Summary
Hepatic progenitor cells (HPCs) appear in response to several types of chronic injury in the human and rodent liver that often develop into liver fibrosis, cirrhosis, and primary liver cancers. However, the contribution of HPCs to the pathogenesis and progression of such liver diseases remains controversial. HPCs are generally defined as cells that can differentiate into hepatocytes and cholangiocytes. In this study, however, we found that HPCs isolated from the chronically injured liver can also give rise to myofibroblasts as a third type of descendant. While myofibroblast differentiation from HPCs is not significant in culture, during tumor development, HPCs can contribute to the formation of the tumor microenvironment by producing abundant myofibroblasts that might form a niche for tumor growth and survival. Thus, HPCs can be redefined as cells with a potential for differentiation into myofibroblasts that is specifically activated during tumor formation.
Keywords: liver, progenitor cell, myofibroblast, tumor microenvironment
Highlights
-
•
HPCs isolated from the chronically injured liver can give rise to myofibroblasts
-
•
The frequency of myofibroblast production from HPCs is low in culture
-
•
HPCs can give rise to a number of myofibroblasts during tumor development
-
•
HPCs themselves can contribute to the formation of the tumor microenvironment
In this article, Suzuki and colleagues found that hepatic progenitor cells (HPCs) have a potential for trilineage differentiation into hepatocytes, cholangiocytes, and myofibroblasts. Although the frequency of myofibroblast production from HPCs is low in culture, HPCs can give rise to a number of myofibroblasts during tumor development and contribute to the formation of the tumor microenvironment.
Introduction
Hepatic progenitor cells (HPCs) have histologic features of biliary lineage cells and appear in response to several types of chronic injury in the adult human liver, including hepatitis C virus infection, hemochromatosis, α-1-antitrypsin deficiency, alcoholic liver disease, and nonalcoholic fatty liver disease (Lowes et al., 1999, Brunt et al., 2010, Nobili et al., 2012). These liver pathologies are associated with an increased risk of liver fibrosis, cirrhosis, and primary liver cancers (Prior, 1988, Deugnier et al., 1993, Tsukuma et al., 1993, Clouston et al., 2005, Fairbanks and Tavill, 2008, Gao and Bataller, 2011, Carpino et al., 2013). Thus, HPCs are suggested to play a critical role in the onset and progression of such liver diseases. Experimental rodent models have been developed (Farber, 1956, Shinozuka et al., 1978, Tatematsu et al., 1984, Lemire et al., 1991, Factor and Radaeva, 1993, Preisegger et al., 1999) to investigate the properties of HPCs in detail. In these models, HPCs (often called oval cells) that appear in response to chronic liver injury induced by potential carcinogens are histologically identified as cells that express biliary markers and proliferate in portal areas of the hepatic lobule, similar to the case for human liver pathologies. Normally, rodent HPCs are thought to be inefficient in the production of hepatocytes during recovery from chronic liver injury, because hepatocytes themselves are highly proliferative (Schaub et al., 2014, Tarlow et al., 2014, Yanger et al., 2014, Jörs et al., 2015). However, in some specific conditions of liver damage, HPCs can actually give rise to hepatocytes in vivo to promote liver regeneration (Español-Suñer et al., 2012, Lu et al., 2015), suggesting that the behavior of HPCs is tightly regulated in accordance with the context of liver injury. Meanwhile, although extensive studies of HPCs have been performed in commonly used rodent models, it remains a matter of debate whether HPCs contribute to the development of liver diseases.
The liver is composed of multiple cellular lineages, including hepatocytes, cholangiocytes (biliary epithelial cells), hepatic stellate cells, Kupffer cells (hepatic macrophages), myofibroblasts, vascular and sinusoidal endothelial cells, and circulating hematopoietic cells. HPCs are defined as cells that can differentiate into two types of hepatic epithelial cells, namely hepatocytes and cholangiocytes, and are thus designated bipotent progenitor cells (Fausto and Campbell, 2003). Meanwhile, mesenchymal cells in the liver may be derived from their own progenitor cells or provided by bone marrow (BM)-derived cells that flow into the liver (Baba et al., 2004, Asahina et al., 2009, Si-Tayeb et al., 2010), although both of these origins remain controversial. In liver diseases, not only epithelial cells but also mesenchymal cells synchronously contribute to the disease occurrence and progression. Thus, it is suggested that the interaction between HPCs and mesenchymal cells is important for the initiation and development of liver diseases, although the mechanisms remain unclear.
In our previous study, HPCs were identified as cells that can form large colonies (LCs) in single-cell cultures of CD133+ biliary lineage cells isolated from the chronically injured adult mouse liver (Suzuki et al., 2008). In fact, CD133+ LC-forming cells have the properties of HPCs, being capable of giving rise to both hepatocytes and cholangiocytes as descendants, while maintaining undifferentiated cells by self-renewing cell divisions. In the present study, we carefully observed clonal cultures of HPCs and found that, in addition to hepatocytes and cholangiocytes, a small number of myofibroblasts were present. Although the frequency of myofibroblast differentiation from HPCs remained low in culture, a number of myofibroblasts were generated from HPCs and formed a microenvironment in tumors arising from p53-deficient (p53−/−) HPCs. Our findings demonstrate that HPCs have the potential for trilineage differentiation into three cell types, namely hepatocytes, cholangiocytes, and myofibroblasts, and that during tumor development HPCs can create a particular microenvironment by producing abundant myofibroblasts that might form a niche for tumor growth and survival.
