Abstract
Background
Cocaine addiction is characterized by patterns of compulsive drug-taking, including preoccupation with obtaining cocaine and loss of control over drug intake. The lateral hypothalamic hypocretin/orexin (HCRT) system has been implicated in drug-taking and the reinstatement of drug-seeking. Evidence suggests HCRT may drive drug-seeking through activation of specific brain regions implicated in stress system dysfunction, including the central amygdala (CeA). The role of HCRT in the persistence of compulsive-like cocaine-taking has yet to be fully elucidated.
Methods
Systemic and intra-CeA microinfusions of the HCRT-receptor 1 antagonist, SB-334867, were administered to rats allowed either short (1 h; ShA) or long (6 h; LgA) access to cocaine self-administration. Animals were tested for fixed and progressive ratio responding for cocaine and stress-induced reinstatement of drug-seeking. Additionally, using electrophysiological techniques on in vitro slices, we investigated GABAergic neurotransmission in the medial CeA and the sensitivity of GABAergic synapses to modulation of the HCRT system in ShA or LgA rats
Results
We found systemic administration of SB-334867 (0, 7.5, 15, 30 mg/kg) dose-dependently decreased cocaine intake specifically in LgA, but not in ShA, rats. Microinjections of SB-334867 (20 nmol) bilaterally into the CeA significantly reduced cocaine intake in LgA rats. We also observed a significant attenuation of yohimbine-induced reinstatement of cocaine-seeking following intra-CeA SB-334867 (10 nmol) administration. Finally, electrophysiological data indicated enhanced GABAergic neurotransmission within the medial CeA in LgA rats, which was blocked with SB-334867 (10μM).
Conclusions
These findings suggest that HCRT neurotransmission within the CeA is implicated in compulsive-like cocaine-seeking.
Keywords: cocaine, hypocretin/orexin, central amygdala, drug dependence, intravenous self-administration, reinstatement
Introduction
Psychostimulants, including cocaine, are widely-abused drugs that exert robust reinforcing and arousal-enhancing effects, contributing to their use and abuse. Cocaine addiction is a disorder in which both humans and animals ultimately transition from nondependent, episodic drug use to compulsive drug-taking. Compulsive-like behavior in humans (1) and rodents (2; 3) is characterized by excessive drug-seeking/taking patterns that are difficult to terminate once responding has been initiated. Compulsive-like behavior in rats is operationally defined as the emergence of escalated drug intake, increased motivation to obtain the drug (i.e. elevated progressive ratio breakpoints), responding despite punishment, reward system deficits during abstinence and increased likelihood of relapse (2–6). Animal models of extended access (≥ 6 hours) to cocaine self-administration mimic compulsive-like behavioral patterns, maintaining face and construct validity for the transition to drug dependence (7; 8). The escalated cocaine intake and increased progressive ratio breakpoints that are observed in long access (LgA; 6 h) animals is in contrast to lower, stable intake observed in animals allowed short access (ShA; 1 h) to cocaine (7; 9). It is hypothesized that escalated cocaine-taking is partially mediated by upregulation of brain motivational and stress systems, particularly within extended amygdala subregions including the central amygdala (CeA), the bed nucleus of the stria terminalis (BNST) and the medial and caudal nucleus accumbens (NAc; 7). Escalated cocaine intake is also associated with a significant blunting of the dopamine response to cocaine and an increase in intracranial self-stimulation reward thresholds that imply reduced rewarding effects of drugs over time (4; 11). Thus, under conditions of extended cocaine access, it is posited that brain stress systems are recruited and sensitized with concomitant reward system deficits and the drug is taken to alleviate negative emotional states associated with these dynamic allostatic changes (10).
Over the past decade, the lateral hypothalamus, largely via the hypocretin/orexin (HCRT) neuropeptide system, has been implicated in both stress and reward-seeking across many drug classes, including cocaine (12; 13). Despite limited cell numbers in the lateral hypothalamus, HCRT neurons project widely throughout the brain, targeting two G-protein-coupled receptors referred to as HCRT-receptor 1 and 2 (HCRT-R1 and -R2, respectively). These receptors have varying affinities for HCRT peptide ligands such that HCRT-1 peptide binds to both receptors, whereas HCRT-2 peptide binds selectively to HCRT-R2. HCRT neuronal projections include reciprocal connections to the extended amygdala, including the CeA, and other basal forebrain regions (14–16) implicated in the stress surfeit component of addiction. To date, HCRT-R1 signaling has been shown to mediate reinforcement of all major drug classes, including psychostimulants, nicotine, alcohol and opioids. However, HCRT-R1 antagonism has yielded no or limited effects on low-effort, fixed ratio (FR1) responding for cocaine self-administration (17–19), particularly in animals allowed ShA to the drug. Nonetheless, HCRT-R1 antagonism readily blocks stress-induced reinstatement of cocaine-seeking, while central HCRT-1 administration reinstates previously extinguished drug-seeking (20–22). The participation of HCRT in stress-related drug-seeking is not surprising given HCRT neurotransmission has been implicated in stress and high-arousal conditions in both humans and animals (12; 23; 24). These observations suggest that HCRT not only plays a significant role in maintaining the hyper-aroused state needed for cocaine-seeking, but also contributes to highly motivated compulsive-like cocaine-seeking. Consistent with this hypothesis, HCRT-R1 antagonists attenuate effortful, compulsive-like responding under a progressive ratio (PR) schedule (18; 25) and at higher FR schedules (e.g. FR5; 16). The current experiments sought to test the hypothesis that HCRT modulates compulsive-like cocaine-taking associated with LgA. We hypothesized that HCRT stress systems are recruited during compulsive-like drug-taking, and that blocking HCRT neurotransmission within the CeA would reduce compulsive-like cocaine-taking and attenuate stress-induced reinstatement of cocaine-seeking in LgA rats.
