Abstract
Traditionally used as a descriptive term, frailty is now a recognized medical syndrome identifying individuals with decreased physiologic reserve. Frailty is characterized by diminished strength, endurance, and reduced physiologic function. Several valid frailty screening tools exist in the literature, and these measures have been used to relate frailty to outcomes important to the older patient with cancer. Frail adults are at increased risk of adverse surgical outcomes and early findings suggest that frailty predicts poor chemotherapy tolerance. While much research is needed to explore the biologic relationships between frailty and cancer, there is an urgent need to implement frailty screening and management into the care of the older patient with cancer in order to improve outcomes in this vulnerable subset. The purpose of this paper is to provide an introduction of frailty to oncologists including a review of the definition, frailty screening tools, its clinical relevance to older patients with cancer, and a brief guide to frailty management.
Keywords: frailty, cancer, older adults
Introduction
Historically, “frail” was a term used to describe a patient who appeared shrunken, weak and vulnerable, someone with clear fragility, evident to even the untrained eye. In the last several years of geriatric oncology literature, the word “frailty” has been used broadly to define any high risk older adult whether marked by disability, functional deficits, multimorbidity, advanced age, poor nutritional status, polypharmacy, cognitive impairment, or mood disorders. The broad use of this term has contributed to some confusion about the definition of frailty. With increasing numbers of medical and surgical interventions in an aging population, there is a need to more accurately quantify age-related physiologic risk to help identify appropriate candidates for these therapies. In response to this need, aging research experts have worked to develop more formal conceptualizations and definitions of frailty. In parallel, they have worked to develop and validate multiple assessment tools to differentiate between frail and vulnerable versus more robust older adults. As such, two prominent conceptualization theories of frailty have evolved over the past decade with the majority of frailty tools developed around these two theories. These methodologies and assessments described below are increasingly utilized to identify patients at high risk of adverse outcomes in many medical, oncological, and surgical settings. Indeed, the importance of frailty screening in older patients with cancer can be appreciated in several studies relating frailty to important oncology outcomes. The purpose of this paper is to provide an introduction of frailty to oncologists including a review of the definition, frailty screening tools, its clinical relevance to older patients with cancer, and a guide to frailty management.
Frailty Definitions: Conceptualization and the Development of Assessment Tools
In general, frailty has been defined as a state of vulnerability to adverse outcomes in older adults. Frailty represents a loss of physiologic reserve to maintain (or regain) homeostasis in the face of a stressor. Motivated by a growing demand to quantify reserve, aging experts have long sought to create a more formal, medical definition of frailty. A consensus conference held in 2013 suggested a medical definition around the concept of physical frailty. Physical frailty was defined as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.”1 While there is broad agreement around this definition of frailty, there is less agreement around the most appropriate tools or assessments to identify frail older adults. Hence, this definition allows for much flexibility in measuring frailty as described below.
Two leading theories of frailty’s pathophysiology exist in the literature: the frailty phenotype and the accumulated deficits theories.2, 3 The phenotypic frailty theory has been conceptualized around an observed condition of weakness, weight loss, and physical decline. It supposes that frailty arises from aging-related cellular and physiological changes that lead to a condition of vulnerability.4, 5 The accumulated deficits frailty theory has been conceptualized as a vulnerability that results from accumulated medical, physical and social conditions that in turn drive the increased vulnerability observed in frailty.6 The phenotypic frailty theory is grounded in an evidence-based biologic pathway of altered energetics, declining physiologic complexity, and loss of homeostatic capability.4 The accumulated deficits frailty theory is based on the conceptual framework that a global system loses robustness as it develops various illnesses or functional declines, termed “deficits.” This theory asserts that, at a certain threshold of deficits, the system fails completely (e.g., dies).6 As such, an accumulated deficit index tool has been developed that combines between 20 and 70 age-related indicators of health including comorbidities, disability, functional impairments, and symptoms into a single index that can be cumulatively scored (e.g., higher number of co-morbidities, the higher the frailty score). The phenotypic frailty theory presupposes that an underlying physiological decline contributes to frailty and ultimately to a variety of co-morbidities. The accumulated deficits frailty theory presupposes that an accumulation of co-morbidities drives frailty. The phenotypic frailty theory argues that the presence of frailty, or age-related physiologic dysfunction, is not dependent on the presence of comorbidity or disability, though they can co-exist, and is therefore assessed using markers other than comorbidity and disability. The accumulated deficits frailty theory intentionally includes comorbidities and disability as “deficits” of age.