Results
Trilineage Differentiation Potential of HPCs
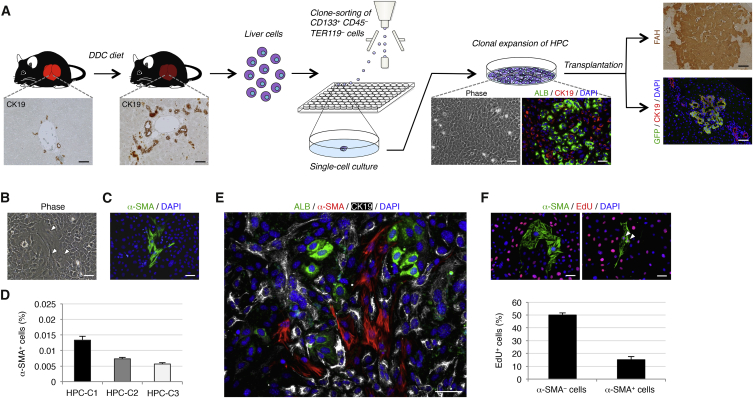
Based on the methods described in our previous study (Suzuki et al., 2008), we obtained HPCs from chronically injured adult mouse liver (Figure 1A). First, we fed mice with a diet containing 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) for 2 weeks to induce HPCs in the chronically injured liver. Next, CD133+CD45−TER119− biliary lineage cells were isolated by flow cytometry and cultured under single-cell culture conditions. About 1% of the isolated cells was able to form LCs, which we designated CD133+ LC-forming cells. These CD133+ LC-forming cells were maintained by self-renewing cell divisions in clonal cultures and spontaneously gave rise to both hepatocytes and cholangiocytes as descendants. Moreover, CD133+ LC-forming cells were able to partially reconstitute the hepatic lobule and form the biliary ductal structures after transplantation into the livers of fumarylacetoacetate hydrolase (Fah)-deficient (Fah−/−) mice and DDC-administered mice, respectively. Thus, we finally concluded that CD133+ LC-forming cells could be identified as HPCs in the chronically injured adult mouse liver.
Figure 1.
HPCs Isolated from the Chronically Injured Adult Mouse Liver Have Trilineage Differentiation Potential
(A) Experimental procedure to isolate and characterize HPCs. Wild-type mice were administered DDC for induction of CK19+ biliary lineage cells that contain a fraction of HPCs in the chronically injured liver. The liver tissues were then dissociated into single cells, and the CD133+CD45−TER119− biliary lineage cells were isolated by flow cytometry and clonally cultured in 96-well plates. HPCs that formed LCs and expanded in clonal culture exhibited the features of epithelial cells and produced hepatocytes and cholangiocytes as descendants, while maintaining undifferentiated cells by undergoing self-renewing cell divisions. Upon transplantation, HPCs marked by expression of GFP were also capable of reconstituting the hepatic lobule as FAH+ hepatocytes and forming the biliary ductal structures by differentiating into CK19+ cholangiocytes. We chose three independent HPC clones for examination.
(B) In clonal cultures of HPCs, a small number of cells with the morphology of mesenchymal cells were present (arrowheads).
(C) Immunofluorescence staining of α-SMA was conducted for cells in a clonal culture of HPCs.
(D) The percentages of cells immunoreactive for α-SMA among HPC clones (HPC-C1, HPC-C2, and HPC-C3) were calculated after counting ∼1 × 105 cells in individual culture dishes.
(E) Co-immunofluorescence staining of ALB with α-SMA and CK19 was conducted for cells in a clonal culture of HPCs.
(F) Co-immunofluorescence staining of α-SMA with EdU was conducted for cells in a clonal culture of HPCs (arrowheads: α-SMA+ EdU+ cells), and the percentages of cells immunoreactive for EdU in α-SMA− or α-SMA+ cells were calculated after counting ∼800 or ∼20 cells, respectively, in individual culture dishes. The data represent means ± SD of three technical replicates (n = 3). DNA was stained with DAPI.
Scale bars, 50 μm. See also Figures S1 and S2.
Isolated HPCs and their derivatives exhibit the features of epithelial cells in clonal cultures. However, among the epithelial cells we found a small number of cells with the morphology of mesenchymal cells (Figure 1B). These mesenchymal cells were characterized as myofibroblasts by their expression of α-smooth muscle actin (α-SMA) (Figure 1C). On average, about 0.01% of cells in cultures of three distinct HPC clones were identified as α-SMA+ myofibroblasts (Figure 1D). These myofibroblasts were totally different from hepatocytes and cholangiocytes expressing albumin (ALB) and cytokeratin 19 (CK19), respectively (Figure 1E). Moreover, the number of proliferating cells in the myofibroblast population was much lower than those in the other cell populations (Figure 1F). These data demonstrated that HPC-derived myofibroblasts were independently present in clonal cultures of HPCs, but the possibility remained that α-SMA+ cells were originally contained in the isolated CD133+CD45−TER119− cells and differentiated into hepatocytes and cholangiocytes. However, immunofluorescence analyses revealed that no α-SMA+ cells were present in the initially isolated CD133+CD45−TER119− cells (Figure S1). Moreover, because hepatocytes and cholangiocytes undergo epithelial-to-mesenchymal transition (EMT) through activation of transforming growth factor β (TGF-β) signaling in culture (Taura et al., 2010, Chu et al., 2011), we examined whether HPC-derived myofibroblasts appeared in the result of EMT by culturing an HPC clone with the TGF-β receptor inhibitor SB431542. Our data showed that there was no significant difference in the frequency of α-SMA+ cells between the cultures of HPCs in the presence and absence of SB431542 (Figure S2). Thus, the generation of myofibroblasts from HPCs may not be due to a spontaneous induction of EMT based on the culture medium containing TGF-β, while the possibility of involvement of EMT cannot completely be excluded. Taken together, our findings indicate that HPCs derived from the chronically injured adult mouse liver have trilineage differentiation potential, while the production ratio of myofibroblasts from HPCs is much lower than those of hepatocytes and cholangiocytes.
