Abstract
Fibromyalgia (FM) patients show characteristically enhanced unpleasantness to painful and non-painful sensations accompanied by altered neural responses. The diagnostic potential of such neural alterations, including their sensitivity and specificity to FM (vs. healthy controls) is unknown. We identify a brain signature that characterizes FM central pathophysiology at the neural systems level. We included 37 FM patients and 35 matched healthy controls, and analyzed fMRI responses to (i) painful pressure and (ii) non-painful multisensory (visual-auditory-tactile) stimulation. We used machine-learning techniques to identify a brain-based FM signature. When exposed to the same painful stimuli, FM patients showed greater Neurologic Pain Signature (NPS, Wager 2013) responses. In addition, a new pain-related classifier (‘FM-pain’) revealed augmented responses in sensory integration (insula/operculum) and self-referential (e.g., medial prefrontal) regions in FM, and reduced responses in the lateral frontal cortex. A ‘Multisensory’ classifier trained on non-painful sensory stimulation revealed augmented responses in insula/operculum, posterior cingulate, and medial prefrontal regions, and reduced responses in primary/secondary sensory cortices, basal ganglia and cerebellum. Combined activity in the NPS, FM-pain, and Multisensory patterns classified patients vs. controls with 92% sensitivity and 94% specificity in out-of-sample individuals. Enhanced NPS responses partly mediated mechanical hypersensitivity, and correlated with depression and disability(puncorrected<0.05); FM-pain and Multisensory responses correlated with clinical pain(puncorrected<0.05). The study provides initial characterization of individual FM-patients based on pathophysiological, symptom-related brain features. If replicated, these brain features may constitute objective neural targets for therapeutic interventions. The results establish a framework for assessing therapeutic mechanisms and predicting treatment response at the individual level.
INTRODUCTION
Fibromyalgia (FM) is characterized by the presence of widespread musculoskeletal pain and tenderness accompanied by fatigue, cognitive/emotional and sleep-related symptoms, occurring without any other medical explanation[77]. Besides its high prevalence and clinical relevance[79], there is an inherent problem associated with the diagnosis of FM in that there is an absence of laboratory findings or well-characterized pathology that is sensitive and specific for the disorder. The existence of FM as a clinical diagnosis has been therefore historically questioned[79]. The last two decades of research, however, have provided consistent evidence to suggest abnormal nervous system findings in FM patients[14]. Of note, neuroimaging studies have shown augmented responses to a variety of painful stimuli in FM[15; 25; 56], and altered brain structure, metabolic activity and resting state functional connectivity in regions that are consistently involved in processing pain, e.g.[23; 28; 34; 36; 38; 44; 50; 57; 58; 60; 61].
In addition to pain-related changes, FM patients show reduced tolerance (augmented unpleasantness) to non-painful sensory stimulation (visual, auditory, olfactory and tactile), along with abnormal brain processing of non-painful sensory stimuli, e.g.[11; 21; 30; 42; 74]. Our group[42; 58] and others[31; 46; 53] have reported evidence suggesting that the brain systems involved in the primary cortical processing of non-painful sensory signals and their integration may play an important role in FM pain. These studies suggest that pain in FM may be associated with (i) hyper-excitability of the nociceptive system, i.e., increased transmission (e.g.[52; 64; 69]), central amplification[15; 25; 56], and/or reduced inhibitory control mechanisms[35; 37] and (ii) reduced opponent non-nociceptive sensory processing[31; 42; 46; 53; 58]. In spite of these brain and behavioral findings, there remains a critical gap between characterizing abnormalities in FM at a group level and identifying neurophysiological markers diagnostic of FM at an individual patient level.
Here we use a multisensory approach to identify a brain signature sensitive to FM status (vs. healthy) at the individual-person level. Notably, we do not imply that the signature should differentiate FM from other chronic pain conditions with a sensitization component. We include tests of both mechanical pain and non-painful sensory brain responses to (i) characterize the sensory processing alterations that are distinctive of FM at the central level, and (ii) address how such characteristic pathophysiological features relate to patients’ core symptoms. As a first approach to assess alterations in central pain processing, we applied the Neurologic Pain Signature (NPS), a multivariate brain activation pattern that was previously validated to be sensitive and specific to predict experimental pain perception at the individual-person level[70]. The NPS accurately predicts experimental pain perception, but does not respond to other unpleasant, highly arousing emotional experiences[70]. Augmented expression of the NPS in FM would indicate enhanced pain-specific cerebral processing in patients. In addition, we applied cross-validated machine learning algorithms to differentiate FM patients from healthy participants based on their brain responses to (i) pressure pain and (ii) combined non-painful visual, auditory, and tactile-motor stimulation. Finally, we combined pain and multisensory brain measures to obtain a cross-validated signature for FM status.
MATERIALS AND METHODS
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Ethics and Institutional Review Board of the Autonomous University of Barcelona (reference number SAF2010-19434). All patients and healthy participants provided written informed consent to participate in the study.
Subjects
Seventy-two subjects participated, including 37 female FM patients (1990 American College of Rheumatology criteria for FM)[78] and 35 female matched healthy controls (see Table 1). Patients and healthy subjects were matched for age, education status and handedness (all right-handed, Table 1). The patients were consecutively recruited during clinical follow-up in the rheumatology service (CIMA, Barcelona) to provide a homogeneous sample with severe and persistent FM symptoms. Vision and hearing were normal upon neurological examination for all patients and healthy participants.
Table 1.
Demographics, Illness Severity and Medication regime.
| FM Patients (N = 37)* |
Healthy Participants (N = 35)* |
Statistics | |
|---|---|---|---|
| Age | |||
| (mean ± SD) | 46.27 ± 7.72 | 43.86 ± 6.05 | t=1.47, p=0.15 |
| Education (years) | 14.19 ± 4.47 | 15.09 ± 4.90 | t=0.81, p=0.42 |
| Illness duration (months) | 80.41 ± 52.05 | ||
| Tender points (number) | 15.92 ± 1.96 | ||
| FIQ (Total Score) | 66.86 ± 15.79 | ||
| FIQ (Functional Capacity) | 4.81 ± 1.84 | ||
| SF-36 (Perception of Health) | 30.33 ± 17.63 | ||
| HADS (Depression) | 8.89 ± 4.72 | ||
| HADS (Anxiety) | 11.54 ± 4.15 | ||
| Clinical Pain (0–100 NRS) | 72.03 ± 14.82 | ||
|
| |||
| Number of FM patients (N = 37) | |||
|
| |||
| Antidepressants** | |||
| SSRI | 15 | ||
| NS-SSRI | 3 | ||
| SNRI | 6 | ||
| NaSSAs | 1 | ||
| TCA and SSRI | 3 | ||
| Anxiolytics | |||
| Benzodiazepines | 18 | ||
| Hypnotics | |||
| Benzodiazepinics, long half-life | 1 | ||
| Benzodiazepinics, medium half-life | 1 | ||
| Benzodiazepinics, short half-life | 3 | ||
| Gabapentin | 10 | ||
| Analgesic drugs* | 33 occasionally (11 stably) | ||
| Non-opiod (Ibuprofen and Paracetamol) | (11) | ||
| Opiod (Tramadol) | (6) | ||
All subjects were right handed.
The total number of patients receiving antidepressant medication was 28. FM, Fibromyalgia Patients; SSRI, Selective Serotonin Reuptake Inhibitor; NS-SSRI, Non-selective Serotonin Reuptake Inhibitor; SNRI, Serotonin-Norepinephrine Reuptake Inhibitor; NaSSAs, Noradrenergic and Specific Serotonergic Antidepressants; TCA, Tricyclic Antidepressant;
Analgesic drugs include ibuprofen, paracetamol and tramadol. Patients were asked to refrain from taking non-stable (rescue) NSAID/analgesic drugs (i.e., ibuprofen, paracetamol and tramadol) 72 hours before the MRI assessment. The number in parenthesis corresponds to subjects taking the analgesic medications under a stable regime.
We administered the following scales in a visit prior to the MRI appointment: Fibromyalgia Impact Questionnaire (FIQ); the 36-Item Short-Form Health Survey, General Perception of Health; the Hospital Anxiety and Depression Scale (HADS).