Measuring Frailty
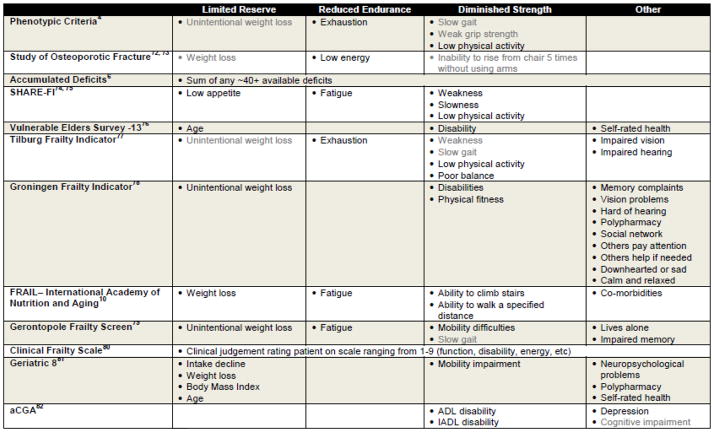
Many frailty screening tools have been developed and reported in the literature; a subset of these has been validated as well (Table 1).1 The tools generally align with one of the two predominant frailty pathophysiology theories described above although to varying degrees.3 The original measures selected for phenotypic frailty were chosen for their ability to assess various points along the proposed central biologic pathway: weak grip strength, unintentional weight loss, low physical activity, slow gait, and exhaustion. It was operationalized by Fried et al. into a validated screening exam whereby those below a population-based cutoff receive a point.4 Those with 3–5 points are deemed frail, and those with 1–2 are intermediate or pre-frail, and those with 0 are deemed robust. Many subsequent frailty measurement tools have been developed and validated based on this conceptualization of frailty. An accumulated deficit index tool to measure frailty was developed by Rockwood and colleagues that combines between 20 and 70 age-related indicators of health including comorbidities, disability, functional impairments, and symptoms into a single index. The index is scored cumulatively such that the higher number of co-morbidities or deficits, the higher the frailty score.6 This methodology has also been widely utilized to develop other frailty-related co-morbidity measurement tools.7, 8 Although most of the frailty assessment tools are derived from these two concepts of frailty, there are multiple additional tools that include cognitive dysfunction, disability, and comorbidities as measures of frailty.3 While some controversy remains as to whether cognitive decline is a core biological component of physical frailty, many tools incorporate cognition allowing identification of frailty and cognitive impairment as potentially co-existing but distinct risks.4, 9 Some frailty tools also measure social and economic vulnerabilities (eg, social isolation, poverty), yet it is not clear if these items should be considered separate risks from age-related physiologic risk.
Table 1.
Examples of Frailty Screening Tools
 = Objective Measure ■ = Self-Reported Measure or Provider Impression
= Objective Measure ■ = Self-Reported Measure or Provider Impression
Selecting a Frailty Tool
The array of frailty tools available to researchers and clinicians can be daunting. Because of the variability in the tools, we recommend selecting a frailty tool for clinical or research applications in patients with cancer based on 1) the feasibility and intention of implementing the tool into practice and 2) the specific clinical or research needs while also considering the limitations of available comparative data. 1) An important consideration for the potential choice of frailty measurement tools is the feasibility of their use in screening. The phenotype frailty tools are, in general, brief, primary screening tools that can be conducted and scored in the absence of a large amount of previously generated clinical or functional information. The accumulated deficits index, on the other hand, requires a substantial collection of comprehensive medical and functional information in order to be calculated, which makes it more difficult to use as a primary screening tool. Electronic medical records or large existing databases, once comprehensively populated with accumulated deficit index items, may facilitate its rapid use. Pending the available resources and pre-existing information, it may be more feasible for practitioners to implement a phenotypic frailty tool to screen for frailty into clinical practice while using the accumulated deficits tool to assess comprehensive risk.
Another key consideration is(are) the specific clinical and research need(s). Some frailty tools are better suited for different needs. For pure risk assessment, a very quick and easy tool that can be used to differentiate risk is the FRAIL tool.10 This is based on five questions (weight loss, fatigue, ability to climb stairs, ability to walk a specified distance, and co-morbidities), and has been demonstrated to identify older adults at risk for earlier mortality. If the frailty assessment will be utilized to study the underlying biology of frailty, or to develop interventions in a pre-frail state, the phenotypic frailty-type tools are likely the correct choice given the comprehensive biological and intervention literature that has been developed around phenotypic frailty. If the patient is not available or well enough to answer questions, or is hospitalized or non-ambulatory, then an accumulated deficits index tool may be the appropriate choice given that much of the required information could be abstracted from medical records.7, 8 Oncologists interested in studying the impact of loss of age-related physiologic reserve or overlapping aging and cancer biologic pathways may want to consider measuring phenotypic frailty. Oncologists wanting to assess broad risks for mortality may want to choose a tool that does not require physical measurements and utilizes abstracted medical records in an accumulated deficits index format to assess this vulnerability.