HPCs Persistently Produce a Small Number of Myofibroblasts in Clonal Subcultures
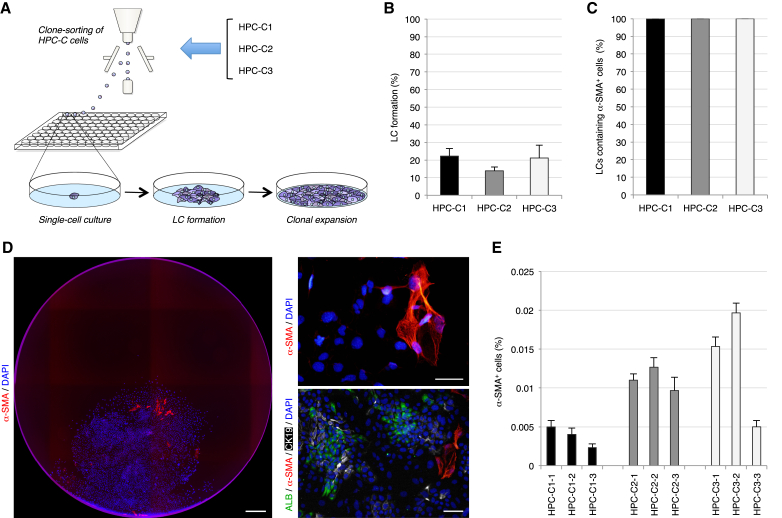
There were two possibilities underlying these unexpected findings, namely that HPCs isolated from the chronically injured liver can temporarily produce myofibroblasts, and that HPCs have a potential to differentiate into myofibroblasts even after self-renewing cell divisions. To investigate these possibilities, we conducted subcloning experiments for three different HPC clones. The cells in cultures of the three HPC clones were trypsinized, subjected to clone sorting by flow cytometry, and cultured under single-cell culture conditions (Figure 2A). About 20% of the re-sorted cells formed LCs again, and all of the LCs contained α-SMA+ myofibroblasts, in addition to ALB+ hepatocytes and CK19+ cholangiocytes (Figures 2B–2D). Moreover, the percentages of α-SMA+ cells within the cultures of three representative subclones of the individual primary HPC clones did not differ significantly from those within cultures of the primary HPC clones (Figure 2E). Thus, our data indicate that HPCs isolated from the chronically injured liver have a potential to undergo self-renewal and infrequently, but persistently, produce myofibroblasts in addition to abundant hepatocytes and cholangiocytes in clonal subcultures.
Figure 2.
HPCs Have a Potential to Persistently Produce Myofibroblasts in Clonal Subcultures
(A) Experimental procedure to perform subcloning experiments for three different HPC clones (HPC-C1, HPC-C2, and HPC-C3). Cells in cultures of each HPC clone underwent clone sorting by flow cytometry and were clonally cultured in 96-well plates. After LC formation and clonal expansion, three representative subclones of the individual primary HPC clones were chosen for examination.
(B and C) The numbers of LCs in the wells of 96-well plates (B) and the numbers of LCs containing α-SMA+ cells in LCs (C) were counted. The percentages are shown.
(D) Immunofluorescence staining of α-SMA and co-immunofluorescence staining of ALB with α-SMA and CK19 were conducted for cells in LCs formed from cells in cultures of a primary HPC clone. DNA was stained with DAPI. Scale bars, 0.5 mm (left panel) and 50 μm (right panels).
(E) The percentages of cells immunoreactive for α-SMA among subclones of the three primary HPC clones were calculated after counting ∼1 × 105 cells in individual culture dishes. The data represent means ± SD of three technical replicates (n = 3).