Previous studies demonstrate that extended access to cocaine self-administration alters plasticity at gamma-aminobutyric acid (GABA)ergic synapses within the medial CeA (27). We further hypothesized that dysregulated HCRT activity in the CeA contributes to the development or maintenance of negative emotional states associated with cocaine withdrawal via enhancement of GABAergic transmission. There is little evidence regarding the interaction between HCRT and GABAergic neurotransmission in the CeA of animals allowed extended cocaine access. Thus, we used in vitro electrophysiological techniques to measure spontaneous GABAergic transmission and the effects of HCRT stimulation and antagonism in CeA neurons from ShA and LgA rats compared with cocaine-naïve controls. We hypothesized that LgA rats would exhibit increased sponanteous GABAergic transmission in the CeA, and that this increased GABAergic transmission would be blocked by HCRT-receptor antagonism.
Combined, the results from our behavioral and electrophysiological studies demonstrate that HCRT within the CeA is dysregulated following LgA to cocaine and support a role for HCRT in the neuroplasticity associated with cocaine addiction.
Material and Methods
Animals
Adult male Wistar rats (N=56; Charles River, Raleigh, NC), weighing 225–275 g at the beginning of the experiments, were group housed in a temperature-controlled (22°C) vivarium on a 12 h/12 h light/dark cycle (lights off at 08:00 h) with ad libitum access to food and water. Behavioral testing occurred once per day during the dark/active cycle. Animals acclimated to the facility for at least 7 days before surgery. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute or the National Institute on Drug Abuse Intramural Research Program.
Surgery
Rats were anesthetized with isoflurane (1.5–2.5%) and prepared with chronic indwelling jugular vein catheters as previously described (5). Catheters were flushed daily with heparinized sterile saline (0.2 ml; 30 USP units/ml). Following self-administration training, a subset of rats underwent stereotaxic surgery under isoflurane anesthesia, and were implanted bilaterally with 23-gauge cannulae (Plastics One, Roanoke, VA) aimed at the CeA (flathead; A/P −2.4, M/L ±4.2, V/D −6.0, mm relevant to bregma). Cannulae were cemented into position using acrylic resin (Lang Dental Manufacturing Co., Wheeling, IL). A stainless steel stylet with a threaded plastic connector (Plastics One) was inserted into each cannula. Rats recovered for 5–7 days before behavioral testing.
Self-administration
Intravenous self-administration sessions were conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT) as previously described (28; 29). After the acquisition of cocaine self-administration, rats were given either 1 h (short access; ShA) or 6 h (long access; LgA) of daily access to FR1 cocaine self-administration for 14 escalation sessions (Figure S1). Following escalation, testing occurred under either FR1 or PR schedules of reinforcement (see supplemental Methods).
Electrophysiological Recordings
Coronal slices (300 μm) were prepared as previously described (30) in an ice-cold high-sucrose solution using a vibrating microtome. Whole-cell voltage-clamp recordings were made with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 2–5 kHz, digitized (Digidata 1440A; Molecular Devices), and stored using pClamp 10 software (Molecular Devices). The intracellular solution used for recordings was composed of (in mM): KCl 145; EGTA 5; MgCl2 5; HEPES 10; Na-ATP 2; Na-GTP 0.2. Recordings (Vhold= −60mV) were performed in the presence of 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM), DL-2-amino-5-phosphonovalerate (AP-5, 30 μM) and CGP55845A (1 μM).
Pharmacological Testing
SB-334867, a selective non-peptide HCRT-R1 antagonist (Tocris Bioscience, Bristol, UK), was dissolved in 5% Emulphor/5% dimethylsulfoxide (DMSO) and diluted with saline (systemic behavioral studies) or 100% DMSO (intra-CeA behavioral studies). SB-334867 was administered either intraperitoneally (0, 7.5, 15 and 30 mg/kg; 2 ml/kg) 30 min prior to behavioral testing, or intracranially (0, 10, 20 nmol; 250 nl) 15 min prior to testing. For cocaine self-administration testing, animals received all doses in a within-subject Latin-Square design. A cocaine self-administration session without SB-334867 treatment was performed between testing days. For electrophysiological studies, HCRT-1 was dissolved in aCSF (1 μM) and SB-334867 was first dissolved into a 50 mM stock in 100% DMSO and subsequently diluted to experimental concentrations (10 μM) in aCSF for a final concentration of <0.1% DMSO. Drug effects were compared to control baseline levels prior to drug application.
Doses for SB-334867 and the HCRT-1 peptide were based on a limited number of pilot studies and previously published data (17; 18; 20; 31–33) and had no discernible behavioral side effects. DMSO vehicle is shown to be acceptable for intra-cerebral injections and does not appear to result in neural tissue damage (34). We saw no aberrant tissue effects or significant change in behavior following intra-CeA administration of DMSO.
Reinstatement Testing
Following escalation, intra-CeA cannulated LgA rats underwent extinction training during which rats received intravenous saline substitution for cocaine until self-administration was reduced to ≤ 20% of their escalated baseline values for the total 6 h session (average 8 LgA sessions). Following extinction training, rats were acclimated to vehicle injections prior to testing and allowed a regular FR1 extinction session. On the day of testing, animals received yohimbine (2 mg/kg) 15 min following the intra-CeA infusion of either drug or vehicle and were tested for reinstatement of cocaine-seeking 10 min later (6 h session). The order of intra-CeA treatments (SB-334867 10 nmol or vehicle) was counterbalanced across animals, with extinction training and ≤ 20% baseline occurring prior to each treatment.
