Summary
Dynamic spatiotemporal modification of chromatin around DNA damage is vital for efficient DNA repair. Normal stem cells exhibit an attenuated DNA damage response (DDR), inefficient DNA repair, and high radiosensitivity. The impact of unique chromatin characteristics of stem cells in DDR regulation is not yet recognized. We demonstrate that murine embryonic stem cells (ES) display constitutively elevated acetylation of histone H3 lysine 9 (H3K9ac) and low H3K9 tri-methylation (H3K9me3). DNA damage-induced local deacetylation of H3K9 was abrogated in ES along with the subsequent H3K9me3. Depletion of H3K9ac in ES by suppression of monocytic leukemia zinc finger protein (MOZ) acetyltransferase improved ATM activation, DNA repair, diminished irradiation-induced apoptosis, and enhanced clonogenic survival. Simultaneous suppression of the H3K9 methyltransferase Suv39h1 abrogated the radioprotective effect of MOZ inhibition, suggesting that high H3K9ac promoted by MOZ in ES cells obstructs local upregulation of H3K9me3 and contributes to muted DDR and increased radiosensitivity.
Keywords: stem cells, DNA damage response, ATM, MOZ, histone acetylation, histone methylation, H3K9ac, H3K9me3, epigenetic, radioprotection
Highlights
-
•
Embryonic stem cells show impaired H3K9 deacetylation at DNA DSB sites
-
•
Downregulation of MOZ-dependent H3K9ac improves ATM activation in stem cells
-
•
MOZ knockdown protects embryonic stem cells from radiation-induced cell death
-
•
MOZ suppression-mediated radioprotection is dependent on methyltransferase Suv39h1
H3K9 acetylation is high in stem cells, but requires downregulation at DNA damage sites. Sharma and colleagues show inefficient H3K9 deacetylation and tri-methylation in embryonic stem cells and establish H3K9 acetyltransferase MOZ as a potential therapeutic target to improve ATM activation and stem cell survival after DNA damage induction.
Introduction
The role of chromatin status on DNA damage response (DDR) and DNA double-strand break (DSB) repair has been extensively investigated in differentiated non-stem and cancer cells. Chromatin structure and remodeling plays an important role in the recruitment/assembly of repair factors and allows their access to DNA lesions (Price and D'Andrea, 2013). Activation of DNA DSB repair machinery is promoted not only by local decondensation of chromatin but also by the binding of heterochromatin-associated proteins and the transient compaction of chromatin (Burgess et al., 2014, Lemaître and Soutoglou, 2014). However, chromatin of differentiated/non-stem and cancer cells is starkly different from the stem/progenitor cells in tissue niches in vivo, such as neural stem cells in the subgranular zone of brain and neural stem cells (NS) cells in culture, intestinal crypt stem cells, testicular stem cells, and embryonic stem cells (ES) in vivo and in culture. These “normal” stem cells are characterized by elevated levels of histone marks associated with active chromatin and lower levels of repressive chromatin (Chen and Dent, 2014). Previously, we have delineated the epigenetic influence of H3K56 acetylation on attenuation of DDR in murine embryonic ES and NS cells, as well as in several tissue niches in vivo, demonstrating H3K56ac as an epigenetic suppressor of DDR that promotes ionizing radiation (IR) hypersensitivity in normal stem cells (Jacobs et al., 2016).
Ataxia telangiectasia mutated (ATM) kinase is involved in multiple DDR processes that include phosphorylation of several histone and non-histone proteins, amplification of the DNA damage signal, repair factor activation, and initiation of the cell-cycle arrest (Shiloh and Ziv, 2013). ATM activation is promoted by its acetylation through Tip60 (Sun et al., 2007). This activation requires the binding of Tip60 to tri-methylated histone H3 lysine 9 (H3K9me3) (Sun et al., 2009), a modification associated with repressive heterochromatin (Grewal and Jia, 2007). H3K9me3 is locally and transiently upregulated around the DNA damage site by the methyltransferase Suv39h1, improving ATM activation via Tip60 (Ayrapetov et al., 2014). Recruitment of Suv39h1 enabling H3K9me3 and assembly of binding partners HP1 and KAP1 is rapid and transient, observed for few minutes after DSB induction. Interestingly, acetylation on the same amino acid residue (H3K9ac) is locally downregulated at the DNA damage sites (Bártová et al., 2011).
We compared karyotypically normal, early-passage, radiosensitive murine ES and NS with the isogenic radioresistant non-stem cells (ED and ND), proliferating at identical rates in culture; to investigate the differential regulation of DNA damage and apoptotic responses of radiosensitive stem and radioresistant non-stem cells (Jacobs et al., 2016). In this study, we demonstrate constitutively high H3K9ac but low H3K9me3 levels in ES that fails to downregulate H3K9ac at the DSB sites (microirradiation tracks), unlike the ED. Suppression of the H3K9 acetyltransferase monocytic leukemia zinc finger protein (MOZ) reduced H3K9ac in ES, protected them from IR-induced apoptosis, and improved ES survival after irradiation. On the contrary, knockdown of MOZ did not protect non-stem or cancer cells from IR-induced cell death. Besides ES, reduction of IR-induced apoptosis after MOZ knockdown in human neuroprogenitor cells suggests that a transient suppression of MOZ may very likely be a useful therapeutic strategy for radioprotection of human neuroprogenitor cells.
