Abstract
Background
Graft-versus-Host Disease (GVHD) is a complication of allogeneic hematopoietic stem cell transplantation (HSCT). Transplacental maternal engraftment (TME), the presence of maternal T cells in peripheral blood prior to transplant, is detectable in a significant proportion of SCID patients. While the presence of TME is associated with a decreased risk of rejecting a maternal graft, it is unknown whether TME plays a role in development of GVHD post HSCT.
Objective
The purpose of this study was to determine whether the presence of pre-transplant TME is associated with post-transplant GVHD in SCID patients.
Methods
This was an institutional retrospective review of 74 patients with SCID transplanted between 1988–2014. The incidence of acute GVHD was compared in patients with TME versus those without TME. Confounding variables such as donor type and conditioning regimen were included in a multivariate regression model.
Results
TME was identified in 35 of 74 children. Post-HSCT acute GVHD developed with an incidence of 57.1% vs 17.9% in those without TME (p<0.001). In univariate analysis, donor type (mother) and GVHD prophylaxis (T cell depletion) were also significant predictors of acute GVHD. In multivariate analysis, TME and chemotherapy conditioning were independent risk factors for the development of aGVHD (RR=2.75, p=0.006 and RR=1.42, p=0.02, respectively).
Conclusion
TME independently predicts the development of post-transplant aGVHD, even when controlling for donor type and conditioning used. The presence of TME should be considered when assessing the risk of aGVHD in SCID patients and designing the approach for GVHD prophylaxis.
Keywords: SCID, transplant, HSCT, maternal, engraftment, GVHD, graft-versus-host, haploidentical, conditioning
Capsule Summary
The presence of transplacental maternal engraftment (TME) varies in pre-transplant SCID patients. This study demonstrates that pre-transplant TME is an independent risk factor for the development of post-transplant acute graft-versus-host disease.
Introduction
Severe combined immunodeficiency (SCID) is a genetically heterogeneous group of immune disorders characterized by a reduced number of T lymphocytes associated with a functional or quantitative defect in B lymphocytes and/or NK cells (1–3). A recent analysis from 11 states in the United States participating in newborn screening for SCID estimates the incidence at 1 in 58,000 live births (4). SCID results in susceptibility to a variety of infections; if untreated it is typically fatal within the first years of life. While enzyme replacement therapy and gene therapy may be of benefit to some SCID patients, the current mainstay of treatment is hematopoietic stem cell transplantation (HSCT), which offers curative immune reconstitution (5).
The ideal donor for HSCT is a human leukocyte antigen (HLA) matched sibling; however, for 75–80% of patients, an HLA-matched sibling will not be available. In these cases, HSCT from an unrelated donor is usually considered. However, for many patients (especially those with rare HLA genotypes), finding a matched unrelated donor is not possible. For others, the delay imposed by the process of finding and collecting cells from an unrelated donor can lead to an increased risk of morbidity and mortality related to infection (6, 7). Therefore, HSCT using a haploidentical related donor can provide substantial benefit for patients who do not have a matched related or unrelated donor source, or who have active infection and need HSCT urgently (6). In choosing a parent for stem cell donation under these circumstances, one factor to consider is the presence or absence of transplacental maternal engraftment (TME).
The human placenta allows for bidirectional passage of nucleated cells between mother and fetus (8, 9); in healthy infants, the immune system eradicates these cells. In contrast, patients with SCID may lack the functional immunity required to reject circulating maternal T cells, resulting in persistent TME in up to 40% of SCID patients (10–13). Although TME may be asymptomatic, some SCID infants with TME can have clinical symptoms of graft vs. host disease (GVHD) prior to HSCT (10, 13).
GVHD in SCID may manifest as cutaneous involvement, characterized by localized or diffuse rashes ranging from fine maculopapular or morbilliform erythema to general erythroderma and alopecia. Liver involvement may also be observed (hepatosplenomegaly with elevated liver enzymes, histological signs of cell-mediated inflammation, cholestasis) (10). GI tract involvement primarily manifests as diarrhea; hematologic manifestations, such as eosinophilia, thrombocytopenia, and even hemophagocytosis, may also be observed (14, 15). Since the presence of TME may indicate a degree of host tolerance for maternal antigens as well as a potential source of rejection of non-maternal cells (16, 17), maternal haploidentical transplants are generally preferred over paternal donors if TME is detected.
Parental mismatched grafts, which are typically a readily available stem cell source for patients without an HLA-matched donor, are used regularly with excellent outcomes in SCID (7, 18). However, GVHD is a common side effect of HSCT; approximately 20% of SCID patients receiving a haploidentical HSCT develop acute GVHD, while 10% develop severe (grade III/IV) acute GVHD (6). Chronic GVHD is observed in approximately 23% of CD34-selected haploidentical HSCT’s for SCID (6). Risk factors for development of GVHD are primarily related to donor factors such as HLA disparity, but host factors may play a role (19, 20).
Given the association of pre-existing TME with pre-HSCT GVHD, we hypothesized that the risk of developing post-HSCT GVHD may be higher in TME(+) SCID patients compared to TME(−) SCID patients. Here we report on the presence of TME and its effects on the development of acute and chronic GVHD in SCID patients transplanted between 1988 and 2014 at the University of California, San Francisco.
Methods
Patients
Eligible patients included patients with a diagnosis of SCID who underwent first hematopoietic stem cell transplantation from 1988 to 2014 at the University of California San Francisco Benioff Children’s Hospital. Diagnoses were made based on genetic testing when available, or clinical criteria as previously published (21). Two patients with Omenn syndrome were not included due to the uncertain mechanism of immune hyperactivity in this setting as well as difficulty in distinguishing post-transplant aGVHD from pre-transplant autoimmunity. A total of 88 records were reviewed; patients were further excluded based on unavailability of TME testing results (n=13), or insufficient follow-up available for diagnosis of aGVHD (n=1). “Leaky” SCID was not considered as exclusionary criteria.