Histology and Data Selection
Following experimentation, cannulated animals were anesthetized and perfused transcardially with 4% formaldehyde. The brain was removed, placed in fixative, and subsequently frozen and sectioned (40 μm) through the rostral-caudal extent of the CeA. The ventral-most extent of the needle track was identified with Neutral Red stain. Data were included in the analyses only if the histology verified accurate placements of infusion needles (Figure S2).
Statistical Analysis
All data are expressed as means and standard errors of the mean (SEM). For behavioral studies, data were analyzed using either a two-way analysis of variance (ANOVA), with group (ShA and LgA) as the between-subjects factor and treatment (0, 7.5, 15, and 30 mg/kg) as the within-subjects factor, or a repeated-measure, one-way ANOVA (reinstatement testing only). When appropriate, post hoc comparisons were performed using Bonferroni’s multiple-comparison test, except when treatments were compared to a single control treatment (0 mg/kg) using Dunnett’s multiple-comparison test. Electrophysiological data were analyzed using a one-way ANOVA with post hoc analyses using Bonferroni’s multiple-comparison test or independent sample, two-tailed t-test. P values <0.05 were considered statistically significant for all tests.
Results
Systemic HCRT-R1 antagonist dose-dependently reduces FR1 cocaine self-administration in LgA rats
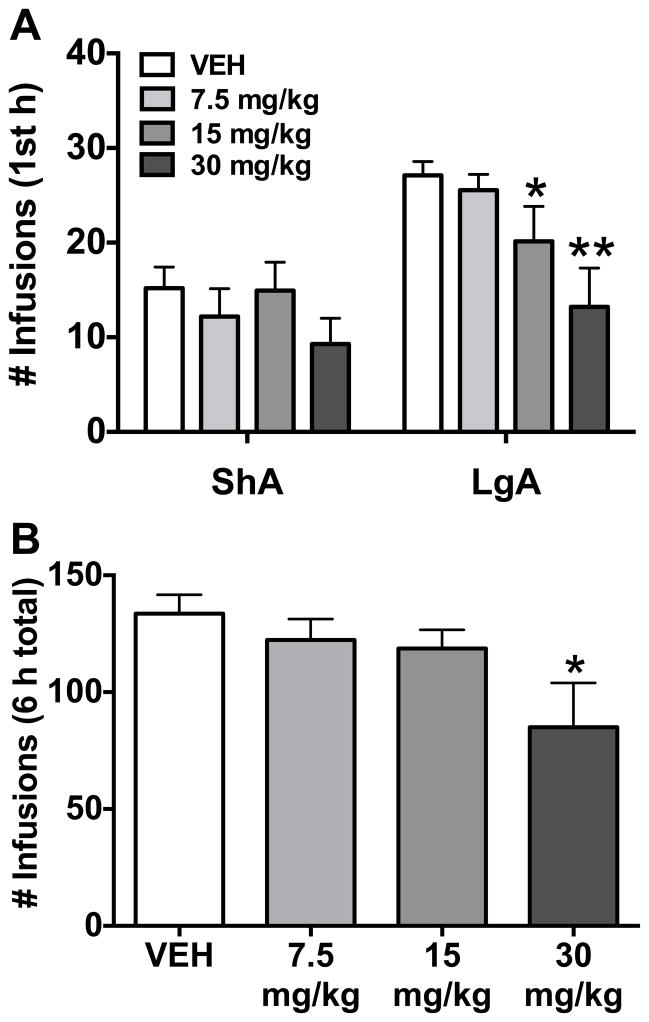
Rats were allowed ShA (1 h; n = 10) or LgA (6 h; n = 9; one rat was excluded due to loss of catheter patency) to cocaine self-administration on an FR1 schedule of reinforcement (Figure S1). Systemic HCRT-R1 antagonist (SB-334867) significantly reduced FR1 cocaine self-administration in the first hour in LgA rats at the doses of 15 and 30 mg/kg (Figure 1A; Group: F(1,17)= 6.79, p< 0.05; Treatment: F(3,51)= 9.86, p< 0.001; Group x treatment: F(3,51)= 3.07, p< 0.05). The SB-334867 treatment did not alter cocaine self-administration in ShA rats, as observed in previous studies. Further analysis of the LgA group showed the significant decrease in cocaine self-administration persisted for the entire 6 h session at the 30 mg/kg dose only (Figure 1B; Treatment: F(3,24)= 3.45, p< 0.05).
Figure 1.
Systemic HCRT-R1 antagonist, SB-334867, dose-dependently reduces fixed ratio (FR) cocaine self-administration in long access (LgA) but not short access (ShA) rats. The bars represent mean number (+ SEM) of cocaine infusions under an FR1 schedule of reinforcement. Administration of SB-334867 (0, 7.5, 15, or 30 mg/kg, i.p.) significantly decreases cocaine intake in LgA (n = 9), but not ShA (n = 10), rats during the first hour (A) and for the total 6 hour session (B; LgA only) of cocaine self-administration at the 30 mg/kg dose. *p < 0.05 and **p < 0.01 versus vehicle (VEH).