Hepatoblasts Isolated from the Developing Mouse Liver Can Rarely Give Rise to Myofibroblasts in Clonal Cultures
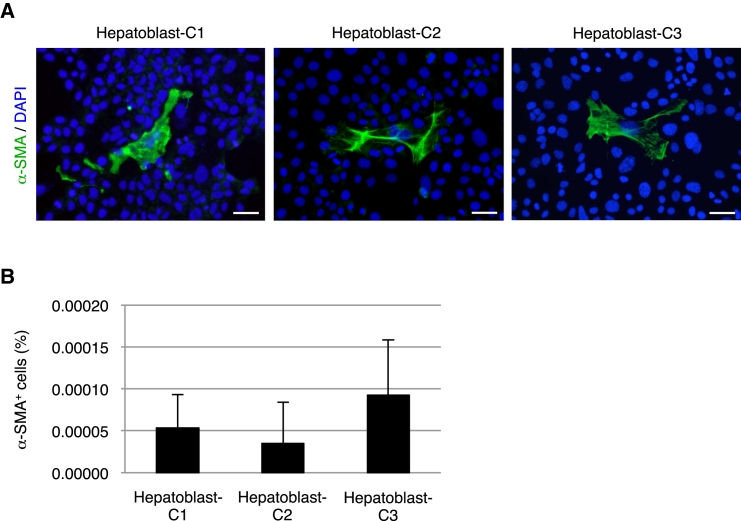
In addition to the injured adult liver, myofibroblasts are also present around the portal areas of the hepatic lobule during liver development. Similar to the HPCs in the chronically injured liver, hepatoblasts that act as hepatic stem/progenitor cells in the developing liver give rise to both hepatocytes and cholangiocytes as descendants and play a central role in liver organogenesis (Lemaigre and Zaret, 2004). Based on the present results, we examined whether hepatoblasts are able to produce myofibroblasts in addition to hepatocytes and cholangiocytes. In our previous study (Suzuki et al., 2002), we established a method to isolate hepatoblasts from the developing mouse liver and to culture these cells under single-cell culture conditions, similar to the case for the HPCs used in this study. Immunofluorescence analyses revealed that clonal cultures of three different clones of hepatoblasts contained α-SMA+ myofibroblasts, while the percentages of these cells were quite low (less than 0.0001%) (Figures 3A and 3B). Thus, similar to HPCs in the chronically injured liver, hepatoblasts also have a potential to produce myofibroblasts, in addition to hepatocytes and cholangiocytes. However, because myofibroblast differentiation from hepatoblasts is rare, it is suggested that these hepatoblast-derived myofibroblasts do not positively contribute to liver development, and that myofibroblasts may be mainly provided by the other types of cells.
Figure 3.
Hepatoblasts Have a Potential to Differentiate into Myofibroblasts
(A) Immunofluorescence staining of α-SMA was conducted for cells in cultures of hepatoblast clones (Hepatoblast-C1, Hepatoblast-C2, and Hepatoblast-C3), following isolation from the developing mouse liver. DNA was stained with DAPI. Scale bars, 50 μm.
(B) The percentages of cells immunoreactive for α-SMA among the hepatoblast clones were calculated after counting ∼3 × 106 cells in individual culture dishes. The data represent means ± SD of three technical replicates (n = 3).
HPC-Derived Myofibroblasts Form a Microenvironment in Tumors Arising from p53−/− HPCs
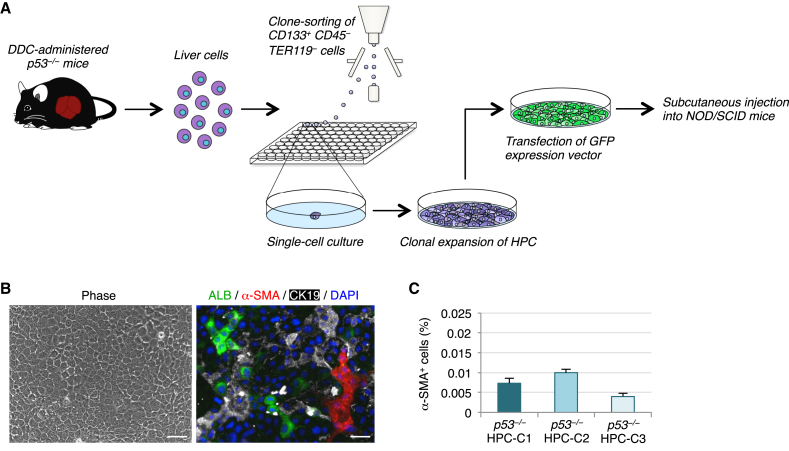
The above data suggest that, because the frequency of myofibroblast production from HPCs is very low, HPC-derived myofibroblasts do not positively contribute to the restoration and maintenance of the liver. Because HPCs are normally observed in a variety of liver pathologies in humans and rodents, we speculated that myofibroblasts derived from HPCs might play a role in liver diseases, especially liver cancers. To examine this possibility, we used HPCs obtained from the chronically injured p53−/− mouse liver that form tumors following subcutaneous injection into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (Suzuki et al., 2008). For this purpose, we isolated CD133+CD45−TER119− cells from the livers of DDC-treated p53−/− mice and cultured these cells under single-cell culture conditions. We observed that CD133+ LC-forming cells were maintained with self-renewing cell divisions in clonal cultures (Figure 4A). Similar to the HPCs obtained from the livers of DDC-treated wild-type mice, p53−/− HPCs and their derivatives were morphologically identifiable as epithelial cells and gave rise to myofibroblasts, as well as hepatocytes and cholangiocytes, in clonal cultures (Figure 4B). Interestingly, the percentages of α-SMA+ myofibroblasts in cultures of three representative p53−/− HPC clones were not much changed compared with those in cultures of the wild-type mice-derived HPC clones (Figure 4C).