Patients were allowed to continue with their stable medical treatment, as listed in Table 1, but were asked to refrain from taking occasional rescue analgesic drugs (i.e., non-steroidal anti-inflammatory drugs, paracetamol, and tramadol) 72 hours prior to scanning session.
Control subjects with relevant medical and neurological disorders, any form of chronic or acute pain, substance abuse, or history of psychiatric illness were not considered for inclusion. Contraindication to MRI including pregnancy was a general exclusion criterion for both groups.
Clinical pain in deep tissue was assessed using a 101-point verbal scale. A score of 0 expressed no pain and a score of 100 the most intense pain imaginable, perceived in the body as a whole, or in most of its extension, rather than referring to any focal tenderness. Patients were asked to report spontaneous pain approximately 1 hour before the scanning session (Table 1). All healthy participants rated “0” for this measure.
Description of fMRI tasks and stimuli used
1. Multisensory task
A block-design fMRI paradigm was used, with alternating 30-second periods of rest (no stimulation) and activation (concurrent visual, auditory and tactile-motor stimulation), completing a total of 4 rest-stimulation cycles[42]. A subset of these data (from 25 controls and 35 patients) was used in a previous publication by our group[42]. The multisensory stimuli consisted of the simultaneous presentation of visual (3Hz, equivalent to 6 color reversals per second) full-field flashing checkerboard composed of a grid of black and white alternating squares (80 ± 10 lux) and auditory stimulation (series of 15 tones of frequencies comprised in the range of 233.1 to 1318.5 Hz, presented at a temporal frequency of 3 Hz, with an intensity of 75 ± 5 dB) and a finger-opposition task during which subjects were instructed to touch the tip of their right thumb with the other fingers (from index to little finger)[42]. In our multisensory assessment, we were interested in a more naturalistic presentation of sensory stimuli (and motor response) that are usually combined in daily life, rather than in modality specific alterations. As a first approach, this approach allowed us to maximize signal power and challenge both sensory and motor systems efficiently.
2. Low and high (healthy participants only) pressure stimulation tasks
Pressure stimulation tasks involved a block design fMRI paradigm consisting of three conditions per stimulation cycle repeated 5 times. Each cycle began with a rest condition with pseudorandom duration (range: 20 to 32 s), followed by a brief auditory stimulus (600-ms tone), followed by a 6-second anticipatory period, and then a 10-second pressure pain period. Each subject was asked to rate pain intensity and unpleasantness immediately after the end of the fMRI scanning sequence (run) using a numerical rating scale (NRS) ranging from 0 (not at all painful/unpleasant) to 100 (worst pain imaginable/most unpleasant imaginable). All participants completed a low-pressure pain task first, with pressure set at 4.5 kg/cm2. Approximately 10 minutes later, 28 healthy participants (out of 35) completed a second, high-pressure pain task, with stimulus intensity individualized based on the calibration session to reliably provoke severe but tolerable pain (5.90 ± 0.62 kg/cm2), comparable to the experience of FM patients at 4.5 kg/cm2.
As in previous studies[25; 41; 56] pressure pain stimuli were delivered using a hydraulic device capable of transmitting controlled pressure to 1 cm2 surface placed on the subjects’ right thumbnail. In a calibration session, each subject was trained to report pain intensity and unpleasantness to different pressure stimuli ranging from 2 to 9 kg/cm2 (or up to tolerance threshold) using the NRS described above. A stimulus of 4.5kg/cm2 was selected to reliably provoke intense pain (above 60 in the NRS, but tolerable) in the patient group. This stimulus is only slightly more intense than what was used to determine tender points during clinical assessment in patients (4 kg/cm2). The ten-second 4.5kg/cm2 was able to evoke, during the calibration assessment, a mean pain intensity of 73.15 ± 19.76 points in the patient group and a 36.47 ± 20.38 points in the healthy control group (between-group effect: t=7.75, p<0.0005). For healthy participants, we also determined the minimum pressure intensity that was required during the calibration session to provoke severe (above 60 in the NRS) but tolerable pain (5.90 ± 0.62 kg/cm2).
Statistical analyses
Behavioral analyses
Two-sample t-tests (for post-scan pain intensity and unpleasantness) were computed in SPSS (IBM SPSS Statistics for Macintosh, Version 20.0).
MRI acquisition and preprocessing
We scanned participants on a Philips Achieva 3.0 TX system (Philips Healthcare, Best, The Netherlands), with an eight-channel phased-array head coil and single-shot echoplanar imaging (EPI). Each functional sequence consisted of gradient recalled acquisition in the steady state (repetition time [TR]= 2.000 ms; echo time [TE]= 35 ms; flip angle= 90°; dummy volumes= 4) within a field of view of 23 cm, a 96×69-pixel matrix, and slice thickness of 4 mm (inter-slice gap, 1 mm). Twenty-two slices parallel to the anterior-posterior commissure provided whole-brain coverage.
Imaging data were processed using MATLAB (v2011b; The MathWorks Inc, Natick, Mass) and Statistical Parametric Mapping software (SPM8; The Wellcome Department of Imaging Neuroscience, London). Preprocessing involved motion correction, spatial normalization and smoothing using a Gaussian filter (full-width half-maximum, 8 mm). Data were normalized to the standard SPM-EPI template provided by SPM8 and resliced to 2 mm isotropic resolution in Montreal Neurological Institute (MNI) space. Regarding motion correction, translation and rotation estimates (x, y, z) were less than 2 mm or 2°, respectively, for all the participants, and no subjects were excluded because of artifacts or head displacement/rotations.
To address the potential effects of head motion on the FM-status prediction results, we computed a single motion index per subject for each fMRI task (see full description of the method in [57]; see also[55]). Briefly, we computed a measure for mean inter-frame motion due to translation (x,y,z), mIMtr, and a measure for mean inter-frame motion due to rotation (pitch, yaw, roll), mIMrot. Whereas mIMtr is a distance, mIMrot is an angle. The combined measurement was based on an average of both, , where r is the approximate average distance of all brain voxels to the rotation principal axis. The multiplicative factor r is necessary to transform the angle mIMrot to its corresponding distance arc. Following previous authors, we set r = 50mm.[55] Specific details about the computation of mIMtr and mIMrot are provided in[57], Supplementary Information file. We did not observe between-group differences in motion during the multisensory paradigm (t=−0.28, p=0.78). However, for the pressure pain task, significant differences were observed in TR-by-TR head motion between FM patients and healthy participants (patients > controls, t=2.49, p=0.02) (also between healthy subjects at the low and high-pressure intensities, t=2.38, p=0.02), whereas no differences emerged between patients and healthy participants when pain was matched (t=0.27, p=0.79). Importantly, the magnitude of the between-group difference in head motion was minimal (95% confidence interval for the head motion difference between patients and healthy participants: 0.01–0.16mm). To account for the potential influence of head motion in the prediction model of FM status (see below, logistic multiple regression), we added the single subject measures of head motion for each task (pain and multisensory, for the sake of completeness) as independent variables in the model, and verified that neither contributed significant variance to explaining FM status (multisensory task: t=−0.84, p=0.40; pain task: t=0.20, p=0.84).
To test whether motion parameters (during the pain task) were sufficiently informative to correctly classify patients from healthy participants, we used support vector machines (SVM) with the 6 motion regressors per subject as the classification features and the subjects’ category (patient vs. healthy participant) as the outcome. The results were not significant (cross-validated accuracy: 60%± 5.7% (SE), p=0.10). We also checked and confirmed that the time course of the ‘nociception-positive NPS’ response (see below) on a TR-by-TR basis was not correlated with motion parameter estimates, for any regressor for any subject (all p-values>0.1).
First level single-subject models for fMRI data
We used a conventional general lineal model approach (GLM) as implemented in SPM8 software to estimate brain responses to (a) multisensory stimulation and (b) pressure stimulation, for each subject.
For the multisensory task, a primary task regressor was created by convolving the sensory stimulation blocks with a canonical hemodynamic response function. The “off” (rest) condition served as an implicit task baseline. Parameter estimates were calculated at each voxel using the general linear model. A high-pass filter was used to remove low-frequency signal fluctuations (1/128 Hz). [Multisensory Stimulation – Baseline] contrast images for each participant were calculated.