Detection of Maternal Engraftment
Maternal engraftment was detected by analyzing patient peripheral blood mononuclear cells using a combination of non-inherited HLAs and fluorescent in situ chromosome analysis (FISH) (prior to 2003, n=34), variable number tandem repeat analysis (2002, N=1), or short tandem repeat (STR) analysis (after 2003, n=39). Quantification of degree of maternal engraftment was available for the subset of patients who underwent STR analysis.
Transplant Procedure
Stem cell products were T-cell depleted ex vivo utilizing a variety of methods if donor HLA allele typing differed from that of recipient at 2 or more loci, or in the case of one patient, at the DRB1 locus only. Soybean agglutination / sheep red blood cells e-rosetting was employed prior to 1996 (n=7) (22). CD34+ selection or a combination of positive and negative selection using the Isolex 300i system (Baxter International Inc.) or the CliniMacs Plus system (Miltenyi Biotec) was employed from 1996 – 2014 (n=44). All T cell depleted transplant recipients received <6 × 104 CD3+ cells/kg, with TME(+) positive patients initially being restricted to <3 × 104 CD3+ cells/kg and later restricted to <1 × 104 CD3+ cells/kg. CD34+ stem cell dose was dependent on donor and graft source, and ranged from 2.4–64 × 106 CD34+ cells/kg, with TCD recipients receiving higher stem cell doses (23). Bone marrow from matched or single allele-mismatched donors was given unmanipulated except for RBC or plasma depletion, depending on ABO mismatch. Patients undergoing matched or single mismatched HSCT typically received GVHD prophylaxis with cyclosporine +/− methotrexate or mycophenolate mofetil. Patients were defined as requiring a second transplant if additional conditioning and stem cell infusion was required following the initial transplant. Stem cell boost refers to patients requiring an additional stem cell infusion without conditioning. Acute and chronic GVHD were diagnosed clinically using established criteria (24). Histopathologic examination was typically used to confirm or refute the presence of GVHD whenever possible.
Statistical Analysis
Statistics were performed using NCSS v8.0 and GraphPad Prism v6.05. Categorical comparisons were made using the Student t-test or, in contingency analysis with low frequencies, Fisher’s exact test; p-values were determined using a two-tailed model. Overall survival was estimated using the method of Kaplan & Meier, compared by the Log-Rank test. Multivariate analysis was performed by logistic regression.
Results
Patient Characteristics
Of 90 patients eligible for analysis, 74 had records of maternal engraftment testing available. Of these, the following genetic etiologies were found: Artemis deficiency (n=19), IL2R-cγ deficiency (n=17), RAG1/2 deficiency (n=13), IL7Ra deficiency (n=7), ADA deficiency (n=2), and 1 patient each with CD3d deficiency, DNA PKcs deficiency, cartilage hair hypoplasia, and reticular dysgenesis (Table 1). For 12 patients, genetic etiology was unknown.
Table 1.
Demographics
| Variable | TME(+) | TME(−) | p | |
|---|---|---|---|---|
| N (%) | 35 (47.3) | 39 (52.7) | ||
| Sex | 0.13 | |||
| Male | 24 (68.6) | 20 (51.3) | ||
| Female | 11 (31.4) | 19 (48.7) | ||
| Age at BMT | 0.62 | |||
| 0–3.5 months | 14 (40) | 18 (46.2) | ||
| 3.5–6 months | 8 (22.9) | 5 (12.8) | ||
| 6–12 months | 9 (25.7) | 9 (23.1) | ||
| >12 months | 4 (11.4) | 7 (17.9) | ||
| Genotype | 0.016 | |||
| Artemis | 8 (22.9) | 11 (28.2) | ||
| ILRcγ | 11 (31.4) | 6 (15.4) | ||
| RAG1/2 | 3 (8.6) | 10 (25.6) | ||
| IL7Ra | 7 (20) | 0 (0) | ||
| ADA | 0 (0) | 2 (5.1) | ||
| Other NK− | 1 (2.9) | 2 (5.1) | ||
| Other NK+ | 5 (14.3) | 8 (20.5) | ||
Transplant Characteristics
Patients were transplanted at a median of 139 days of life (ranging from 13 days to 25.6 months old). Conditioning regimens varied and are described in Table 2. Donors included siblings (n=15), mothers (n=44), fathers (n=7), or unrelated donors (n=8). GVHD prophylaxis was with ex vivo T cell depletion (TCD, with or without additional agents) in a majority of cases (n=50). Other patients received a calcineurin inhibitor with methotrexate (n=18) or without methotrexate (n=6). TME(+) patients were more likely to be treated with a maternal stem cell source (82.9% vs. 38.5%; p=0.001), with ex vivo TCD (85.7% vs. 51.3%; p=0.02). TME(+) patients received unconditioned transplants in 62.9% of cases, compared to 38.5% of TME(−) cases (p=0.103).
Table 2.
Transplant Information.
| Variable | TME(+) | TME(−) | p |
|---|---|---|---|
| N (%) | 35 (47.3) | 39 (52.7) | |
| Conditioning | 0.103 | ||
| Chemotherapy only | 3 (8.5) | 3 (7.7) | |
| Serotherapy only | 6 (17.1) | 8 (20.5) | |
| Both | 4 (11.4) | 13 (33.3) | |
| Unconditioned | 22 (62.9) | 15 (38.5) | |
| Donor | 0.001 | ||
| Maternal | 29 (82.9) | 15 (38.5) | |
| Paternal | 1 (2.9) | 6 (15.4) | |
| Sibling | 4 (11.4) | 11 (28.2) | |
| Unrelated | 1 (2.9) | 7 (17.9) | |
| GVHD Prophylaxis | 0.016 | ||
| TCD | 30 (85.7) | 20 (51.3) | |
| CNI + MTX | 4 (11.4) | 14 (35.9) | |
| CNI Only | 1 (2.9) | 3 (7.7) | |
| CNI + MMF | 0 (0) | 2 (5.1) | |
| 10-Year Overall Survival | 76.0% | 83.7% | 0.43 |
| 10-Year Event-Free Survival | 73.8% | 65.6% | 0.30 |
TCD=T cell depleted; CNI=calcineurin inhibitor; MTX=methotrexate; MMF=mycophenolate mofetil.