There are a number of limitations to the existing frailty tools and the existing frailty literature that must be kept in mind when selecting a frailty tool. The prevalence of frailty varies slightly from study to study depending on the frailty tool used; furthermore, the varying tools often do not identify exactly the same group of people.11, 12 This is due, in part, to the varying factors included in each tool and varying mechanisms for measuring them (e.g., patient survey versus clinical measure versus clinical judgement). This variability admittedly poses a challenge to implementing frailty screening into clinical practice but also an opportunity to contribute to the literature about how different tools compare in cancer populations. Another consequence of this variability among measures is that the ability of each tool to predict poor outcomes depends on the outcome assessed, the tool used, any adaptations to the frailty measures (e.g., self-reported walking versus objectively measured gait speed), and the characteristics of the sample being considered (e.g., surgical candidates, heart failure patients, primary care sample). In some cases, the ability of a frailty tool to improve prediction of poor outcomes over traditional assessments is very significant13, 14 and in others, the improvement may only be modest.15 Few studies have actually compared different tools in the same sample, so it can be difficult to compare tool characteristics across studies.11, 12, 15 Because different factors and measures are included in each tool, there should not be an expectation for the tools to be equivalent. Since the pathophysiology of frailty is still under study, the tools aligning with differing theories of thought should not be considered clashing but complementary to one another. The current literature reporting the application of specific frailty tools to patients with cancer is summarized below and can provide further guidance.
Importance of the Frailty Syndrome Assessment to Cancer Care
Geriatric oncology studies have trailed the rising population of older adults with cancer.16 Among the gaps in knowledge is the need for better risk stratification and treatment selection based on frailty status.17–19 The comprehensive geriatric assessment (CGA) has been used as a “gold standard” in the oncology literature to identify vulnerable and frail adults. The CGA includes an evaluation of medical, functional, psychological, cognitive, and social health. It identifies potentially modifiable interventions to maximize independence, social support, cognition, and quality of life while reducing risks for poor outcomes such as delirium, worsening disability, post-operative complications, rehospitalization, or surgical mortality.20, 21 The CGA identifies not only physical frailty but a wide variety of vulnerabilities. The CGA predicts post-surgical and overall mortality among patients with cancer,9, 22, 23 and a pre-operative geriatric assessment improves surgical outcomes in patients with cancer.24 The CGA is time-consuming (variable but typically ≥ 1 hour), though, and the recommendations made based on the CGA may rely on the availability of specialized team members like geriatric social workers to implement. Because of the resource intensive nature of the CGA, it has not been routinely applied in oncology care of older adults, yet. Many of the physical frailty screens are brief (5–15 minutes), and their ability to predict poor surgical outcomes, chemotherapy toxicity, and CGA-based “frailty” has been the topic of a growing number of studies. Indeed, some studies suggest that patients who are frail are the group that most benefits from CGA.25, 26
Frailty and Surgical Outcomes
Frail adults are more likely than non-frail adults to have surgical complications following elective surgery. The phenotypic frailty criteria have been the most widely studied pre-operative frailty screening tool in patients with cancer. Using phenotypic frailty criteria categorized as pre-frail (2–3 criteria) and frail (4–5 criteria), Makary et al found a step-wise increased risk of 30-day post-operative complications and discharge to an institution among all (cancer and non-cancer), pre-frail and frail elective surgical candidates in addition to traditional surgical risk scores (eg, Lee, Eagle, etc).13 Similarly, presence of frailty as indicated by the phenotypic criteria predicted poor surgical outcomes among older (75+) colorectal cancer (CRC) patients undergoing colon resection27 and among women undergoing a gynecologic oncology surgery.28 An adapted version of the phenotypic frailty criteria predicted survival but not post-operative outcomes among colorectal cancer resection patients.29 Some studies have assessed whether single phenotypic frailty measures predict surgical outcomes. Two of the five phenotypic criteria, unintentional weight loss and weak grip strength, predicted 30-day surgical complications among patients with cancer undergoing major intra-abdominal surgery as well as the five criteria combined.30 Self-reported exhaustion alone predicted major complications, admission to the intensive care unit, discharge to a rehabilitation facility, and decreased 30-day readmissions among adults (≥18) who underwent a pancreaticoduodenectomy.31 Among older (70+) CRC patients undergoing colon resection, grip strength predicted post-operative complications in unadjusted models.32 Fewer studies have evaluated other frailty screening tools in oncologic surgical candidates. In a retrospective study using the National Surgical Quality Improvement Program data, a frailty index modeled after the accumulated deficits index was associated with greater surgical complications and mortality beyond the American Society of Anesthesiologists score among inpatient otolaryngologic operations for non-cancer and cancer indications.33 Among older patients with confirmed glioblastoma, an 11-item frailty index modeled after the accumulated deficits index predicted length of stay, post-operative surgical complications, and survival independent of Karnofsky performance status.34 An index-type screening tool was successfully utilized to identify older trauma surgery patients who were at high risk for surgical complications and mortality.35 In a study assessing predictors of post-operative complications among older adults (70+) requiring non-emergent solid tumor resection, the timed up and go test significantly predicted complications in addition to the American Society of Anesthesiologists score but the Groningen Frailty Index (GFI) and the Vulnerable Elders Survey (VES-13) did not.36 Among adults ≥ 70 undergoing surgery for colorectal cancer, the timed up and go and the Vulnerable Elders Survey as well as instrumental activities of daily living and the Eastern Cooperative Oncology Group Performance Status (ECOG PS) were significantly associated with long-term survival (median 4.6 years) in univariate analyses, though the sample was too small to see if the frailty measure outperformed the other measures in multivariate analyses.37 The growing body of frailty literature highly suggests the critical importance of implementing frailty screening measures into pre-operative assessments to improve risk stratification.