Figure 4.
p53−/− HPCs Give Rise to a Small Number of Myofibroblasts in Culture
(A) Experimental procedure to prepare p53−/− HPCs for in vitro analysis and transplantation. The CD133+CD45−TER119− cells isolated from the livers of DDC-treated p53−/− mice were clonally cultured in 96-well plates. HPCs that formed LCs and expanded in clonal culture were analyzed in culture and used for transplantation into NOD/SCID mice following transfection of a GFP-expressing vector.
(B) Representative morphology of cells in a clonal culture of p53−/− HPCs (left panel). Co-immunofluorescence staining of ALB with α-SMA and CK19 was conducted for cells in a clonal culture of p53−/− HPCs (right panel). DNA was stained with DAPI. Scale bars, 50 μm.
(C) The percentages of cells immunoreactive for α-SMA among p53−/− HPC clones (p53−/− HPC-C1, p53−/− HPC-C2, and p53−/− HPC-C3) were calculated after counting ∼1 × 105 cells in individual culture dishes. The data represent means ± SD of three technical replicates (n = 3).
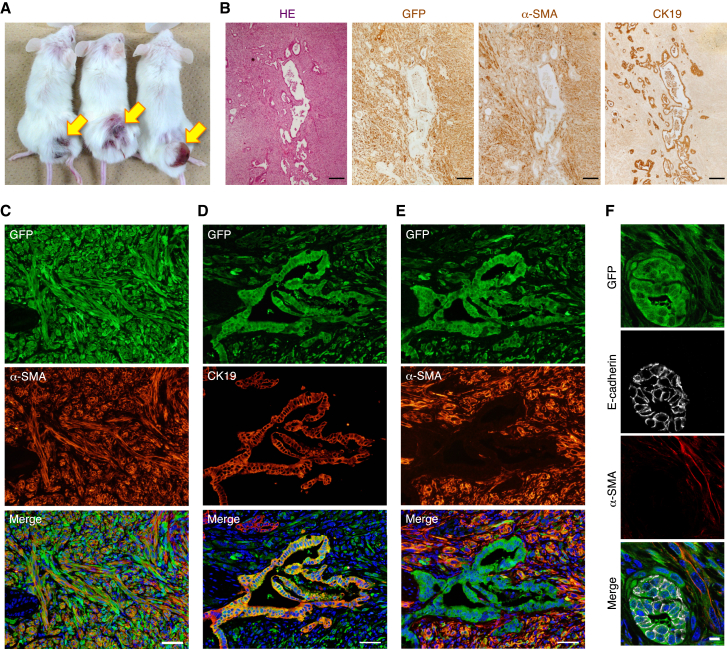
Next, to investigate the role of HPC-derived myofibroblasts in tumor formation, we subcutaneously injected p53−/− HPCs into NOD/SCID recipient mice, after marking the cells by expression of GFP (Figure 4A). At 2 months after transplantation, the three distinct p53−/− HPC clones generated tumors (Figure 5A). Immunofluorescence analyses revealed that the tumors formed by the GFP+ donor cells were composed of both mesenchymal and epithelial tissues constituted by α-SMA+ myofibroblasts and CK19+ biliary lineage cells, respectively (Figures 5B–5E). In addition, and surprisingly, a number of myofibroblasts occupied a wider area of the tumors and tightly surrounded the epithelial tissues within the tumors, although the frequency of myofibroblast production from HPCs was very low in culture (Figures 5B–5E). These properties of p53−/− HPC-derived tumors resemble those of cholangiocarcinoma (Sirica, 2011, Massani et al., 2013), suggesting that, at least in part, p53−/− HPCs can give rise to cholangiocarcinoma. When we transplanted the mixture of wild-type and p53−/− HPC clones into NOD/SCID mice, wild-type HPCs formed only a small number of ductal structures surrounded by p53−/− HPC-derived mesenchymal tissues in the tumor (Figure S3). Because any tumors were not formed after transplantation of only wild-type HPCs (Suzuki et al., 2008), p53−/− HPC-derived myofibroblasts may be possibly capable of supporting the reconstitution of the biliary ductal structures from wild-type HPCs. Moreover, these results suggest that p53 deficiency is required for in vivo production of myofibroblasts from HPCs. Indeed, in the chronically injured liver of DDC-treated wild-type mice, HPC-derived myofibroblasts were not observed (Figure S4). Thus, during tumor development p53−/− HPCs can contribute to the formation of the tumor microenvironment by producing abundant myofibroblasts that might form a particular niche for tumor growth and survival.
Figure 5.
Myofibroblasts Derived from p53−/− HPCs Create the Tumor Microenvironment
(A) Three distinct p53−/− HPC clones gave rise to tumors at 2 months after subcutaneous injection into NOD/SCID mice. Arrows indicate tumors formed from each p53−/− HPC clone.
(B) Serial sections of a tumor arising from a GFP-expressing p53−/− HPC clone were analyzed by H&E staining and immunohistochemical staining of GFP, α-SMA, and CK19.
(C–F) Co-immunofluorescence staining of GFP with α-SMA (C and E) or CK19 (D) and that of GFP with E-cadherin and α-SMA (F) was conducted for p53−/− HPC-derived tumors formed in NOD/SCID recipient mice. Serial sections were used for the staining shown in (D) and (E). DNA was stained with DAPI.
Scale bars represent 200 μm (B), 50 μm (C–E), and 10 μm (F). See also Figures S3 and S4.