Results
Embryonic and Neural Stem Cells Exhibit High H3K9 Acetylation and Low H3K9 Tri-methylation In Vivo and In Vitro
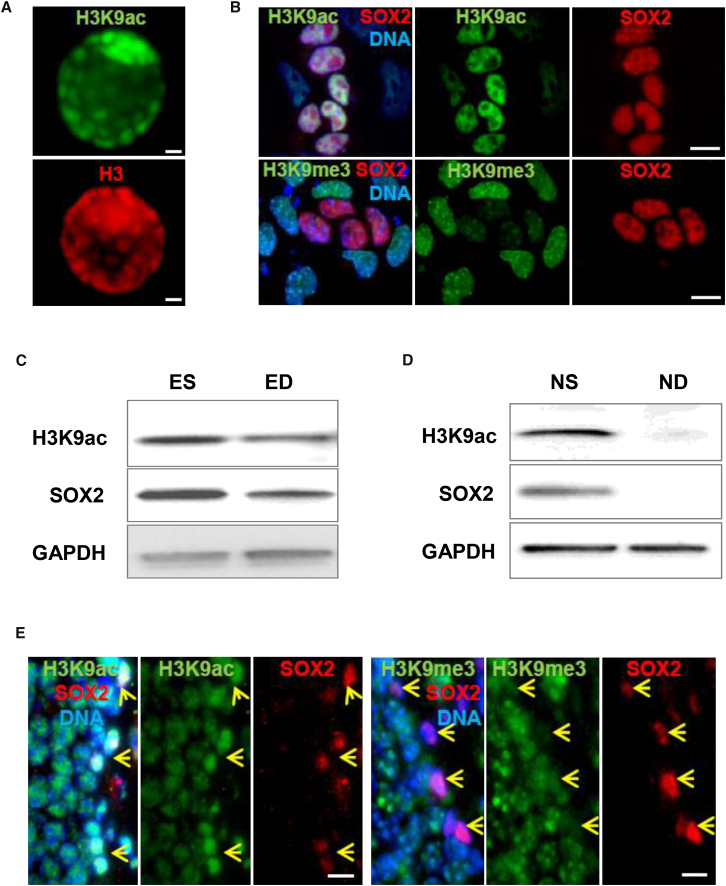
We investigated the H3K9 modifications in embryonic and adult stem cell populations and found that the inner cell mass of the mouse blastocyst (embryonic day 4.5 [E4.5], wild-type C57BL/6) containing the pluripotent ES cells showed markedly high H3K9 acetylation compared with the trophoblast cells (Figure 1A). The basal expression of histone H3 was similar between the two cell types (Figure 1A). ES cells in culture were co-plated with isogenic non-stem ED cells before fixation as described previously (Jacobs et al., 2016). H3K9ac was detected with the stem cell-specific transcription factor SOX2. SOX2-positive cells showed significantly elevated H3K9ac compared with the SOX2-negative ED cells (Figure 1B); however, H3K9me3 was diminished in stem cells in contrast to the non-stem cells (Figure 1B). Although differentiation procedures yield highly enriched non-stem cells in culture, directly derived cells (ED) often retain a minimal SOX expression, probably due to its role in cell fate determination (Robles et al., 2011). Immunoblotting also corroborated high H3K9ac in ES compared with ED (Figure 1C). NS cells, isolated from newborn mouse hippocampi compared with their non-stem cell (ND) counterparts, were also investigated (Jacobs et al., 2016). Immunoblotting revealed high H3K9ac in NS compared with SOX2-negative ND (Figure 1D). To confirm this, we investigated adult NS cells in the dentate gyrus niche of mouse brain. SOX2-positive adult NS/neuroprogenitor cells also displayed high H3K9ac, but reduced H3K9me3 compared with surrounding non-stem cells in the tissue niche (Figure 1E). Similar status was observed in spermatogonial stem cells (Figures S1A and S1B) in mouse testis, suggesting similar H3K9 modifications in several stem cell types. Some PLZF negative non-stem cells (mostly spermatids) also displayed higher H3K9 acetylation, likely due the role in the histone-protamine exchange (Song et al., 2011). Together, these results indicate that H3K9 is differentially modified in murine ES and neuroprogenitor and spermatogonial stem cells, displaying high acetylation (H3K9ac) but low tri-methylation (H3K9me3).
Figure 1.
Embryonic Stem and Neural Progenitor Cells Exhibit High H3K9 Acetylation and Low H3K9 Tri-methylation In Vitro and In Vivo
(A) Mouse blastocysts (E4.5) fixed and stained for H3K9ac or H3.
(B) H3K9ac (upper panel) or H3K9me3 (lower panel) with SOX2 detected in co-cultures of ESCs and EDCs. DNA labeled with DAPI.
(C) H3K9ac, SOX2, and GAPDH detected in lysates of ES and ED using immunoblot.
(D) H3K9ac, SOX2, and GAPDH detected in NS and ND lysates using immunoblot.
(E) Tissue sections of the dentate gyrus in hippocampus obtained from brains of WT C57BL/6 mice and H3K9ac (left panels) or H3K9me3 (right panels) stained with SOX2 and DNA labeled with DAPI. Arrows indicate SOX2-positive stem and progenitor cells with high H3K9ac and low H3K9me3 levels.
Scale bars, 10 μm.