Frailty and Chemotoxicity or Radiotherapy Fatigue
Fewer studies have investigated the relationship between frailty screening tools and chemotoxicity or radiotherapy fatigue although the CGA has been shown to be helpful in predicting toxicity and mortality from chemotherapy.38–41 The GFI predicted mortality from chemotherapy in advanced CRC patients.42, 43 Weak grip strength predicted chemotherapy toxicity but not mortality among older (65+) patients with cancer while the ECOG PS score predicted mortality but not treatment toxicity.44 The Geriatric 8 (G8) and GFI did not predict serious adverse events following first cycle of (radio)chemotherapy among older (65+) patients with cancer.45 Only the Vulnerable Elders Survey score significantly predicted mortality among older patients with stage III/IV colorectal cancer undergoing chemotherapy in regression models controlling for ECOG PS, activities of daily living dependence, and age.46 In retrospective regression models controlling for tumor characteristics, age, body mass index, number of medications, and chemotherapy, both a phenotypic frailty score and a cancer-specific comprehensive geriatric assessment were significantly associated with radiotherapy fatigue while the Karnofsky score was not.47 In a prospective study of patients with solid tumors referred for a geriatric assessment, phenotypic frailty predicted a recommendation to switch to supportive/palliative treatment rather than the initial treatment plan while the ECOG PS scale did not.48 These early studies suggest frailty may predict overall chemotherapy and radiotherapy-related morbidity and mortality, but it is not yet clear whether frailty predicts short-term chemotherapy outcomes.
Frailty Tool versus a Comprehensive Geriatric Assessment
A CGA is a considered a gold standard older adult assessment to identify all geriatric syndromes; however its time-consuming nature and low reimbursement to date have prevented its broad application to older adults. Several studies have attempted to identify the screening test characteristics of various frailty scales for identifying patients with cancer who have an abnormal CGA. Comparison of these studies is difficult because the CGA is not conducted similarly across studies, the threshold used to identify a positive frailty screen was variable, and the studies included different subpopulations.49 With these limitations in mind, the sensitivity of frailty tools to identify older persons with an abnormal CGA ranged from 52% to 97%, the specificity ranged from 44% to 100%.50–55 Higher sensitivity was noted among people with more advanced disease at the expense of lower specificity, and the test characteristics varied by cancer type subgroup analysis.52 A comprehensive review of frailty screening test characteristics determined that none demonstrate the optimal combination of high sensitivity and negative predicted value and an acceptable specificity for predicting abnormal CGA to be considered for favored use.56 Despite the lack of a preferred frailty screening tool, aging experts strongly recommend the use of at least one of these tools, validated in a relevant population, to help identify high-risk older adults who would most benefit from a CGA.26 Screening is of particular importance to subgroups, including those with cancer, that have a high likelihood of benefitting from frailty-reduction strategies.17
Summary of Frailty Assessment in Older Patients with Cancer
Given the growing evidence that physical frailty predicts poor surgical outcomes and early evidence that frailty may help predict individuals who experience chemotherapy toxicity, screening for frailty as an independent risk stratification tool in older patients with cancer has become imperative. Several frailty tools have proven useful in predicting surgical and chemotherapy outcomes, although not all of the validated tools have been studied. As others have highlighted, sensitivity, specificity, positive predictive value, and negative predictive value for predicting the CGA are dependent on the tool being use, the prevalence of frailty in the sample, and the cut-offs chosen.49 Some of the frailty tools aim to measure only biologic risk keeping in line with the consensus definition of frailty as a medical syndrome. Other tools aim to measure social, economic, disability, and psychological risks in addition to biologic risks mirroring the core elements of the CGA. The benefit of using a biologically-based model is that age-related physiologic dysregulation can be studied independent of the effects from these other factors and related to the biological processes of cancer. It requires, however, that these other factors be assessed through other means. The benefit of using a “mini” CGA is that it provides a rapid evaluation of pooled factors but at the expense of having a unifying underlying etiology with which to study its underpinnings.2
Management of Frailty in Cancer Patients
While some studies recommend overall approaches to caring for the older patient with cancer,57 frailty syndrome intervention trials are just starting to emerge in the literature, and none are specific to patients with cancer.58, 59 Furthermore, the trials assess improvement in frailty markers rather than cancer- or surgery-specific outcomes. Addressing weakness through resistance and strength interventions has most consistently improved frailty measures. The duration of exercise interventions tested ranged between 6 weeks and 2.6 years. A positive and significant effect on frailty measures was noted in as short as 6 weeks in one study.60 Protein supplementation through nutritional interventions has had some early success. Nutritional intervention appears to be the most successful when paired with exercise. Multidimensional interventions, similar to those used to address geriatric syndromes identified in a CGA, have also been tested to reduce frailty and ultimately adverse health outcomes. While labor-intensive, they have also improved frailty markers, particularly those including a polypharmacy reduction plan. Early studies have reported the effects of various pharmacotherapies targeting the biologic frailty pathway including symbiotic, DHEA, testosterone, and rhGH.59 On-going trials will test additional pharmacotherapies including ghrelin, allopurinol, vitamin D, and omega-3 fatty acids.58 None of these agents have had enough data to recommend routine use.
