Abstract