For the pressure stimulation task, signal response was modeled using separate regressors for the anticipatory and the pain periods, with a hemodynamic delay of 4 seconds. In three previous studies using similar procedures[22; 41; 56], we systematically observed that the duration of brain responses to 10-second pressure stimuli of similar intensities extends to 16 seconds (average response duration across pain processing regions)[41], which is consistent with observations by different research groups [13; 47; 70]. To account for this, pain-related activation was modeled using a pain condition of 16-sec duration. A high-pass filter was used to remove low-frequency signal fluctuations (1/128 Hz). In agreement with our previous work[42; 56; 58; 70], we did not model autocorrelations. Modeling autocorrelations has the potential disadvantage of producing biased parameter estimates when the AR model assumptions are violated, which can result in reduced efficiency[20; 80]. Of note, models that do not consider autocorrelation have shown to generate unbiased parameter estimates (beta values, which we use here for classification purposes), even if the data are autocorrelated[51; 80] (see also Supplementary Figure 1). [Pressure Stimulation – Baseline] contrast images for each participant were calculated.
We studied brain response alterations during pain processing in FM patients using two complementary approaches: (1) As a first test of pain-related brain responses, we applied the NPS brain signature[70], a multivariate fMRI-based brain pattern that was validated to specifically predict experimental pain (and not other unpleasant/arousing emotional experiences) in humans. The goal of this approach was to use a defined marker or process that has been well characterized in healthy individuals to test for abnormalities in the patient population. An advantage is that the NPS was trained to track pain intensity in a fine-grained way across multiple levels of stimulus intensity, and tested for specificity to pain and generalizability across a number of independent studies. (2) Because the NPS may not be sufficient to capture all pain-related differences between patients and controls, a second approach was to train a classifier optimized to discriminate FM patients from controls. This pattern identifies pain-related signals that may be missed by the NPS. This second approach was also applied to the multisensory task (see below).
Computing Neurologic Pain Signature (NPS) responses
We computed for each subject (FM patient or healthy participant) a single scalar value representing their expression of the NPS pattern in response to pressure pain (using the contrast [Pressure Stimulation minus Baseline] images as detailed below).
For this analysis, we separated NPS regions likely to be related to nociceptive pain (associated with pain-evoked activation in the NPS) from those that play other modulatory roles (associated with pain-evoked deactivation in the NPS). In most of the regions in the NPS, pain is associated with increased overall activity. Such regions include the major targets of ascending nociceptive afferents, including the thalamus, secondary somatosensory regions (SI/SII), posterior, mid and anterior insula and adjacent opercula, midbrain, dorsal anterior cingulate cortex (dACC), inferior frontal gyrus and amygdala (Supplementary Table 1 and Figure 1). We refer to pattern responses in this set of regions as the “Nociception-positive NPS” (NPSp). In a subset of other medial regions, including the perigenual ACC (pgACC) and the PCC (posterior cingulate)/precuneus/paracentral lobule, pain was associated with deactivation in the original NPS pattern. These regions are not strongly linked to nociception and are not direct targets of nociceptive afferents, rather they have been associated with a variety of affective, autonomic, social, self-referential, and decision-making functions[62]. We refer to responses in this set of regions as the “Nociception-negative NPS” (NPSn), and analyze this pattern separately from the NPSp due to its differential functional characteristics and considering the particular role of these regions, mostly the pgACC, in chronic pain. Of note, the local pattern of voxel weights is exactly the same as in the original NPS within the two NPS components (NPSp and NPSn). Supplementary Text 1 provides a detailed description of the rational for computing NPSp and NPSn and the procedure used to characterize them.
Mediation analysis
We tested two separate mediation models to assess whether the relationship between FM status (FM vs. healthy) and pain ratings during the fMRI pain task (intensity -model 1- and unpleasantness –model 2-) were significantly mediated by NPS brain responses. The mediation analysis tested several joint hypotheses: Path a tested whether FM status (FM vs. healthy) predicts NPSp responses. Path b tested whether NPSp responses predict subjective ratings of intensity (or unpleasantness), controlling for FM status. Finally, the Path a×b tested the mediation effect, i.e., whether NPSp responses during pressure pain explain a significant proportion of the co-variation between FM status and subjective pain ratings. The analyses were conducted using the mediation toolbox (http://wagerlab.colorado.edu/tools) that has been used and described extensively in previous work (e.g.[71]) with bias-corrected, accelerated bootstrap tests.
Multivariate pattern-based classification of FM patients vs. healthy controls
We performed two analyses using linear support vector machines (SVMs) to discriminate FM patients and controls based on whole-brain activation patterns. The first analysis used activation patterns during painful pressure at 4.5kg/cm2 pressure stimulation (FM-pain), and the second analysis used activations during non-painful multisensory stimulation (Multisensory). The SVM was implemented in the Spider Toolbox (http://people.kyb.tuebingen.mpg.de/spider). It identifies a hyperplane (direction in multidimensional voxel space) that separates the two groups. Distances from the hyperplane are related to the likelihood a participant belongs to the patient vs. control class, and were used in the logistic regression analysis below.
The FM-pain classifier was based on the [Pressure stimulation minus Baseline] contrast, and the Multisensory classifier was based on the [Multisensory stimulation minus Baseline] contrast. In each analysis, we used leave-two-subject-out cross-validation, which ensured that the patterns we identified were always tested on new, out-of-sample individuals (see Supplementary Information). Accuracy (sensitivity and specificity) was based on the cross-validation, and the final weight map was based on the full sample, and was thresholded using a bootstrap test (q < .05 FDR-corrected; see Supplementary Text 2 for a detailed description of the SVM analysis and the bootstrap test).
Logistic multiple regression to develop a combined classifier for FM status
We used logistic regression to combine results from the three fMRI-based classifiers (NPS, FM-pain and Multisensory) into a single signature of FM status. The predictors in the regression were: (1) the NPSp response; (2) the NPSn response; (3) the cross-validated FM-pain signature response (distance from the hyperplane); and (4) the cross-validated Multisensory signature response.
Logistic regression results were used to calculate sensitivity, specificity, and the area under the Receiver Operating Characteristic (ROC) curve (AUC). We assessed these values for each fMRI-based classifier (NPSp, FM-pain and Multisensory) independently, and for the combined model.
Multivariate brain pattern responses and medication status
In order to examine the relationship between medication status and brain pattern responses, we performed a series of two-sample t-tests to compare between-group differences in pattern response between medicated and non-medicated patients.
Multivariate brain pattern responses and clinical severity
We also tested whether the multivariate fMRI patterns used to classify FM status were correlated with clinical symptom severity. We performed linear regression (stepwise procedure in SPSS), including the 4 brain-derived (cross-validated) pattern response values as predictors (NPSp, NPSn, FM-pain and multisensory) and each of the clinical measures as the dependent variable in one of three regression models (clinical pain, FIQ, and HADS depression scores). We included a fourth predictor representing the presence or absence of anxiolytic or antidepressant medication in each model, considering the significant correlation between NPS responses and antidepressant and anxiolytic medication status (further described in the results section). For completeness, we also assessed zero-order Pearson correlations between brain measures and clinical symptom severity in FM patients.
RESULTS
Enhanced pressure pain sensitivity in FM patients
In response to the low-pressure intensity fMRI task (4.5 kg/cm2), FM patients (vs. healthy participants) reported increased pain intensity (mean ± SD, 71.71 ± 14.47 for FM patients, 48.48 ± 18.31 for healthy participants; between-group effect: t=5.95; p<0.0005) and unpleasantness (68.24 ± 18.84 for FM patients, 44.11± 19.98 for healthy subjects; between-group effect: t=5.24, p<0.0005). In the high-pressure intensity task (~6 kg/cm2 ± 0.62) healthy subjects reported equivalent pain levels as FM patients stimulated at low pressure (t=0.61, p=0.54 for intensity and t=0.54, p=0.59 for unpleasantness).