Transplacental Maternal Engraftment
Pre-transplant TME was identified in 35 of 74 patients (47.3%), and varied significantly based on SCID subtype (p=0.016). TME was more commonly identified in patients with IL7Ra SCID (6/6; 100%) and ILRcγ SCID (11/17; 64.7%). TME was identified in 3 of 13 RAG1/2 SCID patients (23.1%) and 8 of 19 Artemis SCID patients (42.1%). TME was detected in 23/52 (44.2%) NK-positive SCID patients compared to 12/22 (54.5%) NK-negative SCID patients (p=0.45); TME was not detected in the patient with reticular dysgenesis. There was no difference in the rate of TME in the more recent STR era (19/39, 48.7%) compared to pre-STR era (15/35, 42.8%) (p=0.62).
Graft-versus-Host Disease
Pre-transplant GVHD was present in 8 patients. TME was detected in all of these, at levels ranging from 1% to 87% in patients for whom STR analysis was performed. These patients were included in this analysis, but a separate analysis was performed excluding patients with pre-transplant aGVHD, and results were similar (see subset analysis in Appendix Table 1). Post-transplant aGVHD of any grade developed in 36.5% of patients (95%CI=25.5–47.5%). Grade II–IV aGVHD was diagnosed in 28.4% (95%CI=18.1–39.7%) of all patients, and Grade III–IV aGVHD in 9.5% (95%CI=2.8–16.1%) of all patients. Of the 72 evaluable patients who survived >100 days post-transplant, chronic GVHD was diagnosed in 6 patients (8.3%; 95%CI=1.9–14.7%); 3 of these (4.2%; 95%CI=0–8.8%) developed extensive chronic GVHD.
TME and Risk for Post-HSCT Acute GVHD
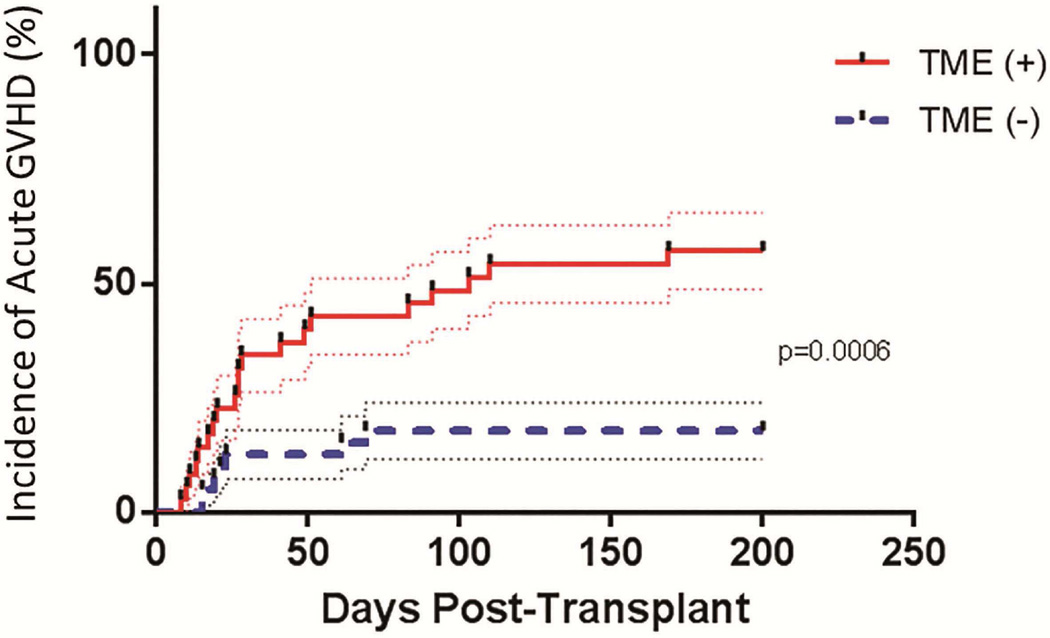
In the 39 patients without TME, 7 developed Grade I–IV aGVHD (17.9%), compared to 20 of the 35 patients with TME (57.1%; RR=3.2; p=0.0006) (Figure 1). The incidence of Grade II–IV aGVHD was 15.4% in the 39 TME(−) patients, compared to 42.9% in the 35 TME(+) patients (RR 2.8, p=0.011). The risk of grade III–IV aGVHD was also 2.8-fold higher in patients with TME, though this did not reach statistical significance (p=0.24) (Table 3).
Figure 1.
Incidence of acute GVHD of any grade in patients with TME (solid line) and without TME (dashed line). TME(+) patients had a significantly higher risk of developing acute GVHD (57.1% of patients) compared to TME(−) patients (17.9%; p=0.0006).
Table 3.
Incidence and Relative Risk of aGVHD, cGVHD.
| All Patients | N (%) | TME(+) N (%) |
TME(−) N (%) |
RR (95%CI) | p |
|---|---|---|---|---|---|
| 74 | 35 | 39 | |||
| aGVHD I–IV | 27 (36.5) | 20 (57.1) | 7 (17.9) | 3.2 (1.5–6.6) | 0.0007 |
| aGVHD II–IV | 21 (28.4) | 15 (42.9) | 6 (15.4) | 2.8 (1.2–6.4) | 0.011 |
| aGVHD III–IV | 7 (9.5) | 5 (14.3) | 2 (5.1) | 2.8 (0.58–13) | 0.24 |
| cGVHD (N=35, 37)^ | 6 (8.1) | 4 (11.4) | 2 (5.4) | 2.1 (0.41–11) | 0.42 |
2 patients in the TME(−) group were not evaluable for cGVHD.