Systemic HCRT-R1 antagonist reduces PR cocaine self-administration in ShA and LgA rats
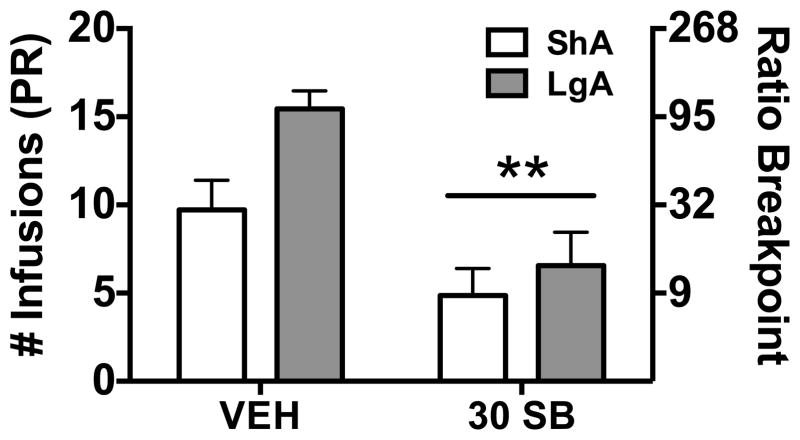
Following FR1 self-administration testing, the same rats allowed ShA (n = 7; 3 rats were excluded due to loss of catheter patency) or LgA (n = 9) were tested on a PR schedule of reinforcement. Both ShA and LgA rats showed a significant reduction in PR responding following a systemic injection of SB-334867 at the highest dose tested under FR1 (30 mg/kg) compared to vehicle-treated animals (Figure 2; Group: F(1,14)= 4.31, p= 0.06; Treatment: F(1,14)= 26.06, p< 0.001; Group x treatment: F(1,14)= 2.24, n.s.). These results are similar to previous reports that HCRT modulates effortful responding for cocaine self-administration in ShA animals (18; 25), but also extend previous findings to conditions of LgA to cocaine self-administration.
Figure 2.
Systemic administration of the HCRT-R1 antagonist, SB-334867, reduces cocaine self-administration in both short access (ShA) and long access (LgA) rats. The bars represent mean number (+ SEM) of cocaine infusions under a progressive ratio (PR; left axis) schedule of reinforcement, which correspond to the ratio breakpoint (right axis). Administration of SB-334867 (30 mg/kg; 30SB) significantly decreased cocaine self-administration in LgA (n = 9) and ShA (n = 7) rats. **p < 0.01 versus vehicle (VEH).
Intra-CeA HCRT-R1 antagonist reduces cocaine self-administration in LgA rats
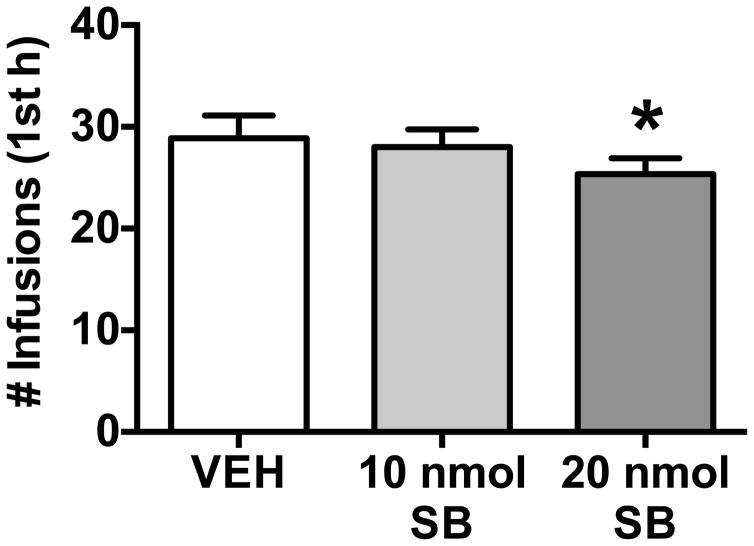
Based on the present results demonstrating systemic SB-334867 injection reduces cocaine self-administration (FR1) in LgA animals, we tested intra-CeA SB-334867 administration in LgA rats. A separate group of rats (n = 9; one rat was excluded due to misplaced cannulae) escalated their cocaine intake over 14 daily sessions and then received bilateral CeA cannulations. These LgA rats were tested under an FR1 schedule following bilateral intra-CeA infusions of SB-334867 (10 and 20 nmol) and vehicle in a Latin-square design. Intra-CeA SB-334867 reduced FR1 cocaine self-administration at the highest dose tested (20 nmol; Figure 3; F(2,16)= 4.07, p< 0.05). This drug effect did not appear to persist for the entire 6 h session (data not shown, F(2,16)= 0.34, n.s.). These results indicate a role for HCRT within the CeA in compulsive-like cocaine-taking associated with extended access to drug self-administration.
Figure 3.
Intra-CeA administration of the HCRT-R1 antagonist, SB-334867, reduces cocaine self-administration in long access rats under a fixed ratio (FR1) schedule of reinforcement. The bars represent mean number (+ SEM) of cocaine infusions during the first hour of a six-hour cocaine self-administration session. Administration of SB-334867 (0, 10, or 20 nmol SB) bilaterally into the CeA significantly decreases cocaine intake in LgA (n = 9) at the highest dose tested (20 nmol SB) versus vehicle (VEH). *p < 0.05 versus vehicle (VEH).
Intra-CeA HCRT-R1 antagonist attenuates stress-induced reinstatement of cocaine self-administration in LgA rats
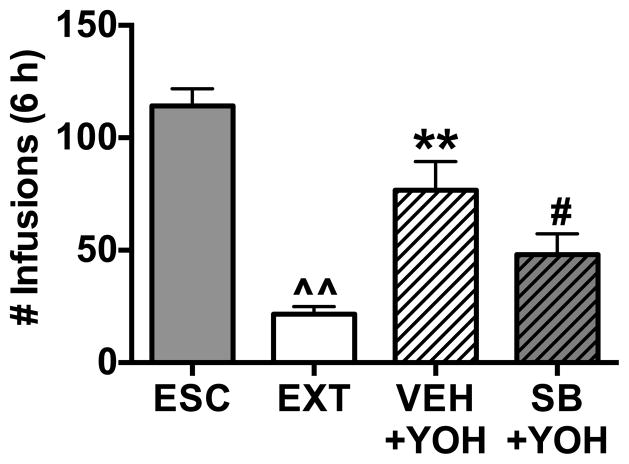
Another separate group of rats (n = 9; one rat was excluded due to misplaced cannula) escalated their cocaine intake over 14 daily sessions (6 h) and then received bilateral CeA cannulations. These LgA animals underwent extinction training until FR1 responding was reduced to ≤20% of their escalated 6 h baseline values. Rats were tested for reinstatement of cocaine-seeking following bilateral intra-CeA infusion of SB-334867 (10 nmol) or vehicle in a counter-balanced order, with extinction sessions occurring between testing days. Intra-CeA drug/vehicle infusions were followed by administration of a pharmacological stressor (yohimbine; 2 mg/kg) that, when administered alone, reinstates cocaine-seeking (19; 35). Intra-CeA SB-334867 pretreatment attenuated stress-induced reinstatement effects of yohimbine on cocaine-seeking compared with vehicle pretreatment in LgA rats (Figure 4; F(3,35)= 23.40, p< 0.001). These results suggest that the HCRT system within the CeA plays a role in stress-induced reinstatement of cocaine-seeking in LgA rats.