Discussion
Myofibroblasts are normally absent from the adult liver but appear during liver injury. In experimental rodent models of liver fibrosis and patients with liver disease, myofibroblasts are generated from activated liver-resident mesenchymal cells, such as hepatic stellate cells and portal fibroblasts (Lemoinne et al., 2013, Mederacke et al., 2013, Xu et al., 2014), but not from hepatocytes and cholangiocytes (Scholten et al., 2010, Taura et al., 2010, Chu et al., 2011). Thus, these two types of mesenchymal cells are considered to be the major sources of myofibroblasts in the fibrotic liver. Meanwhile, although BM-derived collagen-producing cells, fibrocytes, are recruited into the injured liver and are involved in inflammation and fibrosis, the contribution of fibrocytes to myofibroblasts remains controversial (Lemoinne et al., 2013, Xu et al., 2014). Our present data show that HPCs can also be a source of myofibroblasts that may potentially create a microenvironment for tumor development arising through the progression of chronic liver injury. HPCs are generally defined as cells that can give rise to only two types of hepatic epithelial cells, hepatocytes and cholangiocytes. However, we found that HPCs obtained from the chronically injured liver can actually produce a third cell type, myofibroblasts, meaning that HPCs can likely be redefined as cells with at least trilineage differentiation potential. Interestingly, although HPCs can persistently produce myofibroblasts, the production ratio of myofibroblasts from HPCs is much less than those of hepatocytes and cholangiocytes in culture. However, during tumor development HPCs can contribute to the formation of the tumor microenvironment by producing abundant myofibroblasts, which may depend on p53 deficiency in HPCs. Although we have no related data in this study, there is a possibility that p53−/− HPC-derived myofibroblasts could contribute to liver fibrosis as well as liver cancers.
Our present data suggest that HPC-derived myofibroblasts play an essential role for tumor development by generating a particular microenvironment that might act as a niche for tumor growth and survival. However, it remains to be determined whether the microenvironment composed of HPC-derived myofibroblasts can actually support tumor development. Indeed, myofibroblasts may mainly be provided by the hepatic stellate cells and portal fibroblasts during the development and progression of liver fibrosis and cancers. Thus, to understand the specific role of HPC-derived myofibroblasts in liver diseases it will be important to clarify the differences among myofibroblasts derived from HPCs, hepatic stellate cells, and portal fibroblasts. The new knowledge acquired from this approach will enable us to explore and identify the fundamental factors derived from the tumor microenvironment created by HPC-derived myofibroblasts, which might include tumor-associated cytokines and extracellular matrix proteins. Moreover, identification of the mechanism underlying myofibroblast differentiation from HPCs may play a critical role in developing a method to inhibit tumor formation by blocking the generation of myofibroblasts from HPCs. Thus, our findings will help to elucidate the pathologic mechanisms underlying liver fibrosis and cancer and to develop therapeutic strategies for such liver diseases.
Experimental Procedures
Mice
C57BL/6 mice (Clea), Fah−/− mice (Suzuki et al., 2008), p53−/− mice (CDB0001K) (Tsukada et al., 1993), NOD/SCID mice (Charles River Laboratories), CK19-CreERT2 mice (Sekiya and Suzuki, 2012), and R26RYFP mice (Srinivas et al., 2001) were used in this study.
Isolation and Culture of Cells
HPCs and hepatoblasts were prospectively isolated from the chronically injured adult mouse liver and developing mouse liver, respectively, and isolated cells were clonally cultured as described previously (Suzuki et al., 2002, Suzuki et al., 2008). In brief, for isolation of HPCs, single-cell suspensions of liver cells were prepared from C57BL/6 wild-type or p53−/− mice fed a diet containing 0.1% DDC (Sigma-Aldrich) for 2 weeks using a dual-protease digestion protocol. Next, fluorescence-conjugated antibodies against CD133, CD45, and TER119 were used for isolation of CD133+CD45−TER119− cells. For isolation of hepatoblasts, we used fluorescence-conjugated antibodies against c-Met, CD49f, c-Kit, CD45, and TER119, and isolated c-Met+CD49f+/lowc-Kit−CD45−TER119− cells from the liver of E13.5 mouse embryos. CD133+CD45−TER119− cells and c-Met+CD49f+/lowc-Kit−CD45−TER119− cells identified by clone sorting using flow cytometry were cultured in individual wells of 96-well plates to induce colony formation. The HPCs and hepatoblasts that formed LCs were then subcultured and clonally expanded. HPCs and hepatoblasts were cultured in our hepato-medium, comprising a 1:1 mixture of DMEM and F-12, supplemented with 10% fetal bovine serum, 1 μg/mL insulin (Wako), 1 × 10−7 M dexamethasone (Sigma-Aldrich), 10 mM nicotinamide (Sigma-Aldrich), 2 mM L-glutamine, 50 μM β-mercaptoethanol (Nacalai Tesque), penicillin-streptomycin, 20 ng/mL hepatocyte growth factor (Sigma-Aldrich), and 20 ng/mL epidermal growth factor (Sigma-Aldrich). Under our culture conditions, HPCs and hepatoblasts were maintained with self-renewing cell divisions at a certain ratio, and spontaneously and continuously produced their differentiated progeny. We conducted at least three independent experiments for isolation and culture of HPCs and hepatoblasts, and three clones of HPCs and hepatoblasts were randomly selected and used for examination.