Neurologic Pain Signature (NPS) responses in FM patients vs. healthy controls
Figure 1A shows the NPSp pattern. FM patients and healthy subjects (at both stimulation intensities) showed significant NPSp responses, shown in Figure 1B. Responses to the low-pressure fixed intensity (4.5kg/cm2) were greater for FM patients than healthy participants (t=3.24; p=0.002), consistent with hypersensitivity to mechanical pain in FM. When subjective pain was matched between groups by comparing healthy participants experiencing high pressure (6 kg/cm2 ± 0.62) to FM patients experiencing low pressure (4.5kg/cm2), NPSp responses for both groups were virtually identical (t=0.07, p=0.94), suggesting that subjective reports of pain were proportional to pain-specific NPSp responses.
Figure 1.
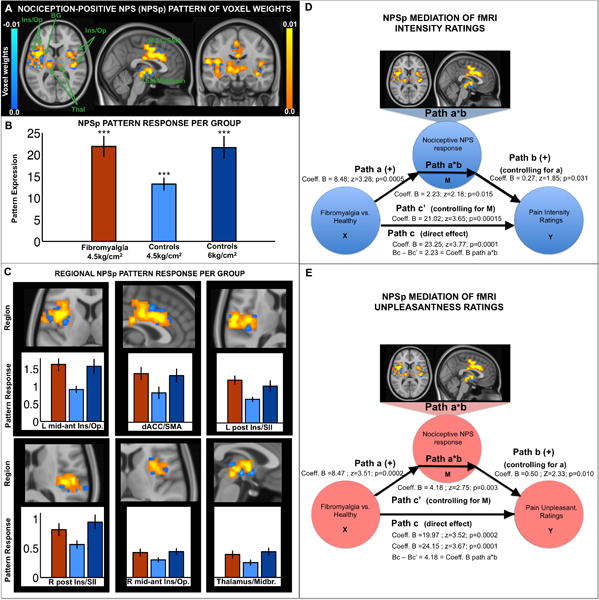
Nociception-positive NPS (NPSp) map of voxel weights (A); pattern response per group (B); and contiguous regions (C). Ins/Op, insula and operculum; BG, basal ganglia; Thal, thalamus. dACC/SMA, dorsal anterior cingulate cortex and supplementary motor area. L, left, R, right. Midbr, midbrain. Error bars represent standard errors of the mean. ***, p<0.0001. Responses to low intensity stimulation in Figure 1B were 21.87 ± 14.00 (t=9.5, p<0.0001) in FM patients and 13.21± 8.02 in healthy participants (t=9.74, p<0.0001). Responses to high intensity stimulation were 21.62 ± 13.31 in healthy participants (t=9.74, p<0.0001). NPSp pattern response significantly mediates (partial mediation) the relationship between clinical category (FM diagnosis present vs. absent) and Pain Intensity (D), and Pain Unpleasantness (E) ratings in response to 4.5kg/cm2 painful pressure. All coefficients in the mediation models have been tested for significance using 10,000 bootstrap tests. One-tail p-values are reported as we had directional a priori hypotheses (FM patients will show greater NPS responses; and, the greater the NPS response the higher the pain ratings).
We also examined local signature responses in each contiguous region of the NPSp (Figure 1C and Supplementary Table 1). Comparisons between FM patients and healthy participants showed significantly greater NPSp responses to low-pressure intensity in FM patients in all regions tested, with one exception (the inferior frontal gyrus). When subjective pain was matched (high pressure in healthy participants vs. low pressure in FM patients), local pattern responses were virtually identical for all regions.
Mediation analyses supported the conclusion that the FM vs. healthy participant difference in pain intensity and unpleasantness was significantly partly mediated by NPSp brain responses (Figure 1D–E).
We also studied pattern responses in the heteromodal regions of the NPSn component, which may have pain modulatory functions but which are not considered nociceptive targets (fully described in Supplementary Figure 2, Supplementary Table 1). In the original NPS map, increased pain was associated with deactivation in these regions (negative weights, blue-colored regions in Supplementary Figure 2). However, NPSn regions in this study showed pain-evoked activation (not deactivation). Patients showed significant pain-evoked activation in both pgACC and PCC/precuneus/paracentral lobule regions of the NPSn, whereas healthy subjects showed significant pain-evoked activation only in the PCC/precuneus/paracentral lobule cluster. For simplicity, pattern response magnitudes are always signed such that increases in pattern response indicate increases in pain activation in these regions. Both FM patients at low pressure and healthy participants at high pressure showed significant NPSn pattern response (FM: 1.56 ± 1.96 (mean ± SD); t=4.82, p<0.00005; healthy participants, low pressure: 0.30 ± 1.18; t=1.49, p=0.15; healthy participants, high pressure: 1.04 ± 1.61; t=3.40, p=0.0021). FM patients showed significantly greater pattern response in NPSn regions than healthy controls (all receiving 4.5kg/cm2, t=3.27, p=0.002; i.e., greater activation in such regions; Supplementary Figure 2). Equating pain perception between groups again eliminated the FM vs. healthy participant difference (t=1.14, p=0.258). Additionally, in healthy participants, NPSn responses were stronger (i.e., greater activation) in the high pressure than in the low-pressure condition (t=2.09, p=0.04). Analyses of individual regions within the NPSn are summarized in Supplementary Figure 2 and Supplementary Table 1. Interestingly, the pgACC showed pain-evoked activation only for patients (and not for healthy participants at high pressure). Both NPSn regions (pgACC and PCC/precuneus) exhibited stronger response for patients than healthy participants at matched pressure. Matching pain intensity across groups resulted in statistically equivalent activation in PCC/precuneus, but a trend (p=0.10, Supplementary Table 1) towards greater activation in the pgACC in patients. Furthermore, there were no between-group differences in pgACC activity between healthy participants at low and high pressure (p=0.48, Supplementary Table 1), suggesting that this region does not contribute to pain intensity encoding in healthy participants.
We finally assessed whether the NPSp response to low-pressure intensity performed significantly better than chance in classifying FM status (present vs. absent) and found that it classified 68%± 5.5 (SE) of the cases correctly (p=0.0029). Also, NPSn responses to low pressure classified FM status with 71%± 5.3 (SE) accuracy (p=0.0004) suggesting that greater pain-evoked activation in NPSn regions at low pressure is an identifying feature of FM. Note that the two classification accuracy values are not statistically different from each other.
A new pain-related classifier map (FM-pain) discriminates FM patients from controls
The FM-pain classification brain pattern (Figure 2, Supplementary Table 2) was characterized by augmented activity in FM patients in regions associated with sensory integration (SII/parietal operculum extending into mid-insula) and self-referential/‘default mode’ network regions (including dorsomedial prefrontal cortex [PFC, all q<0.05 FDR-corrected]). At a lower level of significance (p<0.001), a larger extended network was observed that included augmented pain-related responses in ventromedial PFC/subgenual ACC and PCC. Reduced activity in FM patients was found in a region considered important for pain and emotion regulation, the dorsolateral PFC (q<0.05 FDR-corrected). This pattern, when applied to new test participants, classified FM patients vs. controls with 70% ± 5.4% accuracy, P=0.0009. Sensitivity was 74% (CI: 62%–86%) and specificity was 66% (CI: 53%–79%).
Figure 2.
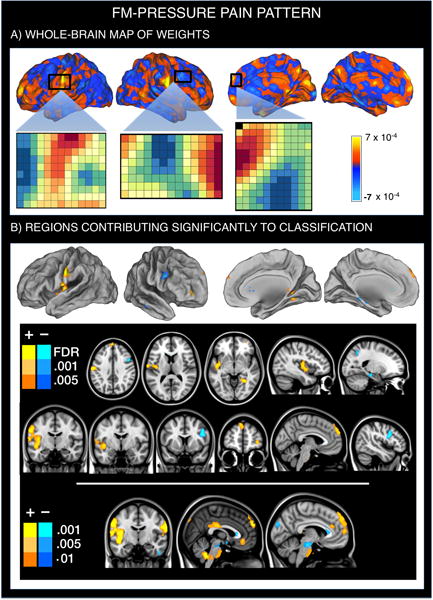
Multivariate brain pattern that predicts fibromyalgia status on the basis of brain activation during painful (pressure) stimulation. Positive weight values reflect higher pain-evoked activation in FM patients relative to healthy participants, whereas negative weight values reflect reduced pain-evoked activation in FM patients. A. SVM pattern of whole-brain voxel weights that optimizes classification of FM patients and healthy participants. We provide the voxel-by-voxel weights for three representative regions (anterior SII, right dorsolateral and dorsomedial PFC) to illustrate the concept of weighted pattern. B. Regions whose voxel weights contributed most reliably to the prediction of FM status (q<0.05 FDR-corrected for the first two rows; p-uncorrected<0.001 to further illustrate the findings).