Figure 4.
Intra-CeA administration of the HCRT-R1 antagonist, SB-334867, blocks yohimbine-induced reinstatement of cocaine seeking in rats with a history of long access (LgA) to cocaine self-administration. The bars represent mean number (+ SEM) of cocaine infusions during the six-hour self-administration session. Rats were allowed to self-administer cocaine during escalation (ESC) and then underwent extinction (EXT) training with a saline self-administration substitution. Rats showed a significant reduction in FR1 responding following EXT. LgA rats were then pretreated with either vehicle (VEH) or SB-334867 (10 nmol; SB) bilaterally into the CeA before receiving a systemic injection of yohimbine (2 mg/kg; YOH). LgA rats showed a significant increase in FR1 responding following VEH + YOH treatments. In the same LgA rats, YOH-induced reinstatement of drug-seeking was attenuated with pretreatment of intra-CeA SB. Order of VEH and SB intra-CeA injections was counterbalanced to reduce order effects. ^^p < 0.01 versus ESC; **p < 0.01 versus EXT, #p < 0.05 versus VEH+YOH.
Baseline GABAergic neurotransmission in the CeA is elevated in LgA rats
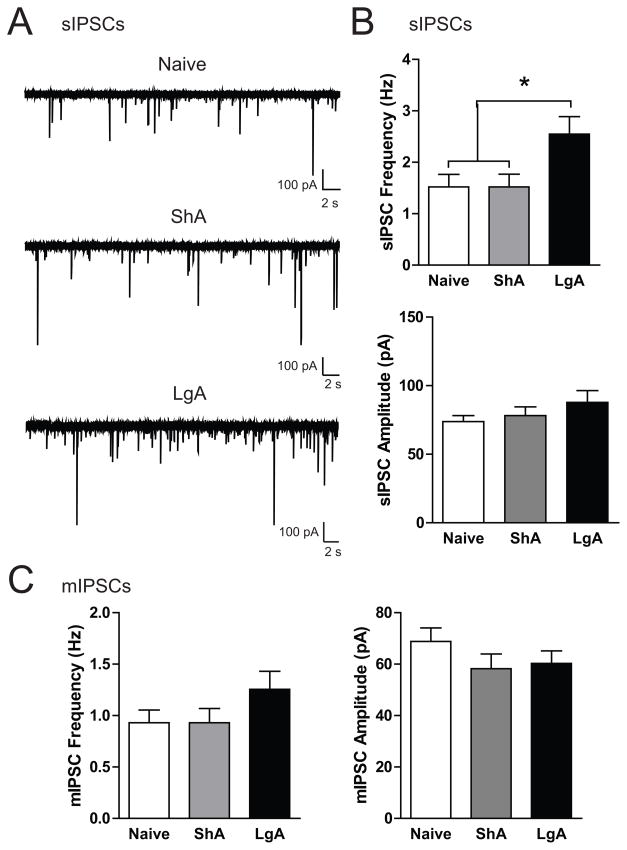
Inhibitory postsynaptic currents (IPSCs) were recorded in medial CeA neurons from naïve, ShA and LgA rats (n = 6, 5, and 5 rats, respectively). CeA neurons from LgA (n = 30) rats had a significantly higher spontaneous IPSC (sIPSC) frequency compared to neurons from naïve or ShA rats (n = 27 each; Figure 5B, upper panel; F(2,81)= 4.41, p< 0.05) with no difference in sIPSC amplitude (Figure 5B, lower panel; F(2,81)= 1.15, n.s.) or rise or decay time (Figure S3A). Miniature IPSCs (mIPSCS) were assessed in the presence of tetrodotoxin (1 μM). Although there was a trend for increased mIPSC frequency in CeA neurons from LgA rats (n = 13) compared to neurons from naïve (n = 15) or ShA rats (n = 13; Figure 5C, left panel; F(2,38)= 1.61, n.s.), the difference was not significant. There was no difference in mIPSC amplitude (Figure 5C, right panel; F(2,38)= 1.18, n.s.) or rise or decay time (Figure S3B). These results suggest that only LgA to cocaine results in elevated baseline GABAergic transmission in the CeA and that these alterations likely occur at the presynaptic and network level.
Figure 5.
Spontaneous GABA transmission is elevated in CeA neurons from rats allowed long access (LgA) to cocaine self-administration. A. Representative traces of spontaneous inhibitory postsynaptic currents (sIPSCs) in CeA neurons from naïve (top), short access (ShA, middle), and LgA rats (bottom). B. Average sIPSC frequency (upper panel) and sIPSC amplitude (lower panel) in CeA neurons from naïve (n = 27), ShA (n = 27), and LgA (n = 30) rats. C. Average miniature IPSC (mIPSC) frequency (left panel) and sIPSC amplitude (right panel) in CeA neurons from naïve (n = 15), ShA (n = 13), and LgA (n = 13) rats. *p< 0.05 versus Naïve and ShA.