Embryonic Stem Cells Fail to Efficiently Downregulate H3K9 Acetylation and Upregulate H3K9 Methylation at the DNA Breaks
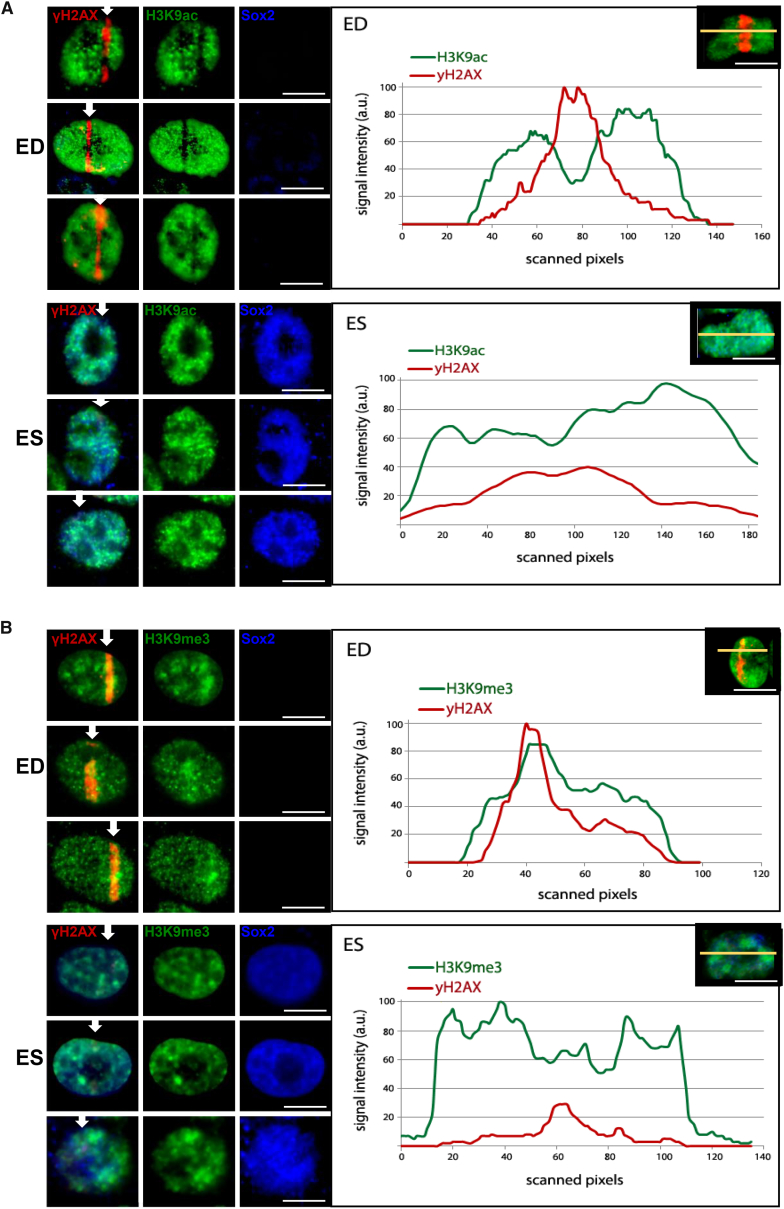
H3K9ac has been shown to be downregulated locally around the DNA damage sites in a fraction of murine ES cells (Bártová et al., 2011). We tested whether in ES such downregulation occurs around DSB sites despite the significantly high global H3K9 acetylation in contrast to the ED. We co-plated ES and ED and irradiated ES in close proximity of ED using laser microirradiation. Induction of DSBs along the microirradiation track was visualized by labeling the DSB marker γH2AX together with staining for H3K9ac while ES were identified by SOX2-positive staining (Figure 2A). Figure S2A shows a representative example of an uncropped image of co-plated ESCs and EDCs in same line of microirradiation. Most of the ED cells showed depleted H3K9ac at the DSB track, quantified in the fluorescent signal-intensity line profile across the irradiated nuclei (Figure 2A, upper panel), which does not occur due to eviction of H3 from the damaged sites (Jacobs et al., 2016). But unlike ED, ES cells did not downregulate H3K9ac at the DNA DSBs with same proficiency as observed in ED (Figure 2A, lower panel). ES displayed significantly reduced and very diffused, if any, γH2AX signal at DNA lesions compared with ED, which is very likely due to reduced ATM activation that has been previously shown in stem cells (Jacobs et al., 2016). Unirradiated ES (Figure S2D) and ED (Figure S2E) displayed uneven H3K9ac profiles throughout the nuclei. Within the DNA damage sites, in and along the track, ED showed clearly reduced H3K9ac at all points of the intensity profile (Figure S2C), while in ES H3K9ac remained high (Figure S2B) and comparable with that in unirradiated cells. We quantified the number of ES and ED cells that showed clear downregulation of H3K9ac at DSBs and found that about 90% of ED locally deacetylated H3K9, while only 40% of analyzed ES showed depletion of H3K9ac (Figure S2F). These results suggest that H3K9 acetylation and local H3K9 deacetylation in response to DNA damage induction are differentially regulated in stem compared with non-stem cells.
Figure 2.
Embryonic Stem Cells Fail to Downregulate H3K9 Acetylation and Upregulate H3K9 Methylation Locally at DNA Damage Sites Compared with Differentiated Cells
ES were co-plated with isogenic non-stem ED cells. Cells were microirradiated subnuclearly in a line across the ES and ED in same region of interest (ROI) crossing through both cell types.
(A) ED and ES microirradiated and fixed immediately after irradiation and H3K9ac and γH2AX detected along with Sox2 (arrow indicates laser ROI). Quantification of fluorescence intensities along the yellow line is depicted in the corresponding graph on the right. ED are shown in the top panel and ES adjacent to ED in the line of irradiation, treated in the same way, in the lower panel.