Immunostaining
Liver and tumor tissues were fixed in 20% formalin, dehydrated in ethanol and xylene, embedded in paraffin wax, and sectioned. After deparaffinization and rehydration of the sections, antigen retrieval was performed by microwaving in 0.01 M citrate buffer (pH 6.0). For immunohistochemistry, the sections were incubated with 0.3% hydrogen peroxide in methanol for 20 min at room temperature to quench endogenous peroxidase activity. Cultured cells were washed with PBS and sequentially fixed with 4% paraformaldehyde for 5 min and 25% acetone in methanol for 1 min at room temperature. The fixed cells were washed in PBS containing 0.1% Tween 20 (Nacalai Tesque) and treated with 0.2% Triton X-100 (Nacalai Tesque) for 1 hr at room temperature. After washing with PBS/Tween 20 and blocking, the tissue sections and cultured cells were incubated with the following primary antibodies: mouse anti-α-SMA (1:10,000; Sigma-Aldrich, A2547), goat anti-ALB (1:2,000; Bethyl Laboratories, A90-134A), rabbit anti-CK19 (1:4,000; Sekiya and Suzuki, 2012), rabbit anti-FAH (1:2,000; Abcam, ab81087), goat anti-GFP (1:2,000; Abcam, ab6673), and rat anti-E-cadherin (1:200; Takara, M108). After washing, the sections and cells were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000; Dako) specific to the species of the primary antibodies for immunohistochemistry or with Alexa 488-, Alexa 555-, and/or Alexa 647-conjugated secondary antibodies (1:1,000; Molecular Probes) plus DAPI for immunofluorescence staining. In addition, 5-ethynyl-2′-deoxyuridine (EdU)-incorporated cells were stained using a Click-iT EdU Alexa Fluor 555 Imaging Kit (Molecular Probes) in accordance with the manufacturer's instructions. EdU (Sigma-Aldrich) was added to the culture medium at 30 min before analyses.
Cell Transplantation
Similar to our previous study (Suzuki et al., 2008), HPCs (8 × 106) obtained from the livers of DDC-treated wild-type mice were intrasplenically injected into the livers of Fah−/− mice. The Fah−/− mice were maintained on drinking water containing 7.5 mg/L NTBC (Swedish Orphan International), but the treatment was stopped just after transplantation. We also marked HPCs by GFP expression through transfection of a GFP-expressing vector and transplanted these cells (8 × 106) into the livers of wild-type mice at 4 days after the beginning of DDC administration. We transplanted HPCs into more than three recipient mice in each experiment, and the donor cells were able to become engrafted and reconstitute the hepatic tissues in the livers of all recipient mice. For analysis of tumor formation, HPCs (3 × 107) obtained from the livers of DDC-treated p53−/− mice were initially marked by GFP expression. The GFP+ HPCs were then suspended in 250 μL of culture medium with 250 μL of Matrigel (BD Biosciences) containing 5 μg/mL vascular endothelial growth factor 165 (Sigma-Aldrich) and subcutaneously injected into NOD/SCID mice. We transplanted p53−/− HPCs into more than three recipient mice, and the donor cells were able to form tumors in all recipient mice.
Study Approval
The experiments were approved by the Kyushu University Animal Experiment Committee, and the care of the animals was in accordance with institutional guidelines.
Author Contributions
S.S., S.M., and K.M.-I. performed the experiments and analyzed the data. A.S. wrote the manuscript. A.S. contributed to the conception, design, and overall project management.
Acknowledgments
We thank Drs. Shinichi Aizawa and Frank Costantini for providing mice, and Yuuki Honda, Mayumi Yamamoto, Nanako Goto, Chiaki Kaieda, Masato Tanaka, and Kanako Motomura for excellent technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Society for the Promotion of Science (grant numbers 23112002, 25713014, and 16H01850), Health Labor Sciences Research Grants in Japan, and the Core Research for Evolutional Science and Technology Program of the Japan Agency for Medical Research and Development (AMED-CREST).
Published: December 1, 2016
Footnotes
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.11.002.