The knowledge gained from the frailty intervention studies offers some guidelines for frailty management in the oncology patient, but much work is needed to evaluate the impact of frailty interventions on cancer outcomes in older adults (Table 2). This work is especially important because the frailty syndrome and cancer share many of the same presenting signs (e.g., wasting) with potential for shared benefits. Addressing weakness through exercise programs improves frailty measures in as little time as 6 weeks. For the oncologic patient, the luxury of time is often not the case. The literature would suggest that a prehabilitation program prior to chemotherapy or surgery, if possible, will reduce the frailty-associated risks of morbidity and mortality. Prehabilitation programs not specific to older, frail cancer adults have shown some benefit in the general cancer population.61 It is not yet clear if concurring exercise and chemotherapy would offer the same benefits to frail adults. Post-operative rehabilitation has been a standard part of all surgical patients who acquire weakness regardless of frailty status. Nutritional supplementation offers modest improvements in frailty measures in the general older adult population and may be more important among patients with cancer who have cachexia. Addressing factors that may hasten the frailty-associated outcomes of disability, delirium, or falls including polypharmacy management, vitamin D deficiency treatment, and addressing gait impairment are likely also important. The positive impact of multidimensional programs on frailty suggests there is added benefit of simultaneously addressing frailty moderators such as limited social support, cognitive impairment, multimorbidity, and mood disorders.
Table 2.
Frailty Management Approach in the Older Patient with Cancer
| Frailty Treatment Goals | Frailty Management |
|---|---|
| Evaluate for Presence of Other Geriatric Syndromes and Vulnerabilities | Conduct a comprehensive geriatric assessment in frail individuals to identify and manage other geriatric syndromes and vulnerabilities that may commonly co-exist with frailty. When available, refer to a geriatrician for this assessment and co-management of the frail patient to optimize risks. |
| Improve Weakness | Regular resistance & strength training, order physical or occupational therapy, add protein supplementation in diet |
| Temper Weight Loss | Adequate caloric intake, nutritionist evaluation, socialized meals, ensure food access, replace/fix dentures, support for meal preparation, liberalize diet and use of seasonings |
| Reduce Polypharmacy | Avoid high-risk medications in the elderly, frequent medication reconciliation, counsel on creating a medication administration routine with oversight, low-dose and short-term trials of new medications with frequent assessment for side effects |
| Address Exhaustion | Consider medication side effects; evaluate loneliness and mood disorders; improve weakness |
| Screen for Social Support Needs | Obtain contact information for caregivers, add homemakers to support caregivers, establish healthcare power of attorney or surrogate; assess for caregiver burnout; accommodate financial strains |
| Screen for Cognitive Impairment | Administer a validated cognitive screening tools; identify surrogate or healthcare power of attorney; consider pharmacotherapy |
| Reduce Risk for Frailty Outcomes (e.g., falls, fracture, disability, hospitalization, delirium, post-operative morbidity, mortality) | Treat Vitamin D deficiency, assess bone mineral density, schedule frequent outpatient visits with access to urgent care visits to reduce emergency room/hospital utilization; update advance directives; implement mobility devices; order physical or occupational therapy; optimize frailty status before surgery and engage inpatient geriatrics consultation when available for hospitalized frail surgical candidates |
| Tailor Treatment Goals to the Patient’s Risk Status and Their Healthcare Goals | The presence of frailty increases risk of poor cancer treatment outcomes and should be a factor considered in the shared decision making process. |
Frailty management is complex and often requires detailed intervention plans tailored to specific patient deficits to fully optimize frailty status. Comprehensive Geriatric Assessments can be helpful in articulating a care plan. Because Geriatricians are uniquely trained to identify and manage frailty, consideration should be given to having Geriatricians help with the co-management of frail patients with cancer when possible. Several successful oncology-geriatrics collaborative models exist that have had positive effects on cancer outcomes and can offer guidance on creating such models in other institutions.62–66
Future Directions
Clinical cancer trials including older, frail adults are in great need despite the complex nature of these studies.67, 68 Including frailty measures will be an important part of these future studies and may help facilitate the development of more individualized guidelines for frail older adults with cancer. Much work is needed to explore the biologic frailty and cancer relationships. For example, many frailty-related biomarkers are also altered in cancer suggesting they may have common pathophysiologic mechanisms.32, 69, 70 In contrast, there exists a cancer-frailty “paradox” – an early finding that cancer is less prevalent among the very frail adults.71 Distinguishing, or not, the loss of reserve due to age-related physiologic dysregulation from the cancer-associated processes is essentially an uncharted area of research. Differentiating these pathways could greatly improve risk stratification and treatment of both conditions.