HCRT-1 enhances GABAergic neurotransmission in the CeA of naïve but not ShA or LgA rats
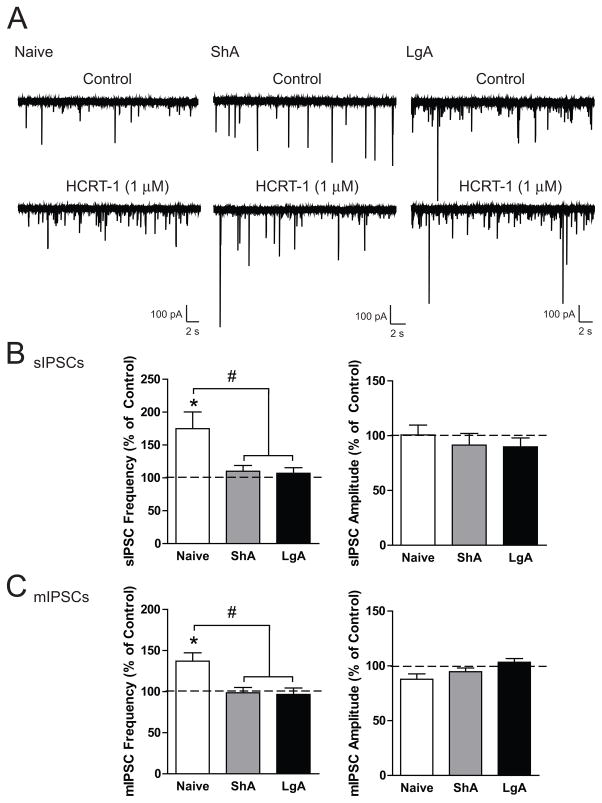
To determine if stimulating HCRT receptors modulates CeA-GABA transmission, we performed recordings in CeA neurons from naïve, ShA and LgA rats (n = 4–6, 3, and 3 rats, respectively) during application of the HCRT-1 peptide. HCRT-1 (1 μM) increased sIPSC frequency in CeA neurons from naïve rats (n = 6) but not from ShA or LgA rats (n = 6 each; Figure 6B, left panel; F(2,15)= 5.549, p<0.05), suggesting that HCRT-1 increases GABA release in the CeA of cocaine-naïve rats only. HCRT-1 produced no change in sIPSC amplitude (Figure 6B, right panel; F(2,15)= 0.39, n.s.) or rise or decay time (Figure S3C). We also performed mIPSC recordings in CeA neurons from naïve, ShA and LgA rats. HCRT-1 produced a significant increase in mIPSC frequency in neurons from naïve rats (n = 8) and not in neurons from ShA or LgA rats (n = 6 each; Figure 6C, left panel; F(2,17)= 7.09, p< 0.01), indicating that HCRT-1 enhances vesicular GABA release in the CeA of cocaine-naïve but not ShA or LgA rats. No change in mIPSC amplitude was noted (Figure 6C, right panel; F(2,17)= 3.47, n.s.) or rise or decay time (Figure S3D). These data indicate that stimulation of HCRT-receptors within the CeA increases both action potential-dependent and spontaneous vesicular GABAergic neurotransmission via a presynaptic mechanism of action that impacts both basal and activity-dependent conditions.
Figure 6.
Stimulation of HCRT-R1 enhances GABAergic neurotransmission in the CeA of naïve but not short access (ShA) or long access (LgA) rats. A. Representative traces of sIPSCs in CeA neurons from naïve (left), ShA (middle), and LgA (right) before and during superfusion of the peptide ligand HCRT-1 (1 μM). B. Average change in sIPSC frequency (left) and sIPSC amplitude (right) in CeA neurons from naïve (n = 6), ShA (n = 6), and LgA (n = 6) rats following superfusion of HCRT-1. C. Average mIPSC frequency (left) and sIPSC amplitude (right) in CeA neurons from naïve (n = 8), ShA (n = 6), and LgA (n = 6) rats following superfusion of HCRT-1. *p < 0.05 by one-sample t-test, #p < 0.05 versus ShA and LgA.
HCRT-R1 antagonist blocks enhanced GABAergic neurotransmission in the CeA of LgA rats
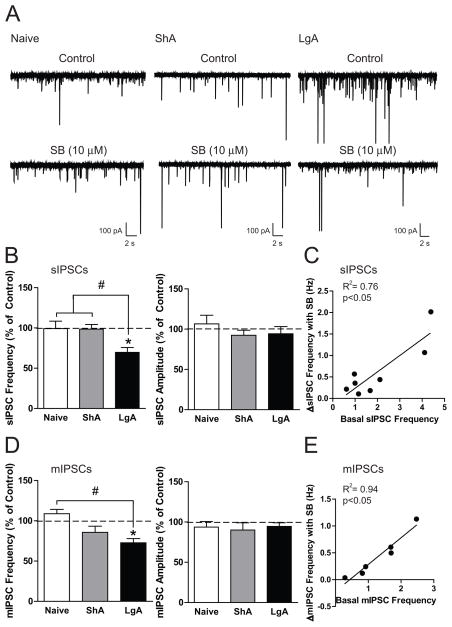
To determine if blocking HCRT-R1 modulates CeA-GABA transmission and if this modulation is altered by cocaine exposure, we performed sIPSC recordings in CeA neurons from naïve, ShA and LgA rats (n = 4, 3, 3 rats, respectively) during SB-334867 application. SB-334867 (10 μM) did not alter sIPSC frequency in CeA neurons from naïve (n = 5) or ShA rats (n = 8) but significantly decreased sIPSC frequency in neurons from LgA rats (n = 8; Figure 7B, left panel; F(2,18)= 6.88, p< 0.01), suggesting that HCRT-R1 regulates basal CeA GABA release in LgA rats. No change in sIPSC amplitude (Figure 7B, right panel; F(2,18)= 0.12, n.s.) or rise or decay time (Figure S3E) was observed in naïve, ShA, or LgA rats. The magnitude of the decrease in sIPSC frequency with HCRT-R1 antagonism was positively correlated with the basal sIPSC frequency (Figure 7C; slope= 0.38 ± 0.1, R2= 0.76, p< 0.01). We also performed mIPSC recordings in CeA neurons from naïve, ShA and LgA rats. SB-334867 produced a significant decrease in mIPSC frequency in CeA neurons from LgA rats (n = 6), a non-significant decrease in mIPSC frequency in neurons from ShA rats (n = 6) and no change in mIPSC frequency in neurons from naïve rats (n = 6; Figure 7D, left panel; F(2,15)= 7.79, p< 0.01). No change in mIPSC amplitude (Figure 7D, right panel; F(2,15)= 0.12, n.s.) or rise or decay time (Figure S3F) was observed in naïve, ShA, or LgA rat neurons. The magnitude of the decrease in mIPSC frequency with HCRT-R1 antagonism was also positively correlated with the basal mIPSC frequency (Figure 7E; slope= 0.50 ± 0.1, R2= 0.94, p< 0.05). These results suggest that the increased GABAergic neurotransmission in LgA rats is partially due to HCRT-R1 and that this increased signaling can be reversed by HCRT-R1 blockade.