(B) ED and ES microirradiated and fixed immediately after irradiation and H3K9me3 and γH2AX detected along with Sox2. Fluorescence intensities along the yellow line is depicted in the corresponding graph on the right. ED are shown in the top panel and ES adjacent to ED in the line of irradiation, treated in the same way, in the lower panel.
Scale bars, 10 μm.
H3K9me3 is locally and transiently upregulated around the DNA damage site by histone methyltransferase Suv39h1, promoting ATM activation via Tip60 (Ayrapetov et al., 2014). We tested whether such upregulation occurs in ES cells. While ED showed a detectable increase in H3K9me on the DSB tracks (Figure 2B, upper panel), ES often failed to upregulate H3K9me3 at the tracks (Figure 2B, lower panel). Quantification of the number of cells with upregulated H3K9me3 at DSBs revealed about 90% of ED with H3K9me3-positive tracks, while only 5% of analyzed ES showed a local increase of H3K9me3. This H3K9 epigenetic modification is quick and transient, with the highest methylation tracks observed within 2 min of irradiation (Figure S2F). These results establish differential regulation of these epigenetic modifications at or around DNA damage sites in stem cells.
Downregulation of MOZ Reduced H3K9 Acetylation and Improved DNA Damage-Induced Activation of ATM and DNA Repair Efficiency in ES cells
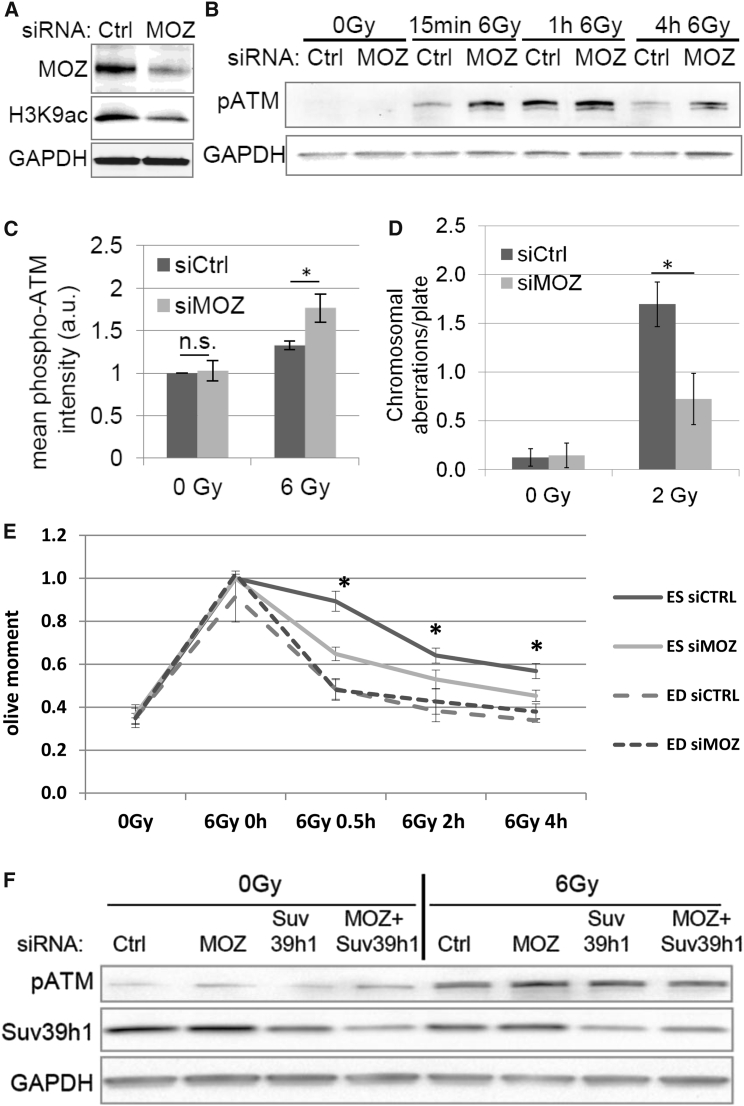
While the precise role of H3K9 deacetylation at DSBs has not been identified as yet, the upregulation of tri-methylation at the same amino acid residue around the DSBs via Suv39h1 has been explicitly shown to be required for the activation of ATM through Tip60 (Ayrapetov et al., 2014). We therefore tested the hypothesis that inefficient H3K9 deacetylation at DNA break site hinders the local upregulation of H3K9me3 and thereby contributes to the reduced ATM activation as observed in stem cells. We investigated whether ATM activation could be promoted/restored in stem cells by downregulation of H3K9ac. Transient knockdown of the acetyltransferase MOZ resulted in a reduction of H3K9ac in stem cells (Figure 3A), while overexpression of deacetylase SIRT6 (Figure S3A) or inhibition of acetyltransferase GCN5 (Figure S3B, left) did not influence H3K9ac levels. Besides, knockdown of GCN5 did not reduce IR-induced apoptosis (Figure S3B, right). Furthermore, a faster and longer activation of ATM (phospho-serine 1981) was observed when MOZ expression was inhibited (Figure 3B). Detection of phospho-ATM with flow cytometry corroborated improved ATM activation with MOZ inhibition (Figure 3C). Total ATM protein levels are similar in ESCs and EDCs (Jacobs et al., 2016). We measured the kinetics of DSB repair after MOZ depletion by comet assay and observed an improvement in DNA repair in stem cells treated with MOZ small interfering RNA (siRNA) (Figure 3E), almost comparable with the DSB repair in irradiated ED without MOZ inhibition. MOZ inhibition in ED had no effect (Figure 3E). The cell-cycling rates of both ES and ED have been shown previously to be identical (Jacobs et al., 2016). Analysis of chromosomal aberrations also revealed an improved repair and reduced residual chromosome and chromatid breaks in MOZ-depleted stem cells after irradiation (Figure 3D). These results suggest that MOZ activity in ES cells antagonizes or interferes with full activation of ATM in response to DNA damage, potentially by sustained maintenance of elevated H3K9ac levels and thereby inhibiting tri-methylation of H3K9. We also observed increased activation of ATM after MOZ suppression to be dependent upon the H3K9 methyltransferase Suv39h1 (Figure 3F). Endogenous SIRT1 (Figure S3C) or SIRT6 (Figure S3D) recruitment could not be detected in ES or ED cells using microirradiation. However, it has previously been shown that SIRT1 is redistributed to the DNA damage in murine ES cells (Oberdoerffer et al., 2008). Using a SIRT6-GFP overexpression approach, we were able to detect rapid recruitment in ED but not in ES (Figure S3E). Instead there was no recovery of the bleached GFP, suggesting a stable binding of SIRT6 to the chromatin in ES cells. Whether this is influenced by MOZ or an independent mechanism contributing to inefficient H3K9 deacetylation requires further investigation.