Univariate analysis was performed examining the following potential confounding factors that may also influence development of acute GVHD: recipient sex, age at transplant, conditioning regimen, donor type, donor ID, GVHD prophylaxis, and SCID type. Of these, donor type (mother) was associated with a higher risk of acute GVHD (RR 3.0; p=0.05), and GVHD prophylaxis using CNI+MTX was associated with a lower risk of acute GVHD (RR 0.25; p=0.04), though this was used primarily in the closely-matched setting. Due to the small number of patients in this study, multivariate analysis was possible only for presence of TME, conditioning type, and donor type (Table 4). TME remained a strong significant independent predictor of acute GVHD (RR 2.75, p=0.006) in multivariate analysis, as did the use of cytotoxic conditioning without serotherapy (RR 1.42, p=0.02). Compared to maternal donors, use of a paternal donor was associated with a statistically significant higher risk of aGVHD (RR 1.42, p=0.02).
Table 4.
Relative risk of acute GVHD of any grade by multivariate analysis.
| Characteristic (N=74) | RR (95%CI) | p | |
|---|---|---|---|
| TME | 2.75 (1.33–5.68) | 0.006 | |
| Conditioning | |||
| Neither | Ref | ||
| Chemo Only | 1.42 (1.06–1.90) | 0.02 | |
| Sero Only | 0.39 (0.12–1.32) | 0.13 | |
| Both | 0.73 (0.31–1.72) | 0.47 | |
| Donor | |||
| Mother | Ref | ||
| Father | 1.42 (1.06–1.90) | 0.02 | |
| Sibling | 0.41 (0.10–1.63) | 0.21 | |
| Unrelated | 0.61 (0.09–4.31) | 0.62 | |
In order to examine more homogeneous populations separately, subset analyses were performed. Groups analyzed included patients who did not have pre-transplant GVHD (N=66), patients receiving transplants from maternal donors (N=44), patients receiving TCD transplants (N=50), patients receiving cytotoxic conditioning (N=23), patients receiving serotherapy (N=31), patients for whom STR analysis was available (N=38), and patients receiving transplants from non-maternal donors (N=30) (Appendix Table 1). In patients receiving maternal donor transplants, TME(+) patients remained at a significantly higher risk for developing acute GVHD (RR 3.8; 95% CI 1.2–9.3; p=0.0097) compared to TME(−) patients, with no TME(−) recipient developing Grade III–IV aGVHD. This was also true for the subset of patients receiving TCD transplants (RR 2.7, 95% CI 1.2–5.9, p=0.009). Method of T cell depletion had no statistically significant effect on development of aGVHD of any grade, although this analysis is confounded by the increased rate of serotherapy usage in grafts depleted using negative selection methodology compared to those using depletion by CD34-positive selection. For patients receiving cytotoxic conditioning, RR for acute GVHD was also increased in TME(+) patients (RR 3.8, 95% CI 1.2–12, p=0.026).
For the 31 patients receiving serotherapy (alemtuzumab or anti-thymocyte globulin) during their conditioning regimen, the rate of GVHD was quite low compared to those who did not receive serotherapy (N=43) (Appendix Table 2). Grade II–IV aGVHD occurred in 12.9% of patients receiving serotherapy, vs 39.5% of those not receiving it (RR 0.33; 95%CI 0.12–0.88; p=0.03).
Lastly, in the subset of patients in whom TME was analyzed by STR (N=38), TME was detected in 19 (50.0%). Similar to the entire cohort, rates of Grade 1–4 and 2–4 aGVHD were higher in TME(+) patients (73.7% vs 15.8% and 47.4% vs 10.5%; p=0.0008 & p=0.03, respectively). Interestingly, the five IL7Rα SCID patients in this subset were all TME(+) and none had aGVHD of any grade, while the other 14 patients in whom TME was detected by STR all developed aGVHD (including 3 patients who also had pre-transplant GVHD). Conversely, in the 19 patients in whom no TME was detected by STR, only 3 patients (15.8%) developed aGVHD (RR=6.3; 95% CI 2.2–18; p<0.0001). No patient with TME less than 10% developed Grade 3–4 aGVHD, while 4 of 13 patients with TME 10% or greater developed Grade 3–4 aGVHD. Of note, the only patients with TME greater than 10% who did not develop GVHD of any grade were the IL7Rα deficient SCID patients.
TME and Risk for Post-HSCT Chronic GVHD
Of the 37 TME(−) patients surviving >100 days, 2 (5%) developed cGVHD (one limited, one extensive). Of the 35 TME(+) patients who survived >100 days, 4 (11%) developed cGVHD (2 limited, 2 extensive) (RR 2.1, p=0.42). Subset analysis showed no statistically significant increase in risk of cGVHD for TME(+) patients in any subset.