Figure 7.
Blockade of HCRT-R1 reduces enhanced GABAergic neurotransmission in the CeA of long access (LgA) rats. A. Representative traces of sIPSCs in CeA neurons from naïve (left), short access (ShA, middle), and LgA rats (right) before and during superfusion of the HCRT-R1 antagonist, SB-334867 (SB; 10 μM). B. Average change in sIPSC frequency (left) and sIPSC amplitude (right) in CeA neurons from naïve (n = 5), ShA (n = 8), and LgA (n = 8) rats following superfusion of SB. C. The correlation between the basal sIPSC frequency and the change in sIPSC frequency with SB in CeA neurons from LgA (n = 8) rats. D. Average mIPSC frequency (left) and sIPSC amplitude (right) in CeA neurons from naïve (n = 6), ShA (n = 6), and LgA (n = 6) rats following superfusion of SB. E. The correlation between the basal mIPSC frequency and the change in mIPSC frequency with SB in CeA neurons from LgA (n = 6) rats. *p< 0.05 by one-sample t-test, #p < 0.05 versus Naive.
Discussion
Escalation of cocaine intake is thought to reflect an important aspect of the transition from initial controlled drug use to uncontrolled drug dependence, or addiction, and has been associated with dysfunction of brain reward and stress systems (36). The HCRT neuropeptide system has been associated with both stress and drug-seeking behaviors (for review, 11; 12). Our overall hypothesis is that compulsive-like cocaine-taking is driven, in part, by negative emotional states during withdrawal via upregulation of brain motivational and stress systems, including HCRT within the CeA.
A role for HCRT in an enhanced state of motivation
In the present study, we report that the HCRT-R1 antagonist SB-334867 decreased cocaine self-administration under an FR1 schedule of reinforcement in rats allowed LgA to cocaine, with no significant effect in rats given ShA to cocaine. When tested under a PR schedule, both ShA and LgA rats showed a reduction in responding for cocaine following SB-334867 administration. For ShA animals, the attenuation of cocaine-taking following SB-334867 treatment under PR, but not FR1, is consistent with what has been observed in previous studies (17; 18). These results suggest that in the case of cocaine self-administration, HCRT may modulate some aspect of drug-seeking/taking behavior other than the acute, positively-reinforcing actions of cocaine. Consistent with this hypothesis, central HCRT-1 increases intracranial self-stimulation thresholds, suggesting a potential inhibitory action on reward systems, pointing to a mechanism other than an increase in positive affect sensitivity (20). We hypothesize that HCRT not only plays a role in maintaining an adequate state of arousal needed for cocaine-seeking, but HCRT is also recruited under conditions of compulsive-like cocaine-taking associated with extended drug access in which enhanced motivational states are required. Consistent with this hypothesis, we demonstrate a role for HCRT neurotransmission in rats allowed extended cocaine access in that HCRT-R1 antagonism attenuated self-administration under both FR1 and PR schedules in LgA rats. Other studies also show HCRT-R1 antagonism attenuates effortful, compulsive-like responding under higher FR schedules (e.g. FR5; 16) even in animals allowed only ShA to cocaine. In the case of ShA self-administration under higher FR schedules, animals are in a condition that requires greater effort and as a result are more sensitive to motivational challenges. Together, these observations suggest that HCRT modulates compulsive-like, motivation-enhanced behavior, and may be associated with the negative emotional state changes that contribute to excessive drug-taking.
HCRT neurotransmission in the CeA is associated with compulsive-like cocaine intake
It is hypothesized that the aversive emotional state comprising anxiety and dysphoric-like effects following repeated cocaine withdrawal and acute or protracted abstinence is associated with addiction (4; 37). Escalated, compulsive-like cocaine-taking associated with addiction is mediated, in part, by the upregulation of brain stress systems (e.g., dynorphin and corticotropin releasing factor), particularly within subregions of the extended amygdala including the CeA. Given the substantial HCRT projections into the CeA and the established role for HCRT in stress (12; 14–16), we hypothesized that HCRT is an additional brain arousal/stress system recruited during compulsive-like drug-taking and that the CeA is a potential site of action for HCRT-mediated neuroplasticity. Our results show that intra-CeA HCRT neurotransmission plays an important role in compulsive-like cocaine self-administration in LgA rats in that when administered directly into the CeA, SB-334867 reduced cocaine-taking in LgA rats. Furthermore, intra-CeA SB-334867 blocked stress-induced reinstatement of cocaine-seeking in LgA rats. These observations suggest that HCRT dysregulation may promote neuroadapations within the CeA of animals allowed escalated cocaine intake. However, it remains to be determined whether HCRT neurotransmission within the CeA also modulates cocaine-seeking in rats allowed ShA to cocaine, particularly under conditions that require increased effort.