A new non-painful multisensory classifier map discriminates FM patients from controls
The Multisensory classification pattern (Figure 3, Supplementary Table 3) showed enhanced activity in FM patients in heteromodal regions associated with multisensory integration (posterior-mid insula/operculum), self-referential/’default mode’ network regions (including the PCC/precuneus and dorsomedial PFC), and an anterior lingual region proximal to the parahippocampal gyrus. Reduced activity in FM patients was found in primary/secondary sensory areas (occipital and superior temporal regions) associated with visual and auditory processing, respectively; lateral cerebellum; basal ganglia (dorsal and ventral putamen and pallidum); diencephalon (consistent with subthalamic and hypothalamic regions); dorsolateral PFC; and midbrain. This pattern of activity, when applied prospectively to new test participants, classified FM patients and healthy participants with a cross-validated accuracy of 89% ± 3.7% (SE), p<0.0000005; sensitivity: 84% (CI: 73%–93%) and specificity: 94% (CI: 87%–100%).
Figure 3.
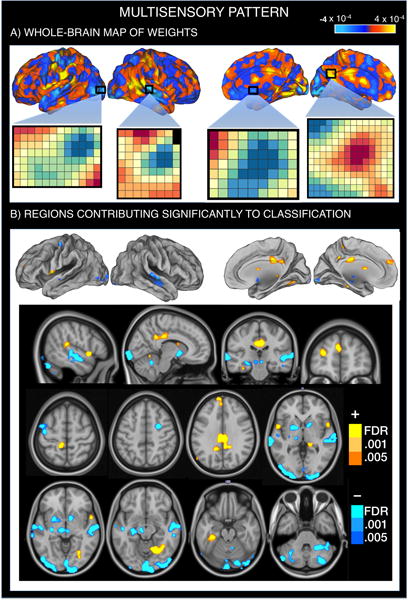
Multivariate brain pattern that predicts fibromyalgia status on the basis of brain activation during multisensory stimulation. Positive weight values reflect higher multisensory-evoked activation in FM patients relative to healthy participants, whereas negative weight values reflect reduced multisensory-evoked activation in FM patients. A. SVM pattern of whole-brain voxel weights that optimizes classification of FM patients and healthy participants. We provide the voxel-by-voxel weights for four representative regions (visual cortex, auditory cortex, basal ganglia and PCC) to illustrate the concept of weighted pattern. B. Regions whose voxel weights contributed most reliably to the prediction of FM status (FDR-corrected). The top row matches the view of Figure 3A, showing that the most reliably predicting voxels correspond to those showing the highest and lowest weights. The last three rows represent sagittal, coronal and axial views showing all predictive voxel clusters.
In order to check for a more global brain functional reorganization in fibromyalgia patients, we also tested whether the FM_pain pattern described above could accurately classify patients vs. controls using images from the multisensory task and vice versa. Indeed, FM_pain pattern responses computed using individual person-level multi-sensory contrast images accurately classified fibromyalgia status (classification accuracy: 86% ± 4.1%; p<0.0001 sensitivity: 95% (88–100%), specificity: 77% (64–88%)). Conversely, Multisensory pattern responses computed using individual pressure pain contrast images also classified fibromyalgia status (classification accuracy: 76% ± 5.0%; p<0.0001 sensitivity: 65% (CI: 51–78%), specificity: 89% (CI: 79–97%)). Thus, the two brain classifiers may in part reflect a more general (task-nonspecific) brain reorganization in fibromyalgia that is not specific to any one sensory modality.
Combined neural classifier using pain and multisensory brain measures
This analysis aimed to predict FM status by combining pattern response values for the NPSp, NPSn, FM-pain and Multisensory patterns using logistic regression. Three of the four pattern responses significantly contributed to the prediction of FM status while controlling for the others (NPSp_t = 2.16 [p= 0.03], FM-pain_t=2.03 [p=0.04] and Multisensory_t= 4.22 [p<0.0005]), whereas the NPSn was not significant (t=0.09, p=0.924). Figure 4 shows the group means in the joint space of NPSp/FM-pain and Multisensory patterns. The combined classifier was able to discriminate patients from healthy participants with a cross-validated accuracy of 93% ± 3.0% (SE), p<0.0000005; sensitivity: 92% (CI: 84%–98%) and specificity: 94% (CI: 87%–100%).
Figure 4.
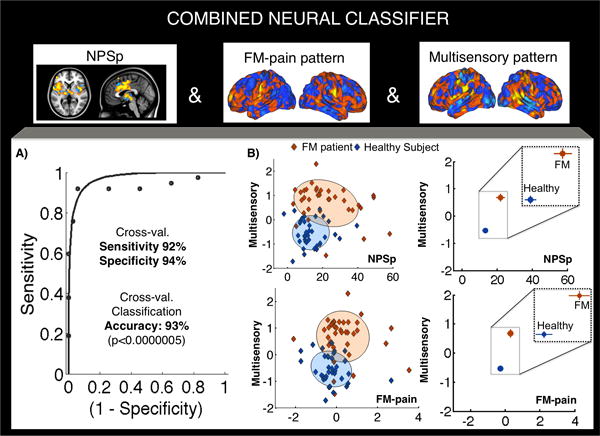
Sensitivity and specificity of the combined neural classifier including NPSp, FM-pain and Multisensory responses (cross-validated) for each subject. Figure 4A shows the receiver-operating characteristic (ROC) plot displaying sensitivity and specificity properties for the combined classifier. Figure 4B (left panels) shows the individual subjects’ data in the join space of NPSp and Multisensory or FM-pain and Multisensory pattern responses. The shadowed areas represent 95% confidence regions for each group. The coefficients of correlation between pattern expression scores for healthy participants are: Multisensory and NPSp, r = 0.17 (p = 0.92), Multisensory and FM-pain, r = −0.26 (p=0.12), NPSp and FM-pain, r = 0.015 (p=0.93). And, for FM patients: Multisensory and NPSp, r = −0.24 (p=0.14), Multisensory and FM-pain, r = −0.09 (p=0.59), NPSp and FM-pain, r = 0.39 (p=0.017). Figure 4B (right panels) represent the group means (and SE) in the same spaces. Outlier tests were performed (≥ 3.5 SDs from the mean of the subject’s group). Only one control showed a value more similar to the Fibromyalgia group than to the Healthy Control group (z=3.93), for the FM-pain pattern.
Associations between brain pattern responses and medication status in FM patients
No significant effect of analgesics (either opioid-dependent [tramadol], or non-opioid dependent [ibuprofen and paracetamol]), hypnotics, or gabapentin was found on pattern response values for any of the four brain patterns, i.e, NPSp, NPSn, FM-pain map and multisensory response (all p > 0.10). A significant association was found between anxiolytic/antidepressant medication and NPS measures. Anxiolytic medication (present in 18 out of 37 patients) was significantly associated with NPSn pattern response values (t=2.29, p=0.03), indicating that medicated patients showed greater pain-evoked activation in pgACC and PCC/precuneus regions than patients who were not receiving anxiolytic medication. In addition, antidepressant medication, which is currently prescribed as a standard treatment for FM and was used in 76% of patients in this sample, was associated with greater NPSp (t=3.74, p=0.001) and NPSn responses (i.e., increased activation; t=2.51, p=0.016). These results indicate that FM patients receiving stable treatment with antidepressants showed significantly greater NPS responses than untreated individuals.
We also found a significant association between antidepressant/anxiolytic medication and clinical severity. Specifically, a variable representing presence of antidepressant/anxiolytic medications (0: no antidepressant neither anxiolytic medication; 1: presence of either antidepressant or anxiolytic medication; 2: presence of both antidepressant and anxiolytic medication) was positively correlated with HADS-depression (r=0.325, p=0.049) and FIQ scores (r=0.341, p=0.039).