Overall Survival and Event Free Survival
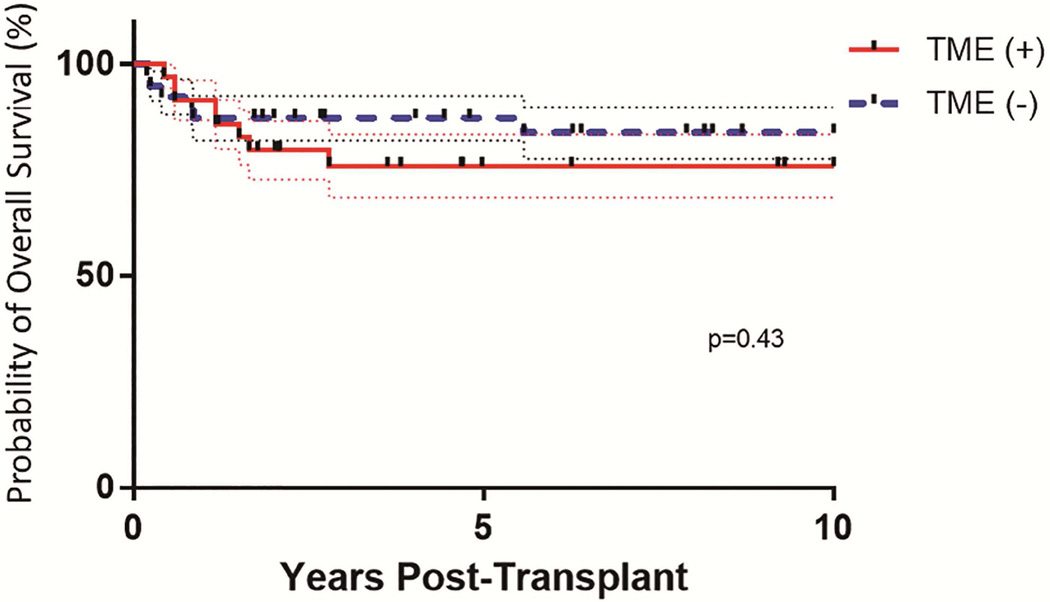
Overall survival for the entire cohort was 80% (95%CI=70.5%–86.3%) (Figure 2), with a median f/u of 7 years (range: 2 months–25 years); the presence or absence of TME was not associated with overall survival (p=0.45).
Figure 2.
Probability of overall survival in patients with TME (solid line) and without TME (dashed line). Overall survival was defined as survival following transplant. There was no statistically significant difference in overall survival between these patient groups.
In the entire cohort, 15 patients required a second transplant, 7 of whom died. An additional 3 patients died following unconditioned stem cell boost or DLI. Six patients died without receiving any post-transplant cell infusions. Thirty-eight patients survived without the need for any post-transplant cell infusions; an additional 12 survived after receiving an unconditioned stem cell boost or DLI.
Long-term event-free survival, defined as survival without the need for second (conditioned) transplant, was 67.6%. In the TME(+) group, 10 of 35 required 2nd transplant or died, compared to 14 of 39 in the TME(−) group (RR=0.80, 95%CI+0.4–1.5, p=0.67). In the subset of maternal transplants with TME (N=29), 13 required a post-transplant cell infusion. In the TME(−) maternal transplants, 9 of 15 required a post-transplant cell infusion (RR=1.34, 95%CI=0.7–2.4, p=0.53).
Discussion
These results demonstrate a higher rate of acute GVHD observed in SCID patients with pre-transplant transplacental maternal engraftment (TME). This association was confirmed in multivariate analysis controlling for conditioning regimen and donor identity, as well as subset analyses evaluating smaller, more homogenous populations. The incidence of TME in this SCID cohort was 47.3%; this is similar to rates (ranging from 40–52%) observed in other studies (3, 6, 10).
In the Primary Immune Deficiency Treatment Consortium (PIDTC) cohort reported by Pai et al, Grade II–IV acute GVHD was observed in 21% of mismatched related transplants; Grade III–IV acute GVHD was observed in 10%, and chronic GVHD was observed in 16% (6). Similarly, in our cohort, 5 of 44 (17%) maternal transplant recipients developed Grade III–IV acute GVHD, and this was strongly predicted by presence or absence of TME (all were TME(+). No TME(−) maternal recipient developed Grade III–IV aGVHD, despite the fact that TME(+) patients received a lower T cell dose. While the biologic reasons for this are not well-understood, one possible hypothesis is that TME(+) patients have active subclinical GVHD, which is then exacerbated following infusion of any T cells with the donor graft.
Interestingly, TME was observed in all seven NK(+) IL7Rα SCID patients, and despite the presence of TME in all of the IL7Ra patients, none of them developed aGVHD. This suggests that, while IL7Rα may be dispensable for the development of phenotypically normal CD16/56(+) NK cells, they may differ functionally from NK cells found in other types of NK+ SCID, such as RAG1/2 and Artemis SCID, where TME was less common. The mechanism for this is unknown, but implies a specific lack of function causing a reduced capacity for cellular rejection in IL7Rα-deficient NK cells.
Another unexpected finding was the association of paternal donors with the development of post-transplant GVHD, independent of the presence of TME. Studies in adult transplants have demonstrated an increased risk of GVHD associated with female versus male donors (possibly mediated by Y-antigens in male recipients) (25); however, little is known regarding maternal-fetal tolerance in the perinatal period. Tolerance to non-inherited maternal antigens has been attributed to better survival using maternal donors, regardless of the recipient sex (26). This tolerance may impart a resistance to aGVHD in patients with maternal donors compared to those with paternal donors. Another proposed mechanism is a so-called graft-versus-graft effect of infused paternal cells inciting an inflammatory reaction against HLA-mismatched, but previously tolerant, maternally engrafted cells. This phenomenon is difficult to evaluate in this cohort, because only one TME(+) patient was transplanted using a paternal donor (the patient developed Grade 2 aGVHD). TME detection methodology differences do not seem to explain this, as all paternal-donor GVHD developed in patients with TME tested by STR.
In the most recent multi-institutional retrospective analysis of SCID patients undergoing transplant, only 37% of recipients had been tested for the presence or absence of TME (6); it was examined in 62% of the first 50 patients enrolled in the more recent prospective PIDTC protocol (3). Given the higher risk of acute GVHD observed in TME(+) SCID patients, an effort should be made to identify TME in the pre-transplant period whenever possible, especially when considering a maternal or paternal donor. When conditions allow, approaches for enhanced GVHD prophylaxis should be considered in these patients. The use of serotherapy-based conditioning in this cohort partially abrogated the risk of GVHD caused by the presence of TME. While serotherapy (anti-thymocyte globulin or alemtuzumab) is often administered in patients considered to be at high risk of rejection, this paradoxically can exclude TME(+) patients who may also benefit from serotherapy (at an appropriately reduced dose for recipients of T cell depleted grafts) due to the reduction in aGVHD risk associated with its use.
