Introduction
Erythema multiforme (EM) is an acute, typically self-limited mucocutaneous eruption characterized by distinctive target lesions. In a subset of patients, EM has a more chronic course, with recurrent episodes or even persistent disease. In some of these patients, EM is related to chronic infections, most commonly herpes simplex virus (HSV), and will respond to antiviral therapy. However, in up to 58% of patients, the etiology is unknown,1 and in these cases treatment is often difficult.
Case report
A 33-year-old woman with a 6-year history of EM was referred to our dermatology clinic. There was no mucosal involvement and no history of orolabial HSV. Treatment with topical corticosteroids, dapsone, valacyclovir, cyclosporine, azathioprine, mycophenolate mofetil, or methotrexate did not permit tapering of prednisone to less than 20 mg/d without a disease flare.
The patient's medical history included several notable features: (1) recurrent long bone fractures with minor trauma since infancy (19 in total); (2) delayed eruption and later retention of primary teeth requiring extraction; (3) weak, easily broken permanent teeth, resulting in complete upper and lower dentures; (4) dental abscesses; (5) T2, N0, M0 papillary thyroid cancer at age 31, now in remission (treated with thyroidectomy and radioactive iodine ablation); (6) ectopic pregnancy; (7) multiple kidney stones; (8) endometriosis; (9) renal cysts; and (10) ovarian cysts. At the time that the EM began, the patient was taking only intermittent ibuprofen. The EM did not improve with discontinuation of this medication. There was no family history of similar cutaneous or extracutaneous disease.
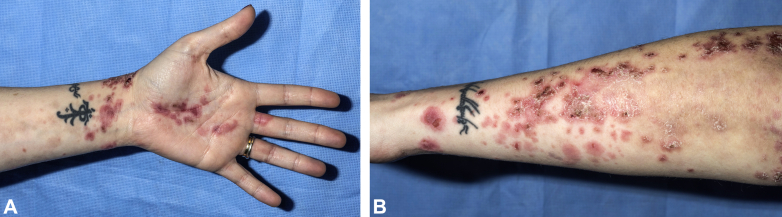
Physical examination did not find any dysmorphic features, with the exception of an elongated philtrum. During periods of prednisone taper or discontinuation, skin examination found numerous 0.5- to 2-cm pink targetoid papules and plaques, many with central vesiculation or hemorrhagic crusting. Lesions were distributed on the hands, forearms, and legs (Fig 1). There was no mucosal involvement. Hair density was normal. Examination of the digits was unremarkable.
Fig 1.
A and B, Representative clinical images.
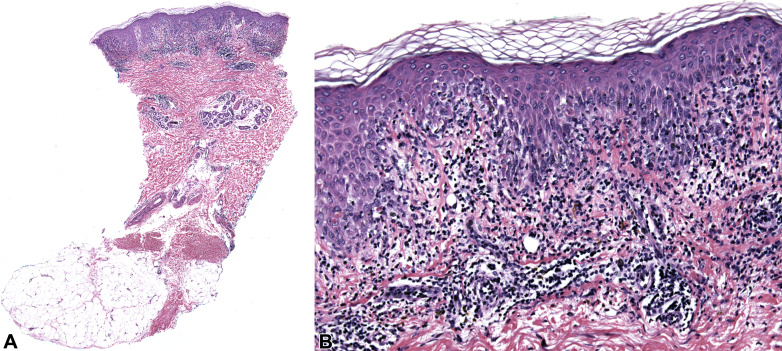
Skin biopsies found interface dermatitis, marked vacuolar alteration along the dermoepidermal junction, necrotic keratinocytes, and lymphocytic inflammation consistent with EM (Fig 2). Direct immunofluorescence was negative. HSV was not detected by polymerase chain reaction in lesional tissue. Basic laboratory workup findings were unremarkable, and serology results for HSV1, HSV2, cytomegalovirus, Epstein-Barr virus IgM and IgG, antinuclear antibody, double-stranded DNA, rheumatoid factor, bullous pemphigoid 180 and 230 antibodies, and desmoglein-1/3 antibodies were negative. There was no evidence of a new malignancy or recurrence of her thyroid cancer.
Fig 2.
Hematoxylin-eosin–stained sections at low (A) and high (B) magnification.
Because of the patient's extensive medical history in the context of recalcitrant EM, we performed whole exome sequencing. This sequencing found a heterozygous missense mutation in the TRPS1 gene (c.2627C>A resulting in p.S876Y). TRPS1 is mutated in type I tricho-rhino-phalangeal syndrome (TRPS), which is inherited in an autosomal dominant fashion and is classically characterized by a triad of sparse hair, a bulbous nasal tip, and brachydactyly together with additional phenotypic features.2 Although the triad was not observed in our patient, other TRPS features including a long philtrum, recurrent fractures, delayed eruption of primary teeth, enamel defects, and malignancy were observed.2
In TRPS, loss-of-function TRPS1 mutations lead to activation of Janus kinase–signal transducer and activator of transcription (JAK-STAT) signaling.3, 4, 5 We hypothesized that dysregulated JAK-STAT signaling was contributing to the pathogenesis of EM in our patient and could be targeted using the JAK 1/3 inhibitor tofacitinib. Tofacitinib, which has been used successfully to treat other inflammatory dermatoses, was initiated at 5 mg twice daily. Treatment resulted in complete resolution of her skin lesions, permitting discontinuation of prednisone. An attempt to lower the dose of tofacitinib to 5 mg once daily resulted in partial recurrence of the skin lesions. The dose was increased back to 5 mg twice daily, and her skin has remained clear for 8 months.