Importantly, after controlling for clinical severity (including clinical pain, HADS and FIQ scores as covariates), the relationship between anxiolytic and antidepressant use and both NPSp and NPSn responses became non-significant (all p>0.1). These findings suggest that the observed relationship between medication use and brain measures may reflect common influences of symptom severity on both measures. That is, greater symptom severity is associated with both increased medication use and larger NPS responses.
Associations between brain pattern responses and clinical symptoms in FM patients
Multiple regression analyses using brain pattern responses (NPSp, NPSn, FM-pain and Multisensory) to predict symptom severity in FM patients showed that several brain measures correlated with FM symptoms, as illustrated in Figure 5. In each multiple regression model we included a predictor representing the presence or absence of anxiolytic/antidepressant medication, to control for medication effects on symptom severity. Greater levels of clinical pain were predicted by a combination of greater FM-pain pattern responses (t=2.14, p=0.039) and greater Multisensory pattern responses (t=2.88, p= 0.007). NPSp and NPSn values were not predictive, either individually or in stepwise multiple regression (all p>0.10). Higher Fibromyalgia Impact Questionnaire scores (FIQ, assessing functional impairment associated with the disease) were predicted by a trend towards stronger NPSn responses (t=1.92, p=0.06), indicating that greater pain-evoked activation in NPSn regions was associated with marginally greater FIQ scores. Other measures were not predictive, either alone or in stepwise regression (all p>0.10). Greater depressive symptomatology (HADS depression) was also predicted by stronger NPSn responses (t=2.09, p=0.04), but not other measures (all p>0.10). Our correlation findings are preliminary and need further replication in larger samples (see Supplementary Table 4).
Figure 5.
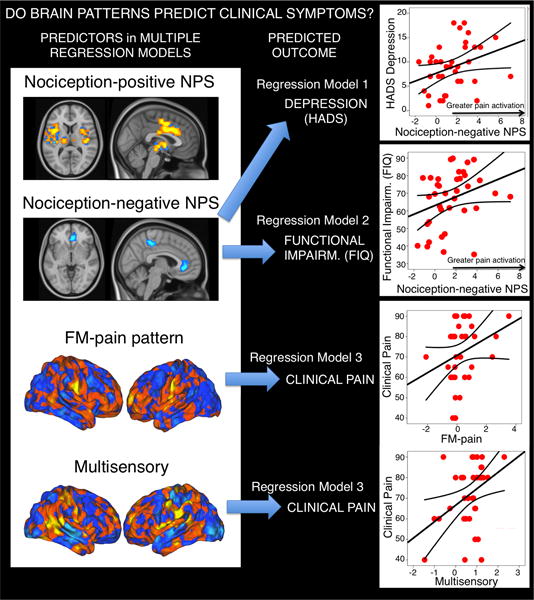
Prediction of symptom severity using brain patterns (NPSp, NPSn, FM-pain and Multisensory) in multiple regression models. The blue arrows indicate statistically significant predictors for each multiple regression analysis model described in the main text. The straight lines in the plots are the standard linear fit lines for each regression model, and the two additional lines in each plot correspond to the confidence intervals for the mean. Of note, the correlation findings reported here are preliminary and need further replication in multiple samples. The right top panel illustrates the correlation between NPSn pattern responses and HADS depression scores in patients (r=0.333, p=0.044); the next panel illustrates the correlation between NPSn pattern responses and functional impairment scores (r=0.309, p=0.063, two-tailed); lastly, FM-pain and Multisensory responses jointly and significantly contributed to the prediction of clinical pain (main text). Here we present the raw correlations between clinical pain and FM-pain pattern responses (r=0.279, p=0.094) and clinical pain and Multisensory pattern responses (r=0.393, p=0.015). To minimize the influence of potential extreme values while retaining the full sample (which is important for evaluating person-level ‘signatures’), we also conducted Spearman rank-correlation tests, which revealed the same pattern of results.
Brain pattern responses and time since diagnosis
There was no significant association between time since diagnosis (surrogate measure for time exposed to the disorder) and brain pattern responses (NPS, FM-pain and multisensory: p>0.1).
DISCUSSION
Diagnosing FM based on objective brain measures is important for several reasons. The status of FM as a disorder with objective physiological dysfunction has been questioned, in part because it is unclear that central[23–25; 28; 32–34; 36; 39; 42; 49; 50; 56; 58] and peripheral changes[52; 64; 69] are sufficient to diagnose FM and explain symptoms. Objective brain-based signatures can reveal patterns of neurophysiological alterations characteristic of FM (vs. healthy), potentially establishing a specific neurological component to the disorder.
Here, we identify a set of three brain patterns based on fMRI responses to pressure pain and non-painful multisensory stimulation. These patterns, taken together, discriminate FM from matched healthy controls with 92% sensitivity and 94% specificity. Though we take this as a provisional ‘signature’ for FM, we do not imply that these patterns are unique to FM or that they differentiate it from other conditions. Multifocal pain and widespread mechanical hypersensitivity are common features of multiple chronic pain conditions[54; 75]. Given the overlap in symptoms and comorbidities across disorders[54; 75; 76], which clinical categories are respected at the neurophysiological level and which clinical features are most likely represented in a continuum, still remain open, empirical questions. Exploratory analyses suggested that the three component patterns are correlated with clinical symptoms. Individual FM patients may vary in the expression of the different brain patterns we identified, which may lead to the characterization of new FM subtypes.
These results show that fMRI may serve as a useful component of multi-modal FM assessments. Previously, heart rate variability[40] and performance in specific fitness tests[3] have shown moderate-to-strong separation of patients and healthy participants. While these measures are useful and are easy to deploy, they are not suitable for addressing the neurophysiological mechanisms of FM. Previous attempts have established neurophysiological differences in pain-related processing in FM patients[15; 24; 25; 56] but have not yet successfully discriminated patients from healthy participants at the individual-person level (cf.[67]). A recently published study based on brain anatomy findings shows ability to discriminate FM patients with from 53% to 76% accuracy in different datasets[61]. Our ability to classify with 93% accuracy in the present study relies strongly on the joint consideration of responses to nociceptive and non-painful multi-sensory stimuli, following previous work[42; 56; 58].
Brain features diagnostic of FM
Combined pain-multisensory neural classifier
Our study provides three distinct neural targets for objective patient characterization. A model combining: (i) enhanced responses in the NPSp, a pain-specific brain network, (ii) a pattern of brain responses to painful pressure (FM-pain pattern) and (ii) a pattern of extra-nociceptive multisensory responses, differentiated FM patients vs. healthy participants with 93% cross-validated accuracy. The three effects are theoretically significant. Stronger NPSp responses mediated mechanical hyperalgesia in FM and were associated with a trend to greater depression; stronger FM-pain and multisensory responses were associated with greater clinical pain. Thus, the combined model is most likely to be useful for clinical and translational purposes, though either feature might also be used alone. Though the NPSn was not significant in the final model, NPSn responses may nonetheless be important for assessing and understanding functional aspects of FM.
Pain-related patterns
The NPSp pattern showed an augmented response in FM when the stimulus intensity was matched, but not when subjective pain was matched. It was a significant mediator of mechanical hypersensitivity in patients. Thus, NPSp responses are enhanced in patients in proportion to their augmented subjective experience of pressure-induced pain, and are consistent with previous evidence supporting peripheral (e.g.,[52; 64; 69]) and central (e.g.,[10; 45; 58; 66]) sensitization in FM. The current study cannot determine whether this enhancement reflects a change in the gain of the NPSp per unit stimulus intensity or simply reflects enhanced afferent input. Future studies could begin to disentangle these alternatives by testing multiple stimulus intensities in both patients and controls.
Rather than being limited to regions associated most strongly with emotional or evaluative aspects of pain (e.g., anterior insula, ACC, lateral PFC), our findings showed a distributed enhancement of nociceptive processing affecting all major NPSp regions. In agreement with previous observations[25; 56] and considering the validation properties of the NPS, the results provide evidence of augmented pain-specific responses in FM, consistent with peripheral/central sensitization.
NPSp responses were predictive of experimental pain but not ongoing clinical pain intensity, consistent with work showing that medial prefrontal-striatal systems rather than classic nociceptive systems are correlated with chronic low back pain[6].