Figure 3.
Suppression of MOZ Decreases H3K9 Acetylation and Improves ATM Activation and DNA Repair Efficiency
ES cells were treated with control siRNA (siCtrl) or MOZ siRNA (siMOZ).
(A) MOZ, H3K9ac, and GAPDH detected in cell lysates using immunoblot.
(B) pATM (phospho-S1981) and GAPDH detected using immunoblot after 6 Gy irradiation at indicated time points.
(C) pATM (phospho-S1981) analyzed with flow cytometry 30 min after 6 Gy irradiation. Values were normalized to the 0-Gy siCtrl sample. Three independent experiments were performed.
(D) Chromosomal aberrations per metaphase plate quantified at 7 hr after 2 Gy irradiation. Three independent experiments were performed.
(E) ES and ED treated with siCtrl or MOZ siRNA were analyzed by comet assay. Values were normalized to the 0-Gy time point. Three independent experiments were performed.
(F) ES cells treated with control, MOZ, Suv39h1, or both MOZ and Suv39h1 siRNA and pATM (phospho-S1981), Suv39h1, and GAPDH detected by immunoblot in lysates 30 min after 6 Gy irradiation.
Error bars denote SD. ∗p < 0.05; n.s., not significant (p > 0.05).
Suppression of H3K9 Acetyltransferase MOZ Reduced IR Sensitivity of Stem Cells
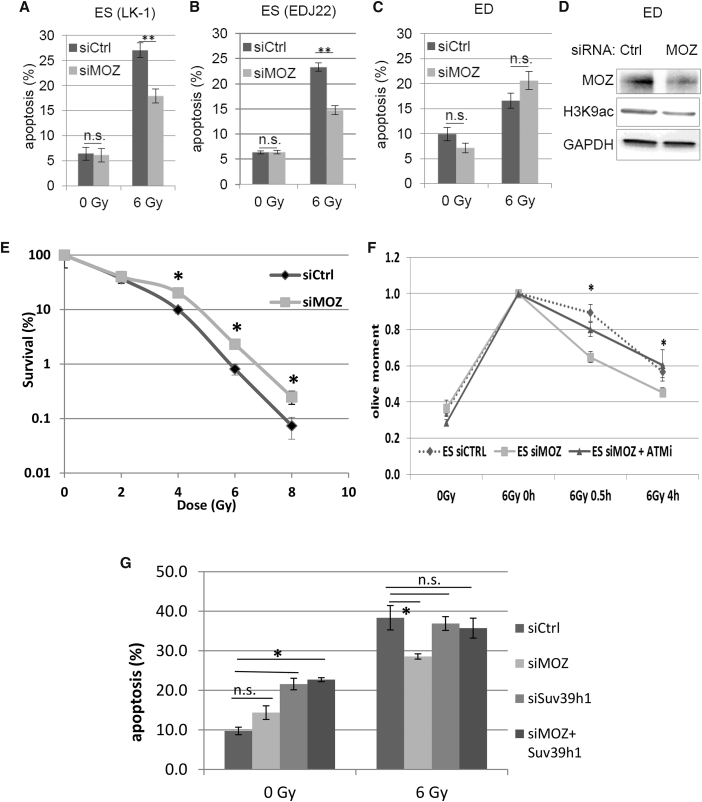
Improvement of DSB repair in stem cells has a potential for development of stem cell-specific radioprotectors. We therefore investigated whether the knockdown of MOZ was effective in improving the overall survival of ES cells after irradiation. Significantly decreased IR-induced apoptosis was observed in ES with depletion of MOZ (Figure 4A), while upregulation of SIRT6 had no influence on apoptosis (Figure S4A). Besides the ES used throughout this study (LK-1), an additional ES cell line, EDJ22, also showed similar radioprotection with the MOZ knockdown (Figure 4B). However, LK-1-derived non-stem ED cells were not protected (Figure 4C), with downregulation of MOZ expression (Figure 4D). In addition, medulloblastoma Daoy HTB-186 cells (Figure S4B) and glioma GL261 cancer cells (Figure S4C) were also not radioprotected after MOZ suppression. Clonogenic survival and radiosensitivity of ES cells improved with MOZ downregulation (Figure 4E). Interestingly, simultaneous downregulation of MOZ and ATM reduced DSB repair efficiency, confirming that improvement in radiosensitivity of stem cells by MOZ inhibition is largely through ATM activation, as ATM inhibition reverses the MOZ inhibition-driven improvement in DSB repair and IR sensitivity phenotype (Figure 4F). MOZ HAT−/− hematopoietic and neural progenitors show a higher expression of p16 leading to senescence (Perez-Campo et al., 2014), and MOZ has been shown to inhibit the p16 pathway in mouse embryonic fibroblasts (MEFs) (Sheikh et al., 2015a). Interestingly, we observed no effect on p16 levels with the MOZ knockdown (Figure S4D), indicating that the analyzed cells were not senescent. It has previously been shown that restoration of the abrogated G1 checkpoint in murine ESCs can reduce their radiosensitivity (Hong and Stambrook, 2004). We observed that despite MOZ knockdown and improved ATM activation, ESCs fail to arrest in G1 (Figure S4E), suggesting that the radioprotection by MOZ suppression is mediated by an improved DNA repair rather than by checkpoint activation. The IR-induced apoptosis of ES cells was reduced by MOZ knockdown only if Suv39h1 was normally expressed, while simultaneous downregulation of MOZ and Suv39h1 did not protect stem cells from apoptosis (Figure 4G). The higher apoptosis rate in the 0-Gy control after inhibition of Suv39h1 alone or in combination with MOZ can likely be due to the cells’ inability to recruit ATM and HP1 to repair DNA damage at the basal level. To confirm the significance of these observations made in murine stem cells, we then analyzed neuroprogenitor cells directly derived from human ESCs. The differentiation was visible in cell morphology (Figure S4F) and confirmed by OCT4 and SOX1 detection (Figure S4G). Human neuroprogenitors were also radioprotected by MOZ knockdown (Figure S4H), corroborating our findings in murine ESCs. Together, these observations indicate a significant role of MOZ in the radiation response of stem cells, suggesting that transient suppression of MOZ can potentially be a useful therapeutic strategy to prevent IR-induced stem cell death.