Discussion
This patient did not show classic features of TRPS despite mutation in TRPS1. In classic TRPS, TRPS1 mutations are typically very detrimental,2 causing skeletal and hair abnormalities by increasing JAK-STAT signaling.3, 4, 5 The S876Y missense mutation in our patient has not been reported in TRPS and may result in a milder/altered phenotype. We believe this mutation is pathogenic based on the observation that it occurs in a semiconserved residue, it is predicted to be detrimental to protein function based on prediction algorithms (UMD-Predictor), and there are several clinical features of TRPS in our patient such as bony and dental anomalies.
In EM, autoreactive, Th1-polarized T cells recognize and attack the epidermis resulting in tissue damage. This response is thought to be driven by cytokines such as interferon-γ and interleukin-2,6 which depend on JAK-STAT signaling to transmit their signals. In some cases of EM, these dysregulated immune responses appear to be reactive and related to infection. In a subset of patients with idiopathic EM, immune dysregulation may be intrinsic and result from mutations or genetic polymorphisms in immune regulatory genes. We hypothesize that the TRPS1 mutation in our patient predisposed her to EM via dysregulation of JAK-STAT signaling in lymphocytes. EM has been reported in a patient with chronic mucocutaneous candidiasis, which is caused by inherited gain-of-function mutations in the STAT1 gene.7 These observations suggest that genetic variation, leading to altered JAK-STAT signaling, may contribute to EM pathogenesis in some patients.
Tofacitinib is approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis (5 mg twice daily) and has shown efficacy in psoriasis. The resolution of EM in our patient is interesting to consider in the context of other Th1 polarized inflammatory dermatoses, many of which depend on interferon signaling and have also shown similarly dramatic responses to JAK inhibition. Such conditions include dermatomyositis, alopecia areata, and vitiligo.8, 9, 10 The data presented here suggest that idiopathic EM may be associated with JAK-STAT activation and that tofacitinib may be a treatment option in these cases.
Acknowledgments
Clinical photographs are courtesy of the National Institutes of Health Undiagnosed Disease Program. The authors also thank Drs N. Rodic and C. Ko of Yale Dermatopathology.
Footnotes
Funding source: The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research (B.A.K).
Conflicts of interest: Dr King has served on advisory boards or is a consultant for Aclaris Therapeutics Inc, Pfizer Inc, Eli Lilly and Company, and Concert Pharmaceuticals Inc. Dr Damsky has no conflicts of interest to declare.
References
- 1.Wetter D.A., Davis M.D. Recurrent erythema multiforme: clinical characteristics, etiologic associations, and treatment in a series of 48 patients at Mayo Clinic, 2000 to 2007. J Am Acad Dermatol. 2010;62:45–53. doi: 10.1016/j.jaad.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 2.Maas S.M., Shaw A.C., Bikker H. Phenotype and genotype in 103 patients with tricho-rhino-phalangeal syndrome. Eur J Med Genet. 2015;58:279–292. doi: 10.1016/j.ejmg.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Fujisawa T., Fukao T., Shimomura Y., Seishima M. A novel TRPS1 mutation in a family with tricho-rhino-phalangeal syndrome type 1. J Dermatol. 2014;41:514–517. doi: 10.1111/1346-8138.12511. [DOI] [PubMed] [Google Scholar]
- 4.Ito T., Shimomura Y., Farooq M., Suzuki N., Sakabe J., Tokura Y. Trichorhinophalangeal syndrome with low expression of TRPS1 on epidermal and hair follicle epithelial cells. J Dermatol. 2013;40:396–398. doi: 10.1111/1346-8138.12111. [DOI] [PubMed] [Google Scholar]
- 5.Suemoto H., Muragaki Y., Nishioka K. Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev Biol. 2007;312:572–581. doi: 10.1016/j.ydbio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Caproni M., Torchia D., Schincaglia E. Expression of cytokines and chemokine receptors in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2006;155:722–728. doi: 10.1111/j.1365-2133.2006.07398.x. [DOI] [PubMed] [Google Scholar]
- 7.Higgins E., Al Shehri T., McAleer M.A. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol. 2015;135:551–553. doi: 10.1016/j.jaci.2014.12.1867. [DOI] [PubMed] [Google Scholar]
- 8.Craiglow B.G., King B.A. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988–2990. doi: 10.1038/jid.2014.260. [DOI] [PubMed] [Google Scholar]
- 9.Craiglow B.G., King B.A. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015;151:1110–1112. doi: 10.1001/jamadermatol.2015.1520. [DOI] [PubMed] [Google Scholar]
- 10.Hornung T., Janzen V., Heidgen F.J., Wolf D., Bieber T., Wenzel J. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med. 2014;371:2537–2538. doi: 10.1056/NEJMc1412997. [DOI] [PubMed] [Google Scholar]