The NPSn pattern includes patterns within medial regions in which greater deactivation was previously associated with increased pain[70]. In this study, however, increased pain was associated with greater activations in healthy participants (specifically for the PCC/precuneus cluster) and FM patients (for both the PCC/precuneus and the pgACC clusters). The pgACC showed increased activation specifically for FM patients, in agreement with previous findings showing that chronic pain patients do not deactivate pgACC/medial PFC and/or surrounding regions of the ‘default mode’ network as strongly as controls do (and sometimes show activation)[2; 5; 6; 12; 27; 63; 72]. Conversely, the PCC/precuneus may activate in response to pressure-evoked pain in both healthy[4; 16; 43; 65] and patient populations[27; 48; 59; 65]. The value of ‘default mode’ network regions as predictors of pain may therefore vary across pain modalities and pain patient groups. We discuss the issue in detail in Supplementary Text 3.
The FM-pain pattern complements the NPS results by showing that FM was best characterized by concurrently augmented pain-evoked activation in regions that show an important role in sensory integration (anterior SII extending to middle insula) and ‘default mode’ regions and reduced activation of the dorsolateral PFC, a region commonly found to play a role in pain and emotion regulation[71; 73]. This pattern, which was (together with the Multisensory) predictive of clinical pain in patients, also emphasizes the importance of some key regions that are not part of the NPS (and are important for self-related, motivational and emotional regulation) as targets for future study in FM.
Supplementary analyses of medication use indicated that it was not sufficient to explain the relationship between FM patient status and alterations in brain activity (Supplementary Text 4).
The Multisensory pattern
The Multisensory pattern is the feature that most strongly discriminates FM patients from healthy participants, with and without controlling for NPS and FM-pain responses, and contributes to predict clinical pain. Central processing of non-painful sensory signals may be of particular importance in maintaining clinical pain in FM, in agreement with previous work [29; 42; 58]. Consistently, Harte and colleagues very recently showed that fMRI data during a visual task could discriminate FM patients vs. healthy controls with 82% accuracy[29]. The Multisensory pattern shows early sensory cortical processing attenuation, which is consistent with reduced processing of fine sensory/discriminative properties of stimulus[1; 18; 46; 53], accompanied by amplification of sensory integration (in agreement with [29]) and self-referential aspects of the response, including potential threat of harm[17].
The FM_pain pattern could accurately classify patients vs. controls using multisensory brain responses and vice versa, which may reflect a more global brain reorganization in FM that may be detected across a range of experimental conditions. Our study and others show overlapping brain functional alterations during rest and task performance in FM and other chronic pain conditions involving sensory integration (e.g.,[19; 36; 42; 58]), salience ([33; 42; 50; 58; 68]) and default-mode network regions (e.g., [6–8; 49; 50; 58]).
Overall, we have found an augmented NPSp response that is consistent with enhanced nociceptive processing, and partly mediates augmented mechanical pain, and, more broadly, evidence for central nervous system alteration in brain processing of sensory stimuli that go beyond nociception, consistent with a central nervous system pathophysiological component in FM.
Several limitations and future directions remain to be addressed in future studies. First, replication of these results with fully independent validation samples is a next step towards translational utility. The validation process is a long, multi-study and multi-publication effort[9; 26]. Second, false positive cases, i.e., patients that are erroneously classified as healthy controls using these brain measures, are always a possibility. Importantly, these brain patterns may not capture other sensory/affective/cognitive processes that may also be altered in FM patients. Third, the brain pattern-symptom severity correlations that we present are preliminary and need replication in multiple samples. Fourth, further studies are needed to test whether our findings generalize to other chronic pain populations or are specific to FM. Lastly, all participants included in the study were females and therefore the findings cannot be generalized to the population of men with fibromyalgia, for which future generalization and validation studies are warranted.
Overall, an important open question concerns the extent to which FM and other chronic pain disorders are pathophysiologically distinct or may share certain brain features independent of primary etiology. Testing the generalizability of the brain patterns we identify here may advance our understanding regarding the common and independent central pathophysiology of distinct chronic pain conditions across domains of sensory processing. Thus, the current approach may help in identifying pain endophenotypes across a spectrum of chronic pain conditions and in providing objective guidance for therapeutic interventions on an individualized basis.
Supplementary Material
Acknowledgments
This work was supported in part by grant R01DA035484 (T.D.W.) and by grant SAF2010-19434 (Ministry of Science and Innovation of Spain, J.D.). Dr. López-Solà’s work was supported by a Beatriu de Pinos-A Postdoctoral Fellowship (2010_BP_A_00136) from the Government of Catalunya.
The Agency of University and Research Funding Management of the Catalonia Government participated in the context of Research Groups SGR 2009/718, 1435 and 1450. Matlab code implementing the analyses presented here is available at wagerlab.colorado.edu.
Footnotes
The authors declare no conflict of interests.
References
- 1.Alanoglu E, Ulas UH, Ozdag F, Odabasi Z, Cakci A, Vural O. Auditory event-related brain potentials in fibromyalgia syndrome. Rheumatol Int. 2005;25(5):345–349. doi: 10.1007/s00296-004-0443-3. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain. 2006;122(3):223–234. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Aparicio VA, Segura-Jimenez V, Alvarez-Gallardo IC, Soriano-Maldonado A, Castro-Pinero J, Delgado-Fernandez M, Carbonell-Baeza A. Fitness Testing in the Fibromyalgia Diagnosis: the al-Andalus Project. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 4.Baciu MV, Bonaz BL, Papillon E, Bost RA, Le Bas JF, Fournet J, Segebarth CM. Central processing of rectal pain: a functional MR imaging study. AJNR American journal of neuroradiology. 1999;20(10):1920–1924. [PMC free article] [PubMed] [Google Scholar]
- 5.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(39):13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(47):12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nature neuroscience. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discovery Medicine. 2011;11(58):197–207. [PubMed] [Google Scholar]
- 10.Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Seminars in arthritis and rheumatism. 2014;44(1):68–75. doi: 10.1016/j.semarthrit.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Carrillo-de-la-Pena MT, Vallet M, Perez MI, Gomez-Perretta C. Intensity dependence of auditory-evoked cortical potentials in fibromyalgia patients: a test of the generalized hypervigilance hypothesis. J Pain. 2006;7(7):480–487. doi: 10.1016/j.jpain.2006.01.452. [DOI] [PubMed] [Google Scholar]
- 12.Ceko M, Gracely JL, Fitzcharles MA, Seminowicz DA, Schweinhardt P, Bushnell MC. Is a Responsive Default Mode Network Required for Successful Working Memory Task Performance? The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(33):11595–11605. doi: 10.1523/JNEUROSCI.0264-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JI, Ha B, Bushnell MC, Pike B, Duncan GH. Differentiating noxious- and innocuous-related activation of human somatosensory cortices using temporal analysis of fMRI. J Neurophysiol. 2002;88(1):464–474. doi: 10.1152/jn.2002.88.1.464. [DOI] [PubMed] [Google Scholar]
- 14.Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clin Proc. 2011;86(9):907–911. doi: 10.4065/mcp.2011.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31(2):364–378. [PubMed] [Google Scholar]
- 16.Creac’h C, Henry P, Caille JM, Allard M. Functional MR imaging analysis of pain-related brain activation after acute mechanical stimulation. AJNR American journal of neuroradiology. 2000;21(8):1402–1406. [PMC free article] [PubMed] [Google Scholar]
- 17.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of cognitive neuroscience. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohrenbusch R, Sodhi H, Lamprecht J, Genth E. Fibromyalgia as a disorder of perceptual organization? An analysis of acoustic stimulus processing in patients with widespread pain. Zeitschrift fur Rheumatologie. 1997;56(6):334–341. doi: 10.1007/s003930050047. [DOI] [PubMed] [Google Scholar]
- 19.Flodin P, Martinsen S, Lofgren M, Bileviciute-Ljungar I, Kosek E, Fransson P. Fibromyalgia is associated with decreased connectivity between pain- and sensorimotor brain areas. Brain connectivity. 2014;4(8):587–594. doi: 10.1089/brain.2014.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friston KAJ, Kiebel S, Nichols T, Penny W. The Analysis of Functional Brain Images. Elsevier; 2007. Statistical Parametric Mapping. [Google Scholar]