Figure 4.
Downregulation of MOZ Expression Diminished Radiation-Induced Apoptosis in a Suv39h1-Dependent Manner and Increased Survival in Embryonic Stem Cells
Cells were treated with control siRNA (siCtrl) or MOZ siRNA (siMOZ).
(A) ES cell line LK-1 irradiated with 6 Gy and apoptosis analyzed after 16 hr by annexin V labeling. Three independent experiments were performed.
(B) As a comparison, ES line EDJ22 was used only in this experiment and analyzed as in (A). Three independent experiments were performed.
(C) ED cells analyzed as in (A). Three independent experiments were performed.
(D) MOZ, H3K9ac, and GAPDH detected in ED lysates by immunoblot.
(E) Clonogenic survival of ESCs analyzed after indicated doses of irradiation. Three independent experiments were performed.
(F) ES treated with siCtrl, MOZ siRNA, or MOZ siRNA with ATM inhibitor analyzed by comet assay. Values were normalized to the 6-Gy time point. Three independent experiments were performed.
(G) ES treated with control, MOZ, Suv39h1, or both MOZ and Suv39h1 siRNA and analyzed as in (A). Three independent experiments were performed.
Error bars denote SD. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant (p > 0.05).
Discussion
The repair of DNA damage requires dynamic chromatin alterations comprising both transient decondensation and compaction of chromatin locally around the DSB sites. Stem cells display a uniquely open, undefined chromatin structure, but our understanding of its consequences in activation of DDR and recruitment of DNA repair factors is still very limited. In this study, we describe that the inhibition of acetyltransferase MOZ reduces H3K9 acetylation and promotes the activation of ATM via the histone methyltransferase Suv39h1, leading to an increase in DNA repair efficiency and decreased IR-induced cell death.
H3K9 modifications have been previously analyzed in ESCs (Lee et al., 2004, Meshorer et al., 2006, Azuara et al., 2006, Loh et al., 2007). This study supports previous observations and additionally demonstrates an increased constitutive H3K9ac along with distinctly reduced H3K9me3 in stem cells, in contrast to the isogenic non-stem ED cells in culture as well as in vivo. Local downregulation of H3K9ac has been reported in ESCs, accompanying recruitment of the transcription factor OCT4 to DNA lesions (Bártová et al., 2011). In the same study, a downregulation of H3K9 acetylation was not observed in one-third of the analyzed stem cells, which is in agreement with our finding of a local decrease of H3K9ac in 40% of the stem cells in culture. Moreover, our work compares the local H3K9 deacetylation at or around DSBs in ES directly with the frequency of H3K9ac downregulation in non-stem ED cells, showing a less efficient deacetylation response in ES cells. While the function of the local H3K9ac downregulation in the DDR is not clear, the regulation of ATM activation by H3K9me3 through Suv39h1 has been extensively studied (Ayrapetov et al., 2014, Sun et al., 2009). We hypothesize that deacetylation at H3K9 is a prerequisite to allow tri-methylation at this same amino acid residue for efficient ATM activation. In accordance, we observed that while ED cells show an increase in H3K9me3 at the DSB tracks, ES did not display such chromatin modification. The lower percentage of ED positive for H3K9me3 compared with deacetylated H3K9 can be explained by the very transient/temporal nature of the process: in fact we observed that the high percentage of ED showing tri-methylation can be observed only within 2 min after the DNA damage induction, and this percentage reduced drastically at 10 min after DSB induction. We thus provide evidence that differential ATM activation through Suv39h1 is influenced by the characteristic chromatin profiles. Suppression of Suv39h1 methyltransferase alone had a limited effect on ATM activation or IR-induced apoptosis of stem cells. This is in accord with our hypothesis that H3K9 deacetylation is required before Suv39h1-mediated methylation of H3K9, and therefore Suv39h1 activity is restricted in stem cells with consistently elevated H3K9ac.