Conclusion
Measures of the frailty syndrome are critical to understanding risk for morbidity and mortality in the older patient with cancer. While there remains controversy in the literature regarding the best frailty assessment tool, many validated tools exist that could be utilized for clinical and research purposes. The selection of the frailty tool will depend on its intended use. Treating the frailty syndrome will likely reduce risk for poor cancer outcomes, particularly surgical outcomes, though much work is needed in this area. Exercise and nutritional interventions that target sarcopenia and protein deficiencies have had the most supportive data. Addressing frailty moderating factors including other geriatric syndromes, lack of social support, multimorbidity, disability, cognitive impairment, and mood disorders are also important in the frailty syndrome management. There is a great need for research exploring the biologic relationships between cancer and the frailty syndrome as well as the impact of frailty interventions on cancer-specific outcomes.
Take Home Points.
Frailty is a defined medical syndrome characterized by diminished strength, reduced endurance, and decreased physiologic function.
Multiple validated frailty measurement tools are available in the literature.
Frailty assessment is generally a useful pre-operative predictor of post-operative complications among patients with cancer beyond traditional risk scoring systems. Frailty assessment is likely helpful is predicting overall chemotherapy tolerance.
Choice of frailty measurement tool should be based on feasibility, whether the frailty assessment will be conducted in the clinical encounter or using previously collected medical record data, the need to screen for frailty versus provide a comprehensive risk score, and on specific clinical or research goals.
Acknowledgments
Dr. Huisingh-Scheetz receives support from the John A. Hartford Foundation.
Footnotes
Disclosures and Conflict of Interest Statements
The authors have no conflicts of interest to report
Author Contributions
All authors contributed to the conception of the article and writing, editing, and final review of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. BMC Med. 2015;13:185. doi: 10.1186/s12916-015-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson TN, Walston JD, Brummel NE, et al. Frailty for Surgeons: Review of a National Institute on Aging Conference on Frailty for Specialists. J Am Coll Surg. 2015 doi: 10.1016/j.jamcollsurg.2015.08.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 8.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stotter A, Reed MW, Gray LJ, Moore N, Robinson TG. Comprehensive Geriatric Assessment and predicted 3-year survival in treatment planning for frail patients with early breast cancer. Br J Surg. 2015;102:525–533. doi: 10.1002/bjs.9755. discussion 533. [DOI] [PubMed] [Google Scholar]
- 10.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 11.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 12.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 13.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sourial N, Bergman H, Karunananthan S, et al. Implementing frailty into clinical practice: a cautionary tale. J Gerontol A Biol Sci Med Sci. 2013;68:1505–1511. doi: 10.1093/gerona/glt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale W, Mohile SG, Eldadah BA, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104:581–589. doi: 10.1093/jnci/djs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz M, Reske T, Cefalu C, Estrada J. Management of elderly and frail elderly cancer patients: the importance of comprehensive geriatrics assessment and the need for guidelines. Am J Med Sci. 2013;346:66–69. doi: 10.1097/MAJ.0b013e31826d59aa. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN) Blood. 2011;118:4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 19.Audisio RA, van Leeuwen B. When reporting on older patients with cancer, frailty information is needed. Ann Surg Oncol. 2011;18:4–5. doi: 10.1245/s10434-010-1327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen BL, Huisman MG, Audisio RA. Surgery in older cancer patients - recent results and new techniques: worth the investment? Interdiscip Top Gerontol. 2013;38:124–131. doi: 10.1159/000343582. [DOI] [PubMed] [Google Scholar]
- 21.Carli F, Brown R, Kennepohl S. Prehabilitation to enhance postoperative recovery for an octogenarian following robotic-assisted hysterectomy with endometrial cancer. Can J Anaesth. 2012;59:779–784. doi: 10.1007/s12630-012-9734-4. [DOI] [PubMed] [Google Scholar]
- 22.Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19:1268–1275. doi: 10.1634/theoncologist.2014-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Indrakusuma R, Dunker MS, Peetoom JJ, Schreurs WH. Evaluation of preoperative geriatric assessment of elderly patients with colorectal carcinoma. A retrospective study. Eur J Surg Oncol. 2015;41:21–27. doi: 10.1016/j.ejso.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 26.Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43:744–747. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 27.Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–143. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Courtney-Brooks M, Tellawi AR, Scalici J, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol. 2012;126:20–24. doi: 10.1016/j.ygyno.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Kristjansson S, Ronning B, Hurria A, et al. A comparison of two pre-operative frailty measures in older surgical cancer patients. Journal of Geriatric Oncology. 2012;3:1–7. [Google Scholar]