Other options include the use of post-transplant GVHD prophylaxis where historically none has been used (TCD haploidentical transplants). Sirolimus is a potentially attractive option in this setting in that it has been shown to preferentially spare regulatory T cells, thus possibly allowing for immune reconstitution while preventing GVHD, though this remains to be tested in a prospective trial and may have risks of sinusoidal obstruction syndrome when used with busulfan-based conditioning (27). Conversely, the low incidence of aGVHD observed in patients without TME provides rationale for a possible de-escalation of GVHD prophylaxis in certain select scenarios, which would potentially allow for earlier immune reconstitution.
In conclusion, since the presence of pre-transplant TME is associated with an increased risk of graft-versus-host disease, further consideration regarding GVHD prophylaxis should be given to these patients; the addition of serotherapy or other immunosuppressive agents may be warranted in these cases. The converse may also be true for TME(−) patients; for those with active infections, a de-escalation of GVHD prophylaxis may allow for earlier immune reconstitution and a reduction in infection-related morbidity and mortality.
One limitation of this analysis is that the underlying mechanisms that may contribute to the increased risk of aGVHD in TME(+) patients cannot be inferred from the available data. In addition, the study is retrospective, and there are multiple potential confounding variables. The small number of patients in this cohort restricted the opportunity for a more robust multivariate analysis. For example, the influence of TME status on the risk for aGVHD in recipients of non-maternal grafts is not clear. Future analysis of SCID patients enrolled on the prospective PIDTC registry study may allow for further examination of other potential variables that may influence development of post-transplant aGVHD in these patients. Further studies are needed to better define the clinical risk factors and biologic mechanisms that mediate the effects of TME on the development of post-transplant aGVHD.
Figure 3.
Probability of event-free survival in patients with TME (solid line) and without TME (dashed line). Event-free survival was defined as survival following transplant without the need for additional (conditioned) transplant. There was no statistically significant difference in event-free survival between these patient groups.
Clinical Implications.
This analysis provides additional data for assessing the risk for GVHD in a high-risk population. The presence of TME will inform timely diagnosis of GVHD as well as prophylactic strategies.
Acknowledgments
Supported in part by grants from the National Institute of Allergy and Infectious Disease (NIAID), the National Center for Advancing Translational Sciences (NCATS) Office of Rare Disease Research (ORDR), and the Rare Disease Clinical Research Network (RDCRN), U54-AI 082973 and R13 AI 094943. The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention by trade names, commercial practices, or organizations imply endorsement by the US Government. The authors thank Nancy Hills for assistance with statistical analysis. Funding for MJC and CCD has been provided by Grant #U54AI082973 from NIAID and the NIH Office of Rare Diseases Research.
Abbreviations
- aGVHD
Acute graft-versus-host disease
- cGVHD
Chronic graft-versus-host disease
- FISH
Fluorescent in situ hybridization
- GVHD
Graft-versus-host disease
- HLA
Human leukocyte antigen
- HSCT
Hematopoietic stem cell transplantation
- NIAID
National Institute of Allergy and Infectious Disease
- NCATS
National Center for Advancing Translational Sciences
- ORDR
Office of Rare Disease Research
- PIDTC
Primary Immune Deficiency Treatment Consortium
- RDCRN
Rare Disease Clinical Research Network
- SCID
Severe combined immunodeficiency
- STR
Short tandem repeat
- TCD
T cell depletion
- TME
Transplacental Maternal Engraftment
Appendix
Appendix Table 1.
aGVHD in TME(+) patients compared to aGVHD in TME(−) patients, restricted to subsets of (a) all patients, (b) patients with no history of pre-transplant GVHD, (c) patients receiving transplants from maternal donors, (d) patients receiving T cell depleted transplants, (e) patients receiving cytotoxic chemotherapy, (f) patients receiving serotherapy, (g) patients for whom TME was determined by STR method, (h) patients receiving transplants from non-maternal donors.
| a | |||||
|---|---|---|---|---|---|
| All Patients | N (%) | TME+ N (%) |
TME− N(%) |
RR (95%CI) | p |
| 74 | 35 | 39 | |||
| aGVHD I–IV | 27 (36.5) | 20 (57.1) | 7 (17.9) | 3.2 (1.5–6.6) | 0.0006 |
| aGVHD II–IV | 21 (28.4) | 15 (42.9) | 6 (15.4) | 2.8 (1.2–6.4) | 0.011 |
| aGVHD III–IV | 7 (9.5) | 5 (14.3) | 2 (5.1) | 2.8 (0.58–13) | 0.24 |
| cGVHD (N=35, 37)^ | 6 (8.1) | 4 (11.4) | 2 (5.4) | 2.1 (0.41–11) | 0.42 |
| b | |||||
|---|---|---|---|---|---|
| No Pre-Transplant GVHD |
N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 66 | 27 | 39 | |||