MOZ regulates H3K9ac at Hox gene promoters, but no global downregulation of H3K9ac was detected in MOZ+/− or MOZ−/− embryos compared with WT mice (Voss et al., 2009). However, with a transient MOZ knockdown approach, we were able to observe a global downregulation of H3K9ac. It is very likely that prolonged absence of functional MOZ could partly be compensated by the activity of other redundant H3K9 acetyltransferases such as GCN5 in MOZ−/− cells. However, we cannot exclude that MOZ has a broader effect on global H3K9 acetylation only in specific types of embryonic or adult stem/progenitor cells.
In this study, transient MOZ suppression did not alter p16 expression. MOZ is important for suppression of p16 that is required to maintain self-renewal capacity, and MOZ HAT−/− hematopoietic cells and NSCs show enhanced p16INK4A expression and an early replicative senescence (Perez-Campo et al., 2014). Similarly, in MEFs MOZ functions as an inhibitor of senescence via the INK4A-ARF pathway, with MOZ−/− MEFs showing a reduction in proliferation only at late passages and no increased levels of apoptosis (Sheikh et al., 2015a). This suggests that only prolonged absence of MOZ leads to cellular senescence, in agreement with our finding that the transient MOZ suppression did not alter p16 expression.
We propose that transient inhibition of MOZ could be beneficial in stem cell survival by improved ATM activation and DNA repair, with a negligible effect on differentiated non-stem or cancer cells. Elevated global H3K9ac, like H3K56ac, is essential for stem cell-specific transcriptome and has possibly other crucial diverse functions in stem cells. Therefore, a total knockout of MOZ from stem cells, or prolonged depletion, would be detrimental for “stemness” state, and also in non-stem cells because of likely induction of senescence in both cell types and lack of DNA damage-induced G1 checkpoint arrest in differentiated cells. It has been suggested that MOZ promotes induction of the G1 cell-cycle arrest in MEF cells through its interaction with p53, improving cellular viability (Rokudai et al., 2009).
Suppression of MOZ protected a fraction of cells from IR-induced apoptosis, increased DNA repair proficiency, and improved survival by a factor of 2 to 3 between 4 and 8 Gy. This also warrants that MOZ is not the sole epigenetic/molecular regulator decisive of the stem cell radiation response. We have recently established that ES and NS cells can be radioprotected by transiently diminishing H3K56ac (Jacobs et al., 2016), which is also characteristically high in stem cells. Besides, we have identified stem cell-specific differential signaling regulation by protein phosphatase PP2A (M.R.F. et al., unpublished data) that contributes to stem cell IR hypersensitivity. We have thus elucidated epigenetic and molecular signaling rheostats that pluralistically regulate and collectively promote IR hypersensitivity of stem cells. Interestingly, use of MOZ inhibition has been suggested earlier for the treatment of certain types of lymphoma and leukemia (Sheikh et al., 2015b) as well as for a potential induction of senescence in cancer stem cells (Perez-Campo et al., 2014). The expression of mutated MOZ has been associated with the formation of medulloblastoma (Wu et al., 2012). We observed that medulloblastoma cells undergo increased apoptosis when MOZ was suppressed, which supports the notion that inhibition of MOZ could have a radiosensitizing effect in certain cancer types but be radioprotective to normal stem cells.
However, development of a pharmacological transient inhibitor of MOZ would be essential to analyze the therapeutic potential of MOZ inhibition on radioprotection in mouse preclinical models. This study proposes MOZ histone acetyltransferase as a promising target for development of radioprotective agent.
Experimental Procedures
RNA Interference
siRNA against MOZ, GCN5, and Suv39h1 was used at 50 nM for 24 hr. See Supplemental Experimental Procedures for a detailed description.
X-Ray Irradiation and Microirradiation
Cells were irradiated with 160 keV X-rays with indicated doses at a dose rate of 1.7 Gy/min. For microirradiation, co-plated embryonic and differentiated cells were cultured for 2 days in 70 μM bromodeoxyuridine and irradiated with a 405- and 633-nm laser. See Supplemental Experimental Procedures for further details.
Apoptosis Assay
Cells were trypsinized at indicated time points after irradiation and labeled using the FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen) according to the manufacturer's instructions. At least 5,000 cells were analyzed by flow cytometry with a Miltenyi flow cytometer.
Clonogenic Assay
Quantification of colony formation was performed according to standard protocols. See Supplemental Experimental Procedures for a detailed description.
Cytogenetic Analysis
The dose of 2 Gy was chosen to allow cells to enter mitosis after irradiation. Five hours after irradiation the cells were arrested in mitosis for 1 hr 45 min. Chromosomes were harvested, DNA stained, and telomeres detected by fluorescence in situ hybridization according to standard protocols. A detailed description is provided in Supplemental Experimental Procedures.
Statistical Analysis
Statistical analysis was performed using the two-sided Student's t test except for the comet assay, which was analyzed using ANOVA. p Values of <0.05 were considered statistically significant, with p < 0.01 highly statistically significant. Error bars represent the SD of the mean.
Cell culture, neutral comet assay, immunoblot analysis, immunocytochemistry, and immunohistochemistry on adult WT mouse tissue were performed using standard protocols. For a detailed description as well as antibody information, see Supplemental Experimental Procedures.
Procedures for all experiments involving animals were approved by the Animal Studies Committee at Washington University Medical Center.