- 30.Revenig LM, Canter DJ, Kim S, et al. Report of a Simplified Frailty Score Predictive of Short-Term Postoperative Morbidity and Mortality. J Am Coll Surg. 2015;220:904–911. e901. doi: 10.1016/j.jamcollsurg.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 31.Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg. 2014;259:960–965. doi: 10.1097/SLA.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronning B, Wyller TB, Jordhoy MS, et al. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol. 2014;5:26–32. doi: 10.1016/j.jgo.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139:783–789. doi: 10.1001/jamaoto.2013.3969. [DOI] [PubMed] [Google Scholar]
- 34.Cloney M, D’Amico R, Lebovic J, et al. Frailty in Geriatric Glioblastoma Patients: A Predictor of Operative Morbidity and Outcome. World Neurosurg. 2016 doi: 10.1016/j.wneu.2015.12.096. [DOI] [PubMed] [Google Scholar]
- 35.Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149:766–772. doi: 10.1001/jamasurg.2014.296. [DOI] [PubMed] [Google Scholar]
- 36.Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in oncogeriatric surgical patients: A multicenter prospective cohort study. Eur J Surg Oncol. 2015;41:844–851. doi: 10.1016/j.ejso.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Ugolini G, Pasini F, Ghignone F, et al. How to select elderly colorectal cancer patients for surgery: a pilot study in an Italian academic medical center. Cancer Biol Med. 2015;12:302–307. doi: 10.7497/j.issn.2095-3941.2015.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamaker ME, Seynaeve C, Wymenga AN, et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: results from the OMEGA study of the Dutch breast cancer trialists’ group. Breast. 2014;23:81–87. doi: 10.1016/j.breast.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Merli F, Luminari S, Rossi G, et al. Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma. 2014;55:38–43. doi: 10.3109/10428194.2013.788176. [DOI] [PubMed] [Google Scholar]
- 41.Marchesi F, Cenfra N, Altomare L, et al. A retrospective study on 73 elderly patients (>/=75years) with aggressive B-cell non Hodgkin lymphoma: clinical significance of treatment intensity and comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:242–248. doi: 10.1016/j.jgo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Aaldriks AA, Maartense E, le Cessie S, et al. Predictive value of geriatric assessment for patients older than 70 years, treated with chemotherapy. Crit Rev Oncol Hematol. 2011;79:205–212. doi: 10.1016/j.critrevonc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. 2013;4:218–226. doi: 10.1016/j.jgo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol. 2011;78:138–149. doi: 10.1016/j.critrevonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Baitar A, Van Fraeyenhove F, Vandebroek A, et al. Geriatric screening results and the association with severe treatment toxicity after the first cycle of (radio)chemotherapy. J Geriatr Oncol. 2014;5:179–184. doi: 10.1016/j.jgo.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Ramsdale E, Polite B, Hemmerich J, et al. The Vulnerable Elders Survey-13 predicts mortality in older adults with later-stage colorectal cancer receiving chemotherapy: a prospective pilot study. J Am Geriatr Soc. 2013;61:2043–2044. doi: 10.1111/jgs.12536. [DOI] [PubMed] [Google Scholar]
- 47.Denkinger MD, Hasch M, Gerstmayer A, Kreienberg R, Nikolaus T, Hancke K. Predicting fatigue in older breast cancer patients receiving radiotherapy. A head-to-head comparison of established assessments. Z Gerontol Geriatr. 2015;48:128–134. doi: 10.1007/s00391-014-0840-5. [DOI] [PubMed] [Google Scholar]
- 48.Farcet A, de Decker L, Pauly V, et al. Frailty Markers and Treatment Decisions in Patients Seen in Oncogeriatric Clinics: Results from the ASRO Pilot Study. PLoS One. 2016;11:e0149732. doi: 10.1371/journal.pone.0149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathoulin-Pelissier S, Bellera C, Rainfray M, Soubeyran P. Screening methods for geriatric frailty. Lancet Oncol. 2013;14:e1–2. doi: 10.1016/S1470-2045(12)70554-5. [DOI] [PubMed] [Google Scholar]
- 50.Smets IH, Kempen GI, Janssen-Heijnen ML, Deckx L, Buntinx FJ, van den Akker M. Four screening instruments for frailty in older patients with and without cancer: a diagnostic study. BMC Geriatr. 2014;14:26. doi: 10.1186/1471-2318-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenig J, Zychiewicz B, Olszewska U, Richter P. Screening for frailty among older patients with cancer that qualify for abdominal surgery. J Geriatr Oncol. 2015;6:52–59. doi: 10.1016/j.jgo.2014.09.179. [DOI] [PubMed] [Google Scholar]
- 52.Biganzoli L, Boni L, Becheri D, et al. Evaluation of the cardiovascular health study (CHS) instrument and the Vulnerable Elders Survey-13 (VES-13) in elderly cancer patients. Are we still missing the right screening tool? Ann Oncol. 2013;24:494–500. doi: 10.1093/annonc/mds331. [DOI] [PubMed] [Google Scholar]
- 53.Luciani A, Dottorini L, Battisti N, et al. Screening elderly cancer patients for disabilities: evaluation of study of osteoporotic fractures (SOF) index and comprehensive geriatric assessment (CGA) Ann Oncol. 2013;24:469–474. doi: 10.1093/annonc/mds471. [DOI] [PubMed] [Google Scholar]
