | Good | ND | ND |
| 112058 | M | Adult | Cercopithecus | Few | Trauma | Emaciated | ND | ND |
| 122194 | Unknown | Adult | Cercopithecus | None | None | Good | ND | ND |
| 72058 | M | Adult | Red colobus | None | Bloat | Thin | ND | ND |
| 112068 | M | Adult | Red colobus | None | Pneumonia | Good | ND | ND |
| 122192 | F | Aged | Red colobus | None | Pneumonia | Good | ND | ND |
| 122193 | F | Adult | Red colobus | None | Pneumonia | Good | ND | ND |
Age in years where known.
Body condition based on palpable bony protuberances, adipose, and skeletal muscle atrophy.
Average counts of eggs consistent with Oesophagostomum in feces collected 1 year prior to death. Number in parentheses is the number of examined fecal samples for egg counts.
Not done.
Frequency of non-solid feces.
Complete gross necropsies were performed on all retrieved carcasses by teams of field biologists, clinical veterinarians, and/or veterinary pathologists. Sections of brain, pituitary gland, adrenal gland, thyroid gland, eye, skin, skeletal muscle, peripheral nerve, tongue, salivary gland, trachea, esophagus, lung, heart, diaphragm, stomach, pancreas, small intestine, cecum, colon, kidney, urinary bladder, testis, uterus, ovary, mammary gland, liver, spleen, bone, bone marrow, and lymph nodes (peripancreatic, tracheobronchial, peripheral, and mesenteric) were collected, as available, and fixed in 10% neutral buffered formalin and selected tissues saved in RNAlater® (Thermofisher, Waltham, MA). Tissues were routinely processed for histopathology, embedded in parafin, sections cut at 3–5 μm and stained with hematoxylineosin. All tissues were reviewed histologically by two board-certified veterinary pathologists (KAT, MJK). Numbers of parasitic granulomas were graded subjectively as numerous, moderate, few, and none based on gross necropsy descriptions, digital images of the abdomen, and numbers of granulomas present within histologic sections.
For PCR, nodules with embedded worms (L3 or L4) in RNAlater® preserved sections of mesentery and intestine from four chimpanzees (Ch-13, Ch-16, Ch-19, and Ch-61) were dissected and DNA extracted using a commercially available kit (DNeasy, Qiagen, Valencia CA) following the manufacturer’s instructions. A 260 bp fragment of the internal transcribed spacer 2 (ITS-2) and contiguous 28s rRNA gene of Oesophagostomum was amplified using primers NC2 and OesophITS2-21 as previously described [Ghai et al., 2014]. PCR was performed using 2.5 μl of extracted DNA within a 25 μl reaction volume containing standard amounts of AmpliTaq® Gold DNA Polymerase and 10 mM each of dNTP (Life Technologies, Grand Island, NY), 25 pmol of each primer, and 1.5 mM MgCl2. Amplification (94°C for 2 min; 35 cycles of 94°C for 1 min, 55°C for 1 min, 72° C for 1 min; and 72°C for 2 min) was performed in a GeneAmp® 9700 model thermocycler (Life Technologies). Negative (sterile water substituted for DNA extract) controls were included in each reaction. Nucleotide sequences were determined on an automated sequencer (Life Technologies 3730XL) and both strands were sequenced using primers NC2 and OesophITS2-21. Forward and reverse sequences were aligned to resolve ambiguous bases using Sequencher 5.0 software (GeneCodes, Ann Arbor MI). Sequences were compared to both known species of Oesophagostomum in GenBank and to each other after trimming to the shortest sequence. Sequences were aligned using Clustal W and evolutionary history inferred by using the Maximum Likelihood method based on the Tamura–Nei model using MEGA version 6 [Tamura et al. 2013]. Sequences were deposited in GenBank under Accession Numbers KT865106-KT865109. Intestinal and mesenteric nodules from other affected chimpanzees, deceased baboons, colobus, and cercopithecid monkeys were only saved in 10% formalin and had been in formalin for extended (>6 months) periods of time prior to importation and thus were unsuitable for PCR.
All observational health data from deceased chimpanzees were extracted from the Gombe chimpanzee module of IMPACT™ (Internet-supported Management Program to Assist Conservation Technology) for 1 year prior to death [Lonsdorf et al., 2016]. Data was available from all-day focal animal follows on individuals of the two habituated communities in the park, Kasekela and Mitumba. Information on presence/absence and severity of fecal frequency and consistency were identified and the frequency of non-solid feces determined from focal animal follows. Frequency of non-solid feces in chimpanzees with numerous granulomas versus moderate and few granulomas was assessed with a two-tailed Mann–Whitney U-test (Systat 13, Systat Software, Inc, Chicago, IL). A P-value less than or equal to 0.05 was considered to be statistically significant. Any clinical signs suggestive of diarrhea or abdominal discomfort were also noted from veterinary summaries provided with necropsy tissues. Similar clinical data were not available from other non-human primates within the study.
Freshly voided feces were collected from individually identified chimpanzees and stored in Para-Pak® (Meridian Bioscience, Cincinnati, OH) containers as previously described [Gillespie et al., 2010]. Oesophagostomum eggs were recovered via sodium nitrate flotation using standardized techniques [Gillespie, 2006]. Eggs were identified based on size, shape, and contents and quantified. Fecal egg counts were conducted on flotation preparations of 1 g of feces [Gillespie, 2006]. Average fecal egg counts for eggs morphologically consistent with Oesophagostomum were determined from all available samples collected from each deceased chimpanzee as available (N =8) during the 1 year prior to the chimpanzees death. Numbers of available samples for fecal egg quantification varied by chimpanzee (N =1–5). Of the four known baboons, no feces were collected for analysis in the 1 year prior to death. Similarly, the remaining two baboons, colobus, and cercopithecid monkeys were not known individuals at the time of death and no corresponding feces were available for analysis.
RESULTS
Nodules with parasites morphologically consistent with Oesophagostomum were present at necropsy along the colon and mesentery of all chimpanzees greater than 1 year of age and all examined baboons (Table I and Fig. 1). At necropsy, many of these nodules are black. Histologically, nodules expand and separate layers of the intestinal wall and separate mesenteric connective tissue (Fig. 2). Nodules in both species were similar and characterized by central necrotic, variably mineralized debris surrounded by a variably thick fibrous capsule with small to moderate numbers of neutrophils, macrophages, lymphocytes, and plasma cells. Within the central debris were sections of 300–700 micron diameter metazoan parasites with a thick smooth eosinophilic cuticle, platymyarian musculature, lateral chords, pseudocoelom, triradiate esophagus, large intestine lined by a few multinucleated cells with a prominent brush border and reproductive tract. One mesenteric granuloma was noted in a single unknown blue monkey. This granuloma was similar morphologically to those described in chimpanzees and baboons but lacked internal parasites. No granulomas were noted in the red colobus. In rare cases (Fig. 3), larvae were present within the intestinal mucosa and could be seen within the submucosa and muscularis, consistent with migration of ingested L3 from the intestinal lumen into the wall and mesentery where they form nodules.
Fig. 1.
Multiple dark nodules visible at necropsy along the colonic surface and within the mesentery of a chimpanzee (Ch-021).
Fig. 2.
Oesophagostomum nodule disrupting and separating the layers of the colonic wall in a baboon (112056). The Oesophagostomum larva (arrow) is surrounded by numerous inflammatory cells and the nodule wall delineated by arrowheads. The muscular layer of the colonic wall is labeled with a “M” and colonic lumen with a “L.” Bar =200 μm.
Fig. 3.
O. stephanostomum (confirmed by PCR and sequencing) larvae migrating through the mucosa and into the wall of the colon in a chimpanzee (Ch-019). Arrows are pointing to representative larvae within the image. Bar =20 μm.
In general, parasitic granulomas were more numerous in older chimpanzees with the exception of two individuals: a 6-year female who had been orphaned and a 13-year female with other significant underlying disease. Body condition at necropsy was thin to emaciated based on palpable bony protuberances, reduced adipose, and skeletal muscle atrophy in 8/10 chimpanzees with moderate to numerous nodules. In five of these eight individuals, there was no evidence of any other significant underlying disease to explain the thin body condition. Nodules were present in all evaluated baboons and considered moderate to numerous in all but one adult (baboon 112070). Exact ages are unknown for baboons and all examined were already adults or considered aged based on teeth wear and lesions. Additionally, little detailed individual history was available from baboons to evaluate presence/absence of diarrhea.
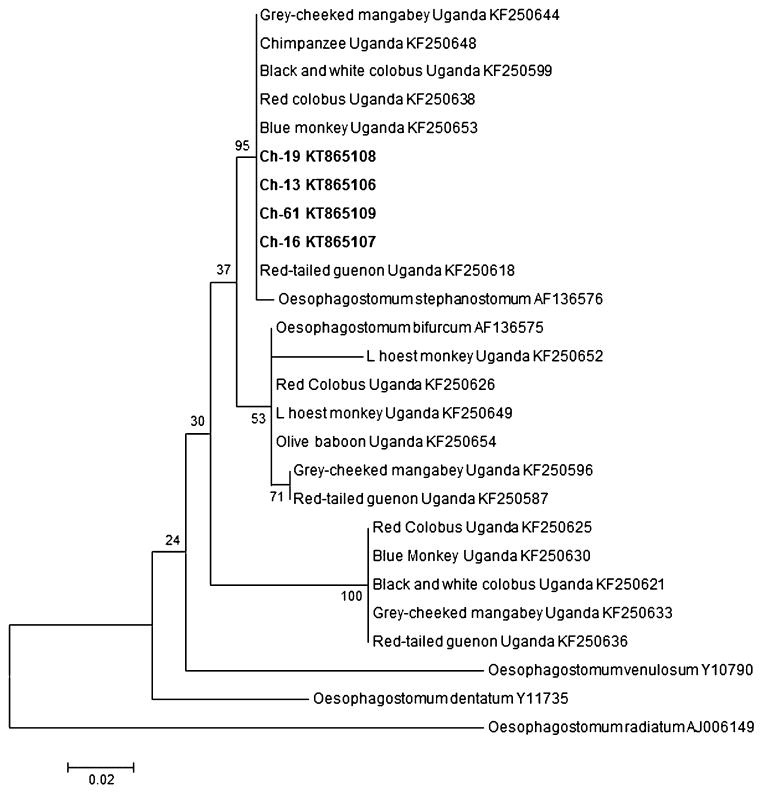
Oesophagostomum was confirmed by PCR in four chimpanzees with affected sections of intestines preserved in RNAlater. Analysis of the 260 bp fragment of the ITS-2 DNA sequence was consistent with Oesophagostomum stephanostomum. Samples from Gombe chimpanzees were >99% identical to each other and 99–100% similar to those reported from chimpanzees, blue monkeys, red colobus, black and white colobus, gray-cheeked mangabey, and red-tailed guenon from Uganda (Fig. 4).
Fig. 4.
Phylogenetic analysis of Oesophagostomum in primates based on ITS2 rDNA (245 bp sequences). Sequences from Gombe National Park chimpanzees are in bold. Genbank accession numbers are after parasite species or country for non-human primate samples. Maximum likelihood phylogeny using Tamura–Nei substitution method in MEGA 6. The percentage of trees in which the associated taxa clustered together is shown next to the branches.
Observational data was available from 14 chimpanzees prior to death with standardized data from focal follows available from 12 chimpanzees. In these individuals, direct observation of defecation occurred 0–10 times with a total of 67 observations during the 1 year prior to death. Frequency of non-solid feces varied from 0% to 75% (Table I) and was unrelated (U =13.5, df =1, P =0.74) to Oesophagostomum load at the time of necropsy. There were no focal follows of two chimpanzees from the Mitumba community. Observational data from veterinary records indicated that one chimpanzee (Ch-016) had diarrhea prior to death. This chimpanzee was emaciated at death and had numerous granulomas with no other significant underlying disease.
Of the deceased chimpanzees, fecal samples with quantified egg counts were available from eight individuals (Table I). Quantification of eggs in fecal samples was not started until 2006 and, thus, was not available for the five chimpanzees that died before this date. Additionally, fecal samples had not been collected from some infants. While the numbers are small and preclude statistical evaluation, three of four chimpanzees with the highest average egg counts for the year prior to their demise had numerous parasitic granulomas at necropsy. In contrast, the four chimpanzees with the lowest egg counts had only moderate numbers of granulomas.
DISCUSSION
Chimpanzees and baboons living within the Gombe ecosystem are infected by Oesophagostomum sp. and infection results in large numbers of mural colonic and mesenteric nodules consistent with multi-nodular oesophagostomiasis in both species. Lesions are similar to those previously described in captive primates, wild-caught primates in sanctuaries and to a few free-ranging chimpanzees [Abbott & Majeed, 1984; Krief et al., 2008; Terio et al., 2011]. In contrast to the large numbers of granulomas noted in Gombe chimpanzees and baboons, only one granuloma of uncertain etiology was noted in a single blue monkey and no granulomas were noted in red colobus.
Oesophagostomum infections clearly occur in red colobus and cercopithecid monkeys at other sites based on fecal studies. While a high prevalence of Oesophagostomum eggs have been noted within feces of chimpanzees [Bakuza & Nkwengulila, 2009; File et al., 1976; Gillespie et al., 2010; McGrew et al., 1989; Murray et al., 2000] and baboons [Murray et al., 2000], systematic parasitological studies of red colobus or blue monkeys have not been conducted at Gombe, to date. Therefore, it is uncertain if the absence of disease in the colobus and cercopithecid monkeys at Gombe is due to an absence of exposure or other factors. In the Taï National Park, Oesophagostomum have been noted in cercopithecid species sympatric with chimpanzees [Kouassi et al., 2015]. In Uganda, Oesophagostomum infects colobus, guenons and blue monkeys but at lower prevalences than chimpanzees although results were described in separate but related studies [Gillespie et al., 2004, 2005; Muehlenbein, 2005]. Further, in a more recent study, red colobus had an Oesophagostomum prevalence of 41% by fecal PCR which was much lower than that noted in sympatric chimpanzees (100%) [Ghai et al., 2014]. In contrast, at Mahale Mountains National Park in Tanzania, Oesophagostomum were noted in all examined species of primates but the prevalence in red-tailed monkeys and red colobus were higher than those noted in baboons [Kooriyama et al., 2012]. Some data support the hypothesis that the more arboreal species have lower exposure to nematode larvae than more terrestrial species; however, the data for Oesophagostomum specifically are less clear [Ghai et al., 2014; Kooriyama et al., 2012]. Thus, it is uncertain if relatively lower exposure in more arboreal primates diminishes the potential for pathogenic infection. Alternatively, differences in gut physiology could affect disease pathogenesis despite high levels of exposure [Stevens & Hume, 2004]. Clearly, further evaluation of Oesophagostomum infection in these species is needed to better understand the ecology of this disease within the Gombe ecosystem.
Previous coprological studies of Oesophagostomumin baboons and chimpanzees, including research at Mahale and Gombe, has noted significant differences in prevalence of eggs between baboons (14% and 17%) and chimpanzees (61% and 73%) [Kooriyama et al., 2012; Murray et al., 2000]. In this study, infection was ubiquitous (100%) among all chimpanzees and baboons greater than 1 year of age based on the presence of nodules at necropsy. Some of the discrepancy may be due to methodological differences. Shedding of eggs requires adult worms within the intestinal lumen and thus stage of infection may account for some of this difference. The trend for higher egg counts in individual chimpanzees with the highest nodule burden suggests that there may be a correlation between severity of infection and shedding and is an area of further needed study. If predictive, then this could be utilized to identify “at risk” individuals for potential intervention. Other factors that affect prevalence include season (lower in the dry season), year and community membership (i.e., Kasekela vs. Mitumba) [Gillespie et al., 2010]. Similar to the current necropsy study, the longest and most intensive previous coprological study at Gombe also found high prevalence of infection in all age classes of chimpanzee [Gillespie et al., 2010]. This is in contrast to another study in these chimpanzees [Murray et al., 2000]; however, the relatively short duration of the sampling period (8 weeks) during dry season months of July and August likely impacted the overall prevalence as they would have been highly influenced by season.
Previous morphologic study of worms obtained at necropsy from nodules in chimpanzees had identified them as Oesophagostomum sp. and probably O. stephanostomum based on the wide anterior portion of the buccal cavity with six denticles projecting into the lumen of the esophageal funnel [Terio et al., 2011]. Partial sequence of the ITS-2 and 28s rRNA gene obtained in this current study was consistent with this classification. While there was minor variation in sequence among Gombe chimpanzees, sequences were similar to those previously reported from non-human primates in Uganda [Ghai et al., 2014]. Additional chimpanzees should be screened by PCR in the future as co-infections with both O. stephanostomum and O. bifurcum have been noted in animals with mophologically indistinguishable eggs [Krief et al., 2010]. As suitable material for PCR was only available from chimpanzees, further research is also needed to assess the diversity of Oesophagostomum sp. among other primates within the Gombe ecosystem, in particular baboons. PCR should also be used to screen colobus and cercopithecid monkeys as it may be more sensitive than light microscopic evaluation in animals with low potential levels of infection [Ghai et al., 2014].
At other sites, Oesophagostomum infections in wild primates do not appear to affect overall health [Krief et al., 2008]. At Gombe, several individuals presented for necropsy with high nodule burdens also had a history of thin body condition, concurrent diarrhea, and or a history of clinical signs consistent with abdominal pain. Self-medication has been proposed at several sites as one possible mechanism for preventing pathogenic infections [Fowler et al., 2007; Huffman, 1997; Krief et al., 2008]. While adults are more likely to be observed with diarrhea [Lonsdorf et al., 2016] which corresponds to differences in prevalence of pathogenic parasitism [Gillespie et al., 2010], in this study there was no association between diarrhea frequency in the 6 and 12 months prior to death and nodule burden at necropsy. This could be due to the relatively crude categories of nodule burden utilized or smaller number of fecal samples available for analysis in the months immediately prior to death. As illness progresses, some but not all, chimpanzees disappear within the forest or separate from their community decreasing the frequency of observation [Gillespie et al., 2008]. After they are noticed to be ill, data is recorded in veterinary records rather than the standardized observations within the IMPACT™ database so some of the relevant data is not collated in the same manner. Other noted clinical signs such as thin condition and apparent abdominal discomfort are non-specific and could be due to other factors. Nonetheless, it remains that several individual chimpanzees in thin condition and with diarrhea had no other significant lesions noted on necropsy to explain these signs suggesting that lesions in these individuals were clinically significant.
This study integrated data from several aspects of the Gombe Ecohealth Program including observational, parasitological and necropsy data. Continued studies of Oesophagostomum infections in this ecosystem are needed to better understand the ecology and pathogenesis of disease, particularly the role that different primates play in maintenance and transmission. Previous research from the Gombe Ecohealth Program has evaluated observational and parasitological data at the population but not previously been integrated with necropsy data on the individual level. As data from future necropsies accumulates, additional analysis of individual animal trends will be possible allowing us to test whether the population level associations also occur within individuals and can be linked to pathogenic mechanisms. Continuing to integrate data from multiple sources and disciplines will be critical toward improving our understanding of disease epidemiology and pathogenesis using Gombe as a model ecosystem.
Acknowledgments
Contract grant sponsor: US Fish and Wildlife Great Ape Conservation Fund; contract grant sponsor: Arcus Foundation; contract grant sponsor: National Institutes of Health; contract grant numbers: R01 AI58715, R00 HD057992; contract grant sponsor: Morris Animal Foundation; contract grant number: MAF D09ZO-041; contract grant sponsor: Emory University; contract grant sponsor: Lincoln Park Zoo’s Davee Center for Epidemiology and Endocrinology; contract grant sponsor: Lester E. Fisher for the Study and Conservation of Apes.
The authors wish to thank Baraka Gilagiza, Drs. Anna Mosser, Ephata Kaaya, Titus Mlengeya, Emily Wroblewski, Jared Bakuza, and Kate Detwiler as well as the field staff at Gombe Stream Research Center for assistance with research and necropsies, The Jane Goodall Institute and Tanzania National Parks (TANAPA) for support of the health monitoring program, and the histopathology laboratory at the University of Illinois Veterinary Diagnostic Laboratory for excellent technical assistance. We thank Nadia Ahmed, Elizabeth Canfield, Kristen Cross, Robert Girodano, Naomi Hauser, and Michele Moses for laboratory assistance. Permission and support to carry out research at Gombe were granted by the Government of Tanzania, Tanzania National Parks, Tanzania Commission for Science and Technology, and the Tanzania Wildlife Research Institute. Fixed tissues were imported in accordance with CITES and USFWS regulations. This work was funded in part by the US Fish and Wildlife Great Ape Conservation Fund, the Arcus Foundation, the National Institutes of Health (R01 AI58715, R00 HD057992), Morris Animal Foundation (MAF D09ZO-041), Emory University, the Lincoln Park Zoo’s Davee Center for Epidemiology and Endocrinology, and the Lester E. Fisher for the Study and Conservation of Apes.
References
- Abbott DP, Majeed SK. A survey of parasitic lesions in wild-caught, laboratory-maintained primates: (Rhesus, Cynomolgus, and Baboon) Veterinary Pathology. 1984;21:198–207. doi: 10.1177/030098588402100212. [DOI] [PubMed] [Google Scholar]
- Ashford RW, Reid GDF, Wrangham RW. Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Annals of Tropical Medicine and Parasitology. 2000;94:173–179. doi: 10.1080/00034980057518. [DOI] [PubMed] [Google Scholar]
- Bakuza JS, Nkwengulila G. Variation over time in parasite prevalence among free-ranging chimpanzees at Gombe National Park, Tanzania. International Journal of Parasitology. 2009;30:43–53. [Google Scholar]
- Bezjian M, Gillespie TR, Chamna CA, Greiner EC. Coprologic evidence of gastrointestinal helminths of forest baboons, Papio anubis, in Kibale National Park, Uganda. Journal of Wildlife Diseases. 2008;44:878–887. doi: 10.7589/0090-3558-44.4.878. [DOI] [PubMed] [Google Scholar]
- File SK, McGrew WC, Tutin CEG. The intestinal parasites of a community of feral chimpanzees, Pan troglodytes schweinfurthii. Journal of Parasitology. 1976;62:259–261. [PubMed] [Google Scholar]
- Fowler A, Koutsioni Y, Sommer V. Leaf-swallowing in Nigerian chimpanzees: evidence for assumed self-medication. Primates. 2007;48:73–76. doi: 10.1007/s10329-006-0001-6. [DOI] [PubMed] [Google Scholar]
- Gasser RB, de Gruijter JM, Polderman AM. Insights into the epidemiology and makeup of Oesophagostomum bifurcum from human and non-human primates using molecular tools. Parasitology. 2006;132:453–460. doi: 10.1017/S0031182005009406. [DOI] [PubMed] [Google Scholar]
- Ghai RR, Chapman CA, Omeja PA, Davies TJ, Goldberg TL. Nodule worm infection in humans and wild primates in Uganda: cryptic species in a newly identified region of human transmission. PloS Neglected Tropical Diseases. 2014;8:e2641. doi: 10.1371/journal.pntd.0002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Gillespie TR, Leendertz FB, Macfie EJ, Travis DA, Whittier CA, Williamson EA. Best practice guidelines for health monitoring and disease control in great ape populations. Gland, Switzerland: IUCN SSC Primate Specialist Group; 2015. p. 56. [Google Scholar]
- Gillespie TR. Non-invasive assessment of gastrointestinal parasite infections in free-ranging primates. International Journal of Primatology. 2006;27:1129–1143. [Google Scholar]
- Gillespie TR, Greiner EC, Chapman CA. Gastrointestinal parasites of the guenons of western Uganda. Journal of Parasitology. 2004;90:1356–1360. doi: 10.1645/GE-311R. [DOI] [PubMed] [Google Scholar]
- Gillespie TR, Greiner EC, Chapman CA. Gastrointestinal parasites of the colobus monkeys of Uganda. Journal of Parasitology. 2005;91:569–573. doi: 10.1645/GE-434R. [DOI] [PubMed] [Google Scholar]
- Gillespie TR, Nunn CL, Leendertz FH. Integrative approaches to the study of primate infectious disease: implications for biodiversity conservation and global health. Yearbook of Physical Anthropology. 2008;51:53–69. doi: 10.1002/ajpa.20949. [DOI] [PubMed] [Google Scholar]
- Gillespie TR, Lonsdorf EV, Canfield EP, et al. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. American Journal of Physical Anthropology. 2010;143:534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman MA. Current evidence for self-medication in primates: a multidisciplinary perspective. Yearbook of Physical Anthropology. 1997;40:171–200. [Google Scholar]
- Huffman MA, Gotoh S, Turner L, Yoshida K. Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates. 1997;38:111–125. [Google Scholar]
- Kooriyama T, Inaba A, Nishida T, Iwaki T. Case report of helminths and lung mite infection in the red-tailed monkey, Cercopithecus ascanus schmidti, in Mahale Mountains National Park, Tanzania. Primates. 2010;51:183–188. doi: 10.1007/s10329-009-0185-7. [DOI] [PubMed] [Google Scholar]