Author Contributions
G.G.S. conceived the project. G.G.S. and B.M. designed the experiments. B.M., M.R.F., S.R., and C.L.Z performed the experiments. D.E.H. and G.G.S. helped with inputs and interpretation of the data. B.M. and G.G.S. wrote the paper.
Acknowledgments
We thank Dr. Mariana Beltcheva of the human stem cell core facility for technical support in human stem cell culture, and Dr. Leonard Guarente for providing the SIRT6-GFP-plasmid. Funding was provided by NIH-R01CA174966; Department of Radiation Oncology and the Siteman Cancer Center of Washington University School of Medicine.
Published: December 13, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.11.004.
Supplemental Information
References
- Ayrapetov M.K., Gursoy-Yuzugullu O., Xu C., Xu Y., Price B.D. DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin. Proc. Natl. Acad. Sci. USA. 2014;111:9169–9174. doi: 10.1073/pnas.1403565111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuara V., Perry P., Sauer S., Spivakov M., Jørgensen H.F., John R.M., Gouti M., Casanova M., Warnes G., Merkenschlager M. Chromatin signatures of pluripotent cell lines. Nat. Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- Bártová E., Šustáčková G., Stixová L., Kozubek S., Legartová S., Foltánková V. Recruitment of Oct4 protein to UV-damaged chromatin in embryonic stem cells. PLoS One. 2011;6:e27281. doi: 10.1371/journal.pone.0027281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R.C., Burman B., Kruhlak M.J., Misteli T. Activation of DNA damage response signaling by condensed chromatin. Cell Rep. 2014;9:1703–1717. doi: 10.1016/j.celrep.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Dent S.Y.R. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat. Rev. Genet. 2014;15:93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S.I.S., Jia S. Heterochromatin revisited. Nat. Rev. Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Hong Y., Stambrook P.J. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc. Natl. Acad. Sci. USA. 2004;101:14443–14448. doi: 10.1073/pnas.0401346101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K.M., Misri S., Meyer B., Raj S., Zobel C.L., Sleckman B.P., Hallahan D.E., Sharma G.G. Unique epigenetic influence of H2AX phosphorylation and H3K56 acetylation on normal stem cell radioresponses. Mol. Biol. Cell. 2016;27:1332–1345. doi: 10.1091/mbc.E16-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Hart S.R.L., Skalnik D.G. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004;38:32–38. doi: 10.1002/gene.10250. [DOI] [PubMed] [Google Scholar]
- Lemaître C., Soutoglou E. Double strand break (DSB) repair in heterochromatin and heterochromatin proteins in DSB repair. DNA Repair (Amst.) 2014;19:163–168. doi: 10.1016/j.dnarep.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Loh Y.H., Zhang W., Chen X., George J., Ng H.H. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E., Yellajoshula D., George E., Scambler P.J., Brown D.T., Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer P., Michan S., McVay M., Mostoslavsky R., Vann J., Park S.K., Hartlerode A., Stegmuller J., Hafner A., Loerch P. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Campo F.M., Costa G., Lie-A-Ling M., Stifani S., Kouskoff V., Lacaud G. MOZ-mediated repression of p16INK4a is critical for the self-renewal of neural and hematopoietic stem cells. Stem Cells. 2014;32:1591–1601. doi: 10.1002/stem.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price B.D., D’Andrea A.D. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152:1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles V., Marti M., Izpisua Belmonte J.C. Study of pluripotency markers in zebrafish embryos and transient embryonic stem cell cultures. Zebrafish. 2011;8:57–63. doi: 10.1089/zeb.2010.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokudai S., Aikawa Y., Tagata Y., Tsuchida N., Taya Y., Kitabayashi I. Monocytic leukemia zinc finger (MOZ) interacts with p53 to induce p21 expression and cell-cycle arrest. J. Biol. Chem. 2009;284:237–244. doi: 10.1074/jbc.M805101200. [DOI] [PubMed] [Google Scholar]
- Sheikh B.N., Phipson B., El-Saafin F., Vanyai H.K., Downer N.L., Bird M.J., Kueh A.J., May R.E., Smyth G.K., Voss A.K., Thomas T. MOZ (MYST3, KAT6A) inhibits senescence via the INK4A-ARF pathway. Oncogene. 2015;34:5807–5820. doi: 10.1038/onc.2015.33. [DOI] [PubMed] [Google Scholar]
- Sheikh B.N., Lee S.C., El-Saafin F., Vanyai H.K., Hu Y., Pang S.H., Grabow S., Strasser A., Nutt S.L., Alexander W.S. MOZ regulates B-cell progenitors and, consequently, Moz haploinsufficiency dramatically retards MYC-induced lymphoma development. Blood. 2015;125:1910–1921. doi: 10.1182/blood-2014-08-594655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y., Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- Song N., Liu J., An S., Nishino T., Hishikawa Y., Koji T. Immunohistochemical analysis of histone H3 modifications in germ cells during mouse spermatogenesis. Acta Histochem. Cytochem. 2011;44:183–190. doi: 10.1267/ahc.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Xu Y., Roy K., Price B.D. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol. Cell. Biol. 2007;27:8502–8509. doi: 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Jiang X., Xu Y., Ayrapetov M.K., Moreau L.A., Whetstine J.R., Price B.D. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat. Cell Biol. 2009;11:1376–1382. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A.K., Collin C., Dixon M.P., Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and Segment Identity. Dev. Cell. 2009;17:674–686. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Wu X., Northcott P.A., Dubuc A., Dupuy A.J., Shih D.J.H., Witt H., Croul S., Bouffet E., Fults D.W., Eberhart C.G. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482:529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.