- 54.Baitar A, Van Fraeyenhove F, Vandebroek A, et al. Evaluation of the Groningen Frailty Indicator and the G8 questionnaire as screening tools for frailty in older patients with cancer. J Geriatr Oncol. 2013;4:32–38. doi: 10.1016/j.jgo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Augschoell J, Kemmler G, Hamaker ME, Stauder R. PPT and VES-13 in elderly patients with cancer: evaluation in multidimensional geriatric assessment and prediction of survival. J Geriatr Oncol. 2014;5:415–421. doi: 10.1016/j.jgo.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13:e437–444. doi: 10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- 57.Ugolini G, Ghignone F, Zattoni D, Veronese G, Montroni I. Personalized surgical management of colorectal cancer in elderly population. World J Gastroenterol. 2014;20:3762–3777. doi: 10.3748/wjg.v20.i14.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bendayan M, Bibas L, Levi M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part II. Ongoing and unpublished randomized trials. Prog Cardiovasc Dis. 2014;57:144–151. doi: 10.1016/j.pcad.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Marsh AP, Chmelo EA, Katula JA, Mihalko SL, Rejeski WJ. Should physical activity programs be tailored when older adults have compromised function? J Aging Phys Act. 2009;17:294–306. doi: 10.1123/japa.17.3.294. [DOI] [PubMed] [Google Scholar]
- 61.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 62.Hurria A, Lichtman SM, Gardes J, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55:1604–1608. doi: 10.1111/j.1532-5415.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 63.Bouzereau V, Le Caer F, Guardiola E, et al. Experience of multidisciplinary assessment of elderly patients with cancer in a French general hospital during 1 year: a new model care study. J Geriatr Oncol. 2013;4:394–401. doi: 10.1016/j.jgo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Tremblay D, Charlebois K, Terret C, Joannette S, Latreille J. Integrated oncogeriatric approach: a systematic review of the literature using concept analysis. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan KY, Tan P, Tan L. A collaborative transdisciplinary “geriatric surgery service” ensures consistent successful outcomes in elderly colorectal surgery patients. World J Surg. 2011;35:1608–1614. doi: 10.1007/s00268-011-1112-9. [DOI] [PubMed] [Google Scholar]
- 66.Owusu C, Studenski SA. Shared care in geriatric oncology: primary care providers’ and medical/oncologist’s perspectives. J Am Geriatr Soc. 2009;57(Suppl 2):S239–242. doi: 10.1111/j.1532-5415.2009.02501.x. [DOI] [PubMed] [Google Scholar]
- 67.Hurria A, Dale W, Mooney M, et al. Designing Therapeutic Clinical Trials for Older and Frail Adults With Cancer: U13 Conference Recommendations. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hempenius L, Slaets JP, Boelens MA, et al. Inclusion of frail elderly patients in clinical trials: solutions to the problems. J Geriatr Oncol. 2013;4:26–31. doi: 10.1016/j.jgo.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Hubbard JM, Jatoi A. Incorporating biomarkers of frailty and senescence in cancer therapeutic trials. J Gerontol A Biol Sci Med Sci. 2015;70:722–728. doi: 10.1093/gerona/glu046. [DOI] [PubMed] [Google Scholar]
- 70.Corona G, Polesel J, Fratino L, et al. Metabolomics biomarkers of frailty in elderly breast cancer patients. J Cell Physiol. 2014;229:898–902. doi: 10.1002/jcp.24520. [DOI] [PubMed] [Google Scholar]
- 71.Kanapuru B, Simonsick EM, Ershler WB. Is cancer incidence decreased in the frail elderly? Evidence from a prospective cohort study. J Geriatr Oncol. 2013;4:19–25. doi: 10.1016/j.jgo.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 74.Romero-Ortuno R, Soraghan C. A Frailty Instrument for primary care for those aged 75 years or more: findings from the Survey of Health, Ageing and Retirement in Europe, a longitudinal populationbased cohort study (SHARE-FI75+) BMJ Open. 2014;4:e006645. doi: 10.1136/bmjopen-2014-006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero-Ortuno R. The Frailty Instrument of the Survey of Health, Ageing and Retirement in Europe (SHARE-FI) predicts mortality beyond age, comorbidities, disability, self-rated health, education and depression. Eur Geriatr Med. 2011;2:323–326. doi: 10.1016/j.eurger.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 77.Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc. 2010;11:344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Peters LL, Boter H, Buskens E, Slaets JP. Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc. 2012;13:546–551. doi: 10.1016/j.jamda.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Vellas B, Balardy L, Gillette-Guyonnet S, et al. Looking for frailty in community-dwelling older persons: the Gerontopole Frailty Screening Tool (GFST) J Nutr Health Aging. 2013;17:629–631. doi: 10.1007/s12603-013-0363-6. [DOI] [PubMed] [Google Scholar]
- 80.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velghe A, Petrovic M, De Buyser S, Demuynck R, Noens L. Validation of the G8 screening tool in older patients with aggressive haematological malignancies. Eur J Oncol Nurs. 2014;18:645–648. doi: 10.1016/j.ejon.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Overcash JA, Beckstead J, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol. 2005;54:129–136. doi: 10.1016/j.critrevonc.2004.12.002. [DOI] [PubMed] [Google Scholar]