Supplemental Information
References
- Asahina K., Tsai S.Y., Li P., Ishii M., Maxson R.E., Jr., Sucov H.M., Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba S., Fujii H., Hirose T., Yasuchika K., Azuma H., Hoppo T., Naito M., Machimoto T., Ikai I. Commitment of bone marrow cells to hepatic stellate cells in mouse. J. Hepatol. 2004;40:255–260. doi: 10.1016/j.jhep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Brunt E.M., Blomenkamp K., Ahmed M., Ali F., Marcus N., Teckman J. Hepatic progenitor cell proliferation and liver injury in α-1-antitrypsin deficiency. J. Pediatr. Gastroenterol. Nutr. 2010;51:626–630. doi: 10.1097/MPG.0b013e3181e7ff55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino G., Renzi A., Onori P., Gaudio E. Role of hepatic progenitor cells in nonalcoholic fatty liver disease development: cellular cross-talks and molecular networks. Int. J. Mol. Sci. 2013;14:20112–20130. doi: 10.3390/ijms141020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A.S., Diaz R., Hui J.J., Yanger K., Zong Y., Alpini G., Stanger B.Z., Wells R.G. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston A.D., Powell E.E., Walsh M.J., Richardson M.M., Demetris A.J., Jonsson J.R. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- Deugnier Y.M., Guyader D., Crantock L., Lopez J.M., Turlin B., Yaouanq J., Jouanolle H., Campion J.P., Launois B., Halliday J.W. Primary liver cancer in genetic hemochromatosis: a clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology. 1993;104:228–234. doi: 10.1016/0016-5085(93)90856-8. [DOI] [PubMed] [Google Scholar]
- Español-Suñer R., Carpentier R., Van Hul N., Legry V., Achouri Y., Cordi S., Jacquemin P., Lemaigre F., Leclercq I.A. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Factor V.M., Radaeva S.A. Oval cells-hepatocytes relationships in Dipin-induced hepatocarcinogenesis in mice. Exp. Toxicol. Pathol. 1993;45:239–244. doi: 10.1016/S0940-2993(11)80399-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks K.D., Tavill A.S. Liver disease in alpha 1-antitrypsin deficiency: a review. Am. J. Gastroenterol. 2008;103:2136–2141. doi: 10.1111/j.1572-0241.2008.01955.x. [DOI] [PubMed] [Google Scholar]
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- Fausto N., Campbell J.S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörs S., Jeliazkova P., Ringelhan M., Thalhammer J., Dürl S., Ferrer J., Sander M., Heikenwalder M., Schmid R.M., Siveke J.T. Lineage fate of ductular reactions in liver injury and carcinogenesis. J. Clin. Invest. 2015;125:2445–2457. doi: 10.1172/JCI78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaigre F., Zaret K.S. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr. Opin. Genet. Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lemire J.M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am. J. Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- Lemoinne S., Cadoret A., El Mourabit H., Thabut D., Housset C. Origins and functions of liver myofibroblasts. Biochim. Biophys. Acta. 2013;1832:948–954. doi: 10.1016/j.bbadis.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Lowes K.N., Brennan B.A., Yeoh G.C., Olynyk J.K. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am. J. Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W.Y., Bird T.G., Boulter L., Tsuchiya A., Cole A.M., Hay T., Guest R.V., Wojtacha D., Man T.Y., Mackinnon A. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massani M., Stecca T., Fabris L., Caratozzolo E., Ruffolo C., Furlanetto A., Morton S., Cadamuro M., Strazzabosco M., Bassi N. Isolation and characterization of biliary epithelial and stromal cells from resected human cholangiocarcinoma: a novel in vitro model to study tumor-stroma interactions. Oncol. Rep. 2013;30:1143–1148. doi: 10.3892/or.2013.2568. [DOI] [PubMed] [Google Scholar]
- Mederacke I., Hsu C.C., Troeger J.S., Huebener P., Mu X., Dapito D.H., Pradere J.P., Schwabe R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili V., Carpino G., Alisi A., Franchitto A., Alpini G., De Vito R., Onori P., Alvaro D., Gaudio E. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56:2142–2153. doi: 10.1002/hep.25742. [DOI] [PubMed] [Google Scholar]
- Preisegger K.H., Factor V.M., Fuchsbichler A., Stumptner C., Denk H., Thorgeirsson S.S. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab. Invest. 1999;79:103–109. [PubMed] [Google Scholar]
- Prior P. Long-term cancer risk in alcoholism. Alcohol Alcohol. 1988;23:163–171. [PubMed] [Google Scholar]
- Schaub J.R., Malato Y., Gormond C., Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten D., Osterreicher C.H., Scholten A., Iwaisako K., Gu G., Brenner D.A., Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya S., Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J. Clin. Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R.M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978;38:1092–1098. [PubMed] [Google Scholar]
- Si-Tayeb K., Lemaigre F.P., Duncan S.A. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sirica A.E. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Zheng Y.W., Kaneko S., Onodera M., Fukao K., Nakauchi H., Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J. Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Sekiya S., Onishi M., Oshima N., Kiyonari H., Nakauchi H., Taniguchi H. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology. 2008;48:1964–1978. doi: 10.1002/hep.22558. [DOI] [PubMed] [Google Scholar]
- Tarlow B.D., Finegold M.J., Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology. 2014;60:278–289. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Ho R.H., Kaku T., Ekem J.K., Farber E. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorene and partial hepatectomy. Am. J. Pathol. 1984;114:418–430. [PMC free article] [PubMed] [Google Scholar]
- Taura K., Miura K., Iwaisako K., Osterreicher C.H., Kodama Y., Penz-Osterreicher M., Brenner D.A. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tomooka Y., Takai S., Ueda Y., Nishikawa S., Yagi T., Tokunaga T., Takeda N., Suda Y., Abe S. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene. 1993;8:3313–3322. [PubMed] [Google Scholar]
- Tsukuma H., Hiyama T., Tanaka S., Nakao M., Yabuuchi T., Kitamura T., Nakanishi K., Fujimoto I., Inoue A., Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. Engl. J. Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu X., Koyama Y., Wang P., Lan T., Kim I.G., Kim I.H., Ma H.Y., Kisseleva T. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front. Pharmacol. 2014;5:167. doi: 10.3389/fphar.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K., Knigin D., Zong Y., Maggs L., Gu G., Akiyama H., Pikarsky E., Stanger B.Z. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.