- 21.Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9(5):417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Gimenez M, Pujol J, Ali Z, Lopez-Sola M, Contreras-Rodriguez O, Deus J, Ortiz H, Soriano-Mas C, Llorente-Onaindia J, Monfort J. Naproxen Effects on Brain Response to Painful Pressure Stimulation in Patients with Knee Osteoarthritis: A Double-blind, Randomized, Placebo-controlled, Single-dose Study. The Journal of rheumatology. 2014;41(11):2240–2248. doi: 10.3899/jrheum.131367. [DOI] [PubMed] [Google Scholar]
- 23.Gracely RH, Ambrose KR. Neuroimaging of fibromyalgia. Best Pract Res Clin Rheumatol. 2011;25(2):271–284. doi: 10.1016/j.berh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain: a journal of neurology. 2004;127(Pt 4):835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 25.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis and rheumatism. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 26.Group BDW. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. 2001 doi: 10.1067/mcp.2001.113989. 0009-9236 (Print) [DOI] [PubMed] [Google Scholar]
- 27.Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. The journal of pain: official journal of the American Pain Society. 2013;14(6):579–589. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. doi: 10.1097/j.pain.0000000000000593. 1872-6623 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain. 2009;141(3):215–221. doi: 10.1016/j.pain.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichesco E, Kairys A, Chang E, Ramirez G, Clauw DJ, Harris RE, Harte SE. Proceedings of the Neuroscience Meeting Planner Vol. Program No. 268.06. Society for Neuroscience; 2013. Further evidence for sensory hypersensitivity in fibromyalgia: Sensitivity to visual stimuli and response to pregabalin. Online. [Google Scholar]
- 32.Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T. Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache. 2012;52(3):441–454. doi: 10.1111/j.1526-4610.2011.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. The journal of pain: official journal of the American Pain Society. 2014;15(8):815–826 e811. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis and rheumatism. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Kim J, Loggia ML, Cahalan C, Garcia RG, Vangel MG, Wasan AD, Edwards RR, Napadow V. Fibromyalgia is characterized by altered frontal and cerebellar structural covariance brain networks. NeuroImage Clinical. 2015;7:667–677. doi: 10.1016/j.nicl.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70(1):41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 38.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(11):3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severity. Arthritis research & therapy. 2011;13(6):R185. doi: 10.1186/ar3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Sola M, Pujol J, Hernandez-Ribas R, Harrison BJ, Ortiz H, Soriano-Mas C, Deus J, Menchon JM, Vallejo J, Cardoner N. Dynamic assessment of the right lateral frontal cortex response to painful stimulation. Neuroimage. 2010;50(3):1177–1187. doi: 10.1016/j.neuroimage.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 42.López-Solà MPJ, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodríguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered fMRI responses to non-painful sensory stimulation in fibromyalgia patients. Arthritis & Rheumatology. 2014 doi: 10.1002/art.38781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda L, Ono M, Koyama T, Oshiro Y, Sumitani M, Mashimo T, Shibata M. Human brain activity associated with painful mechanical stimulation to muscle and bone. Journal of anesthesia. 2011;25(4):523–530. doi: 10.1007/s00540-011-1173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCrae CS, O’Shea AM, Boissoneault J, Vatthauer KE, Robinson ME, Staud R, Perlstein WM, Craggs JG. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. Journal of pain research. 2015;8:47–52. doi: 10.2147/JPR.S71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clinical rheumatology. 2007;26(4):465–473. doi: 10.1007/s10067-006-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montoya P, Sitges C, Garcia-Herrera M, Rodriguez-Cotes A, Izquierdo R, Truyols M, Collado D. Reduced brain habituation to somatosensory stimulation in patients with fibromyalgia. Arthritis and rheumatism. 2006;54(6):1995–2003. doi: 10.1002/art.21910. [DOI] [PubMed] [Google Scholar]
- 47.Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol. 2005;93(4):2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- 48.Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosomatic medicine. 2001;63(3):365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis and rheumatism. 2012;64(7):2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neter JNCJ, Li W. Applied linear statistical models. McGraw-Hill; Irwin: 2005. [Google Scholar]
- 52.Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310–2316. doi: 10.1016/j.pain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozgocmen S, Yoldas T, Kamanli A, Yildizhan H, Yigiter R, Ardicoglu O. Auditory P300 event related potentials and serotonin reuptake inhibitor treatment in patients with fibromyalgia. Ann Rheum Dis. 2003;62(6):551–555. doi: 10.1136/ard.62.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis and rheumatism. 2013;65(2):291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pujol J, Lopez-Sola M, Ortiz H, Vilanova JC, Harrison BJ, Yucel M, Soriano-Mas C, Cardoner N, Deus J. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS One. 2009;4(4):e5224. doi: 10.1371/journal.pone.0005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pujol J, Macia D, Blanco-Hinojo L, Martinez-Vilavella G, Sunyer J, de la Torre R, Caixas A, Martin-Santos R, Deus J, Harrison BJ. Does motion-related brain functional connectivity reflect both artifacts and genuine neural activity? Neuroimage. 2014;101:87–95. doi: 10.1016/j.neuroimage.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 58.Pujol J, Macia D, Garcia-Fontanals A, Blanco-Hinojo L, Lopez-Sola M, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F, Deus J. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. 2014;155(8):1492–1503. doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134(2):396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. The journal of pain: official journal of the American Pain Society. 2011;12(4):436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson ME, O’Shea AM, Craggs JG, Price DD, Letzen JE, Staud R. Comparison of machine classification algorithms for fibromyalgia: neuroimages versus self-report. 2015 doi: 10.1016/j.jpain.2015.02.002. 1528-8447 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in cognitive sciences. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Annals of neurology. 2014;75(2):196–208. doi: 10.1002/ana.24065. [DOI] [PubMed] [Google Scholar]
- 65.Smith JK, Humes DJ, Head KE, Bush D, White TP, Stevenson CM, Brookes MJ, Marciani L, Spiller RC, Gowland PA, Francis ST. fMRI and MEG analysis of visceral pain in healthy volunteers. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23(7):648–e260. doi: 10.1111/j.1365-2982.2011.01712.x. [DOI] [PubMed] [Google Scholar]
- 66.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1–2):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 67.Sundermann B, Burgmer M, Pogatzki-Zahn E, Gaubitz M, Stuber C, Wessolleck E, Heuft G, Pfleiderer B. Diagnostic classification based on functional connectivity in chronic pain: model optimization in fibromyalgia and rheumatoid arthritis. Acad Radiol. 2014;21(3):369–377. doi: 10.1016/j.acra.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, La Cesa S, Tarsitani L, Mainero C, Sarzi-Puttini P, Cruccu G, Caramia F, Di Franco M. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clinical and experimental rheumatology. 2016;34(2 Suppl 96):129–133. [PubMed] [Google Scholar]
- 69.Uceyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain: a journal of neurology. 2013;136(Pt 6):1857–1867. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- 70.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152(2):384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 73.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in cognitive sciences. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Wilbarger JL, Cook DB. Multisensory hypersensitivity in women with fibromyalgia: implications for well being and intervention. Arch Phys Med Rehabil. 2011;92(4):653–656. doi: 10.1016/j.apmr.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolfe F. Fibromyalgianess. Arthritis and rheumatism. 2009;61(6):715–716. doi: 10.1002/art.24553. [DOI] [PubMed] [Google Scholar]
- 76.Wolfe F, Michaud K. Outcome and predictor relationships in fibromyalgia and rheumatoid arthritis: evidence concerning the continuum versus discrete disorder hypothesis. The Journal of rheumatology. 2009;36(4):831–836. doi: 10.3899/jrheum.080897. [DOI] [PubMed] [Google Scholar]
- 77.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis and rheumatism. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 78.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee Arthritis and rheumatism. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 79.Wolfe F, Walitt B. Culture, science and the changing nature of fibromyalgia. Nat Rev Rheumatol. 2013;9(12):751–755. doi: 10.1038/nrrheum.2013.96. [DOI] [PubMed] [Google Scholar]
- 80.Wooldridge J. Introductory econometrics: A modern approach. Cengage Learning; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