| aGVHD I–IV | 24 (36.4) | 16 (59.3) | 8 (20.5) | 2.9 (1.4–5.8) | 0.0018 |
| aGVHD II–IV | 19 (28.8) | 13 (48.1) | 6 (15.4) | 3.1 (1.4–7.2) | 0.0057 |
| aGVHD III–IV | 6 (9.1) | 4 (14.8) | 2(5.1) | 2.9 (0.57–15) | 0.22 |
| cGVHD (N=27, 37)^ | 5 (7.6) | 3 (11.1) | 2 (5.4) | 2.1 (0.37–11) | 0.64 |
| c | |||||
|---|---|---|---|---|---|
| Maternal Donors | N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 44 | 29 | 15 | |||
| aGVHD I–IV | 22 (50.0) | 19(65.5) | 3(20.0) | 3.8 (1.2–9.3) | 0.0097 |
| aGVHD II–IV | 17(38.6) | 14(48.3) | 3 (20.0) | 2.4 (0.82–7.1) | 0.10 |
| aGVHD III–IV | 5 (11.4) | 5 (17.2) | 0 (0) | 7.0 (0.36–135) | 0.15 |
| cGVHD (N=29, 14)^ | 4 (9.1) | 3 (10.3) | 1 (7.1) | 1.4 (0.17–13) | 0.74 |
| d | |||||
|---|---|---|---|---|---|
| TCD | N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 50 | 30 | 20 | |||
| aGVHD I–IV | 25 (50.0) | 20 (66.6) | 5 (25.0) | 2.7 (1.2–5.9) | 0.009 |
| aGVHD II–IV | 19 (38.0) | 15 (50) | 4 (20.0) | 2.5 (0.97–6.4) | 0.04 |
| aGVHD III–IV | 6 (12.0) | 5 (16.6) | 1 (5.0) | 3.3 (0.42–28) | 0.38 |
| cGVHD (N=30, 18)^ | 5 (10.0) | 4 (13.3) | 1 (5.6) | 2.7 (0.32–22) | 0.64 |
| e | |||||
|---|---|---|---|---|---|
| Cytotoxic Conditioning | N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 23 | 7 | 16 | |||
| aGVHD I–IV | 8 (34.8) | 5 (71.4) | 3 (18.8) | 3.8 (1.2–12) | 0.026 |
| aGVHD II–IV | 5 (21.7) | 3 (42.9) | 2 (12.5) | 3.4 (0.73–16) | 0.14 |
| aGVHD III–IV | 2 (8.7) | 2 (28.6) | 0 (0) | NA | NA |
| cGVHD (N=7, 15)^ | 2 (9.1) | 1 (14.3) | 1 (6.7) | 2.1 (0.16–30) | 1.0 |
| f | |||||
|---|---|---|---|---|---|
| Serotherapy Conditioning |
N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 31 | 10 | 21 | |||
| aGVHD I–IV | 7 (22.6) | 3 (30.0) | 4 (19.0) | 1.6 (0.43–5.7) | 0.65 |
| aGVHD II–IV | 4 (12.9) | 2 (20.0) | 2 (9.5) | 2.1 (0.34–13) | 0.58 |
| aGVHD III–IV | 1 (3.2) | 1 (10.0) | 0 (0) | NA | 0.32 |
| cGVHD (N=10, 20)^ | 3 (9.7) | 1 (10.0) | 2 (10.5) | 1.0 (0.08 – 13) | 1.0 |
| g | |||||
|---|---|---|---|---|---|
| STR Analysis | N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 38 | 19 | 19 | |||
| aGVHD I–IV | 17 (44.8) | 14 (73.7) | 3 (15.8) | 4.7 (1.6 – 14) | 0.0008 |
| aGVHD II–IV | 11 (28.9) | 9 (47.4) | 2 (10.5) | 4.5 (1.1–18) | 0.03 |
| aGVHD III–IV | 5 (13.2) | 4 (21.1) | 1 (5.3) | 4.0 (0.49–33) | 0.34 |
| cGVHD (N=19, 17)^ | 4 (11.1) | 2 (10.5) | 2 (11.8) | 0.89 (0.14 – 5.7) |
1.0 |
| h | |||||
|---|---|---|---|---|---|
| Non-Maternal Donors | N (%) | TME+ N (%) |
TME− N(%) |
RR | p |
| 30 | 6 | 24 | |||
| aGVHD I–IV | 6 (20) | 1 (16.7) | 5 (20.1) | 0.8 (0.11–5.6) | 0.82 |
| aGVHD II–IV | 4 (13.3) | 1 (16.7) | 3 (12.5) | 1.3 (0.17–10.7) | 0.79 |
| aGVHD III–IV | 2 (6.7) | 0 (0) | 2 (8.3) | 0.7 (0.04 – 13) | 0.82 |
| cGVHD (N=6, 23)^ | 2 (6.9) | 1 (16.7) | 1 (4.3) | 4.6 (0.34–62) | 0.25 |
cGVHD outcome was restricted to evaluable patients surviving >100 days post-HSCT.
Appendix Table 2.
aGVHD in patients receiving serotherapy compared to those not receiving serotherapy in (a) all patients, and (b) TME(+) patients only.
| a) All patients | |||||
|---|---|---|---|---|---|
| Serotherapy Conditioning |
N (%) | Serotherapy N (%) |
No Serotherapy N(%) |
RR | p |
| 74 (100) | 31 (41.9) | 43 (58.1) | |||
| aGVHD I–IV | 27 (36.5) | 7 (22.6) | 20 (46.5) | 0.49 (0.23–1.0) | 0.05 |
| aGVHD II–IV | 21 (28.4) | 4 (12.9) | 17 (39.5) | 0.33 (0.12–0.88) | 0.03 |
| aGVHD III–IV | 7 (9.5) | 1 (3.2) | 6 (14.0) | 0.23 (0.029–1.8) | 0.23 |
| cGVHD (N=30, 42)^ | 6 (8.3) | 3 (10.0) | 3 (7.1) | 1.4 (0.30–6.5) | 0.69 |
| b) TME(+) patients only | |||||
|---|---|---|---|---|---|
| TME(+) Only | N (%) | Serotherapy N (%) |
No Serotherapy N(%) |
RR | p |
| 35 (47.3) | 10 (28.6) | 25 (71.4) | |||
| aGVHD I–IV | 20 (57.1) | 3 (30.0) | 17 (68.0) | 0.44 (0.16–1.2) | 0.10 |
| aGVHD II–IV | 15 (42.9) | 2 (20.0) | 13 (52.0) | 0.38 (0.11–1.4) | 0.15 |
| aGVHD III–IV | 5 (14.3) | 1 (10.0) | 4 (16.0) | 0.63 (0.79–4.9) | 0.66 |
| cGVHD (N=10, 25)^ | 4 (11.4) | 1 (10.0) | 3 (12.0) | 0.83 (0.98–7.1) | 0.87 |
cGVHD outcome was restricted to evaluable patients surviving >100 days post-HSCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fischer A, Cavazzana-Calvo M, De Saint Basile G, DeVillartay JP, Di Santo JP, Hivroz C, et al. Naturally occurring primary deficiencies of the immune system. Annual review of immunology. 1997;15:93–124. doi: 10.1146/annurev.immunol.15.1.93. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annual review of immunology. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. Epub 2004/03/23. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak CC, Cowan MJ, Logan BR, Notarangelo LD, Griffith LM, Puck JM, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the primary immune deficiency treatment consortium prospective study 6901. Journal of clinical immunology. 2013;33(7):1156–1164. doi: 10.1007/s10875-013-9917-y. Epub 2013/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA : the journal of the American Medical Association. 2014;312(7):729–738. doi: 10.1001/jama.2014.9132. Epub 2014/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahlstrom JT, Dvorak CC, Cowan MJ. Hematopoietic Stem Cell Transplantation for Severe Combined Immunodeficiency. Current pediatrics reports. 2015;3(1):1–10. doi: 10.1007/s40124-014-0071-7. Epub 2015/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–446. doi: 10.1056/NEJMoa1401177. Epub 2014/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–516. doi: 10.1056/NEJM199902183400703. Epub 1999/02/18. [DOI] [PubMed] [Google Scholar]