Enhanced HCRT modulation of GABAergic signaling in the CeA is associated with extended access to cocaine and can be reversed by an HCRT-R1 antagonist
Electrophysiological studies support our behavioral findings in that HCRT in the CeA acts, in part, by modulating GABAergic neurotransmission and this modulation is selectively altered in rats with a history of cocaine exposure. Notably, LgA rats displayed increased GABA release in the CeA that was not observed in naïve or ShA rats. The significant increase in action potential-dependent GABA release, as well as the trend for increased action potential-independent vesicular GABA release, suggest that LgA to cocaine engages the HCRT system to upregulate both stimulated and spontaneous GABA, with potentially distinct mechanisms of release (38). These findings corroborate previous work reporting increased evoked GABA release in the CeA following cocaine self-administration (27). The previous study found increased evoked GABA release in both ShA and LgA rats, a discrepancy that may be due to the stimulation paradigm (27) as opposed to the spontaneous neurotransmission examined in this study. We also found that the HCRT system can directly modulate GABA signaling in the CeA and that this modulation is altered by exposure to cocaine. Stimulation of HCRT-R1 increases GABA release in the CeA of naïve rats, but this increase is absent in both ShA and LgA rats, suggesting that the HCRT system is recruited early in cocaine exposure, but does not impact GABA signaling until after more prolonged exposure. Consistent with this interpretation, antagonism of HCRT-R1 significantly reduced GABA release in CeA neurons from LgA (versus ShA or naïve) rats. Collectively, these results suggest that the CeA GABA system is a critical site for neuroadaptations following prolonged cocaine-taking and demonstrate that elevations in GABA signaling following extended cocaine access are due, in part, to recruitment of HCRT.
In summary, we showed that HCRT-R1 blockade reduced cocaine self-administration and stress-induced reinstatement in LgA rats, suggesting a role for HCRT modulation of compulsive-like intake associated with dependence. Furthermore, these behavioral data are supported by electrophysiological studies demonstrating enhanced GABAergic transmission in the CeA of LgA rats that is reduced with HCRT-R1 antagonism. However, the present results do not preclude additional functional mediation of escalated cocaine-taking by HCRT-R2 in the CeA and/or other stress-related brain regions (e.g., BNST, NAc). HCRT-R2 or dual receptor antagonists should be considered for future experiments. Nonetheless, the current findings clearly suggest HCRT-R1 neurotransmission contributes to the etiology of compulsive-like cocaine intake.
Supplementary Material
Acknowledgments
Funding: This research was supported by grants from the National Institute on Drug Abuse (DA004043, DA004398), National Institute of Alcohol Abuse and Alcoholism (AA007456, AA023002, AA006420, AA021491), and by the Pearson Center for Alcoholism and Addiction Research. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berlin GS, Hollander E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr. 2014;19:62–68. doi: 10.1017/S1092852913000722. [DOI] [PubMed] [Google Scholar]
- 2.Jonkman S, Pelloux Y, Everitt BJ. Drug intake is sufficient, but conditioning is not necessary for the emergence of compulsive cocaine seeking after extended self-administration. Neuropsychopharmacology. 2012;37:1612–1619. doi: 10.1038/npp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 5.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- 6.Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology. 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantsch JR, Yuferov V, Mathieu-Kia A-M, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF. The dark side of emotion: the addiction perspective. Eur J Pharmacol. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willuhn I, Burgeno LM, Groblewski PA, Phillips PEM. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. JNeurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, Wree A. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- 17.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340:801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de LL. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. \iNature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Borgland SL, Chang S-J, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallupi M, Wee S, Edwards S, Whitfield TW, Jr, Oleata CS, Luu G, et al. Kappa Opioid Receptor-Mediated Dysregulation of Gamma-Aminobutyric Acidergic Transmission in the Central Amygdala in Cocaine Addiction. Biological Psychiatry, Developmental Impact of Cocaine. 2013;74:520–528. doi: 10.1016/j.biopsych.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A Combination of Buprenorphine and Naltrexone Blocks Compulsive Cocaine Intake in Rodents Without Producing Dependence. Sci Transl Med. 2012;4:146ra110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorrilla EP, Wee S, Zhao Y, Specio S, Boutrel B, Koob GF, Weiss F. Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addict Biol. 2012;17:300–308. doi: 10.1111/j.1369-1600.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, et al. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodnik ZD, Bernstein DL, Prince CD, España RA. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behavioural Brain Research. 2015;291:377–384. doi: 10.1016/j.bbr.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corcoran A, Richerson G, Harris M. Modulation of respiratory activity by hypocretin-1 (orexin A) in situ and in vitro. Adv Exp Med Biol. 2010;669:109–113. doi: 10.1007/978-1-4419-5692-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dergacheva O, Philbin K, Bateman R, Mendelowitz D. Hypocretin-1 (orexin A) prevents the effects of hypoxia/hypercapnia and enhances the GABAergic pathway from the lateral paragigantocellular nucleus to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2011;175:18–23. doi: 10.1016/j.neuroscience.2010.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blevins JE, Stanley BG, Reidelberger RD. DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav. 2002;71:277–282. doi: 10.1016/s0091-3057(01)00659-1. [DOI] [PubMed] [Google Scholar]
- 35.Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.