- Kooriyama T, Hasegawa H, Shimozuru M, et al. Parasitology of five primates in Mahale Mountains National Park, Tanzania. Primates. 2012;53:365–375. doi: 10.1007/s10329-012-0311-9. [DOI] [PubMed] [Google Scholar]
- Kouassi RY, McGraw SW, Yao PK, et al. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d’Ivoire. Parasite. 2015;22:1. doi: 10.1051/parasite/2015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Jamart A, Mahé S, et al. Clinical and pathologic manifestation of oesophagostomosis in African great apes: does self-medication in wild apes influence disease progression? Journal of Medical Primatology. 2008;37:188–195. doi: 10.1111/j.1600-0684.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- Krief S, Vermeulen B, Lafosse S, et al. Nodular worm infection in wild chimpanzees in Western Uganda: a risk for human health? PLoS Neglected Tropical Diseases. 2010;4:e630. doi: 10.1371/journal.pntd.0000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Travis D, Pusey AE, Goodall J. Using retrospective health data from the Gombe chimpanzee study to inform future monitoring efforts. American Journal of Primatology. 2006;68:897–908. doi: 10.1002/ajp.20296. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Gillespie TR, Wolf TM, et al. Socio-ecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes schweinfurthii) at Gombe National Park, Tanzania. American Journal of Primatology. 2016 doi: 10.1002/ajp.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew MC, Tutin CEG, Collins DA, File SK. Intestinal parasites of sympatric Pan troglodytes and Papio spp. at two sites: Gombe (Tanzania) and Mt. Assirik (Senegal) American Journal of Primatology. 1989;17:147–155. doi: 10.1002/ajp.1350170204. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. American Journal of Primatology. 2005;65:167–179. doi: 10.1002/ajp.20106. [DOI] [PubMed] [Google Scholar]
- Murray S, Stem C, Boudreau B, Goodall J. Intestinal parasites of baboons (Papio cynocephalus anubis) and chimpanzees (Pan troglodytes) in Gombe National Park. Journal of Zoo and Wildlife Medicine. 2000;31:176–178. doi: 10.1638/1042-7260(2000)031[0176:IPOBPC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rose JH, Small AJ. Observations on the development and survival of the free-living stages of Oesophagostomum dentatum both in their natural environments out-of-doors and under controlled conditions in the laboratory. Parasitology. 1980;81:507–517. doi: 10.1017/s0031182000061898. [DOI] [PubMed] [Google Scholar]
- Stevens EC, Hume ID. Comparative physiology of the vertebrate digestive system. 2. Cambridge: Cambridge University Press; 2004. p. 420. [Google Scholar]
- Storey PA, Faile G, Hewit E, et al. Clinical epidemiology and classification of human oesophagostomiasis. Transasctions of the Royal Society of Tropical Medicine and Hygeine. 2000;94:177–182. doi: 10.1016/s0035-9203(00)90267-0. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stetcher G, Peterson D, Filipski A, Kumar S. MEGA 6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, et al. Pathologic lesions in chimpanzees (Pan troglodytes schweinfurthii) from Gombe National Park, Tanzania, 2004–2010. Journal of Zoo and Wildlife Medicine. 2011;42:597–607. doi: 10.1638/2010-0237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis DA, Lonsdorf EV, Mlengeya T, Raphael J. A science-based approach to managing disease risks for ape conservation. American Journal of Primatology. 2008;70:745–750. doi: 10.1002/ajp.20566. [DOI] [PubMed] [Google Scholar]
- Waruiru RM, Munyua WK, Thamsborg SM, et al. Development and survival of infective larvae of gastrointestinal nematodes of cattle on pasture in central Kenya. Veterinary Research Communications. 1998;22:315–323. doi: 10.1023/a:1006112802459. [DOI] [PubMed] [Google Scholar]
- Zommers Z, Macdonald DW, Johnson PJ, Gillespie TR. Impact of human activities on chimpanzee ground use and parasitism (Pan troglodytes) Conservation Letters. 2013;6:264–273. [Google Scholar]