- 8.Scaradavou A, Carrier C, Mollen N, Stevens C, Rubinstein P. Detection of maternal DNA in placental/umbilical cord blood by locus-specific amplification of the noninherited maternal HLA gene. Blood. 1996;88(4):1494–1500. Epub 1996/08/15. [PubMed] [Google Scholar]
- 9.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88(11):4390–4395. Epub 1996/12/01. [PubMed] [Google Scholar]
- 10.Muller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood. 2001;98(6):1847–1851. doi: 10.1182/blood.v98.6.1847. Epub 2001/09/06. [DOI] [PubMed] [Google Scholar]
- 11.Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. The Journal of pediatrics. 1993;123(4):564–572. doi: 10.1016/s0022-3476(05)80951-5. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 12.Thompson LF, O'Connor RD, Bastian JF. Phenotype and function of engrafted maternal T cells in patients with severe combined immunodeficiency. Journal of immunology. 1984;133(5):2513–2517. Epub 1984/11/01. [PubMed] [Google Scholar]
- 13.Denianke KS, Frieden IJ, Cowan MJ, Williams ML, McCalmont TH. Cutaneous manifestations of maternal engraftment in patients with severe combined immunodeficiency: a clinicopathologic study. Bone Marrow Transplantation. 2001;28(3):227–233. doi: 10.1038/sj.bmt.1703128. Epub 2001/09/06. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak CC, Wright NB, Wong WB, Kristovich KM, Matthews EW, Weinberg KI, et al. Safety of hematopoietic stem cell transplantation in children less than three years of age. Pediatr Hematol Oncol. 2008;25(8):705–722. doi: 10.1080/08880010802243524. Epub 2008/12/10. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak CC, Sandford A, Fong A, Cowan MJ, George TI, Lewis DB. Maternal T-cell engraftment associated with severe hemophagocytosis of the bone marrow in untreated X-linked severe combined immunodeficiency. J Pediatr Hematol Oncol. 2008;30(5):396–400. doi: 10.1097/MPH.0b013e318168e7a0. Epub 2008/05/07. [DOI] [PubMed] [Google Scholar]
- 16.Palmer K, Green TD, Roberts JL, Sajaroff E, Cooney M, Parrott R, et al. Unusual clinical and immunologic manifestations of transplacentally acquired maternal T cells in severe combined immunodeficiency. The Journal of allergy and clinical immunology. 2007;120(2):423–428. doi: 10.1016/j.jaci.2007.02.047. Epub 2007/05/08. [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly RJ, Keever CA, Small TN, Brochstein J. The use of HLA-non-identical T-cell-depleted marrow transplants for correction of severe combined immunodeficiency disease. Immunodeficiency reviews. 1989;1(4):273–309. Epub 1989/01/01. [PubMed] [Google Scholar]
- 18.Friedrich W, Honig M. HLA-haploidentical donor transplantation in severe combined immunodeficiency. Hematology/oncology clinics of North America. 2011;25(1):31–44. doi: 10.1016/j.hoc.2010.11.003. Epub 2011/01/18. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Seminars in hematology. 2006;43(1):3–10. doi: 10.1053/j.seminhematol.2005.09.001. Epub 2006/01/18. [DOI] [PubMed] [Google Scholar]
- 20.Ball LM, Egeler RM, Party EPW. Acute GvHD: pathogenesis and classification. Bone Marrow Transplantation. 2008;41(Suppl 2):S58–S64. doi: 10.1038/bmt.2008.56. Epub 2008/07/24. [DOI] [PubMed] [Google Scholar]
- 21.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. The Journal of allergy and clinical immunology. 2014;133(4):1092–1098. doi: 10.1016/j.jaci.2013.09.044. Epub 2013/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dror Y, Gallagher R, Wara DW, Colombe BW, Merino A, Benkerrou M, et al. Immune reconstitution in severe combined immunodeficiency disease after lectin-treated, T-cell-depleted haplocompatible bone marrow transplantation. Blood. 1993;81(8):2021–2030. Epub 1993/04/15. [PubMed] [Google Scholar]
- 23.Dvorak CC, Hung GY, Horn B, Dunn E, Oon CY, Cowan MJ. Megadose CD34(+) cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(10):1125–1133. doi: 10.1016/j.bbmt.2008.07.008. Epub 2008/09/23. [DOI] [PubMed] [Google Scholar]
- 24.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. Epub 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 25.Ciurea SO, Champlin RE. Donor selection in T cell-replete haploidentical hematopoietic stem cell transplantation: knowns, unknowns, and controversies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(2):180–184. doi: 10.1016/j.bbmt.2012.08.007. Epub 2012/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112(7):2990–2995. doi: 10.1182/blood-2008-01-135285. Epub 2008/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–4431. doi: 10.1182/blood-2008-07-169342. Epub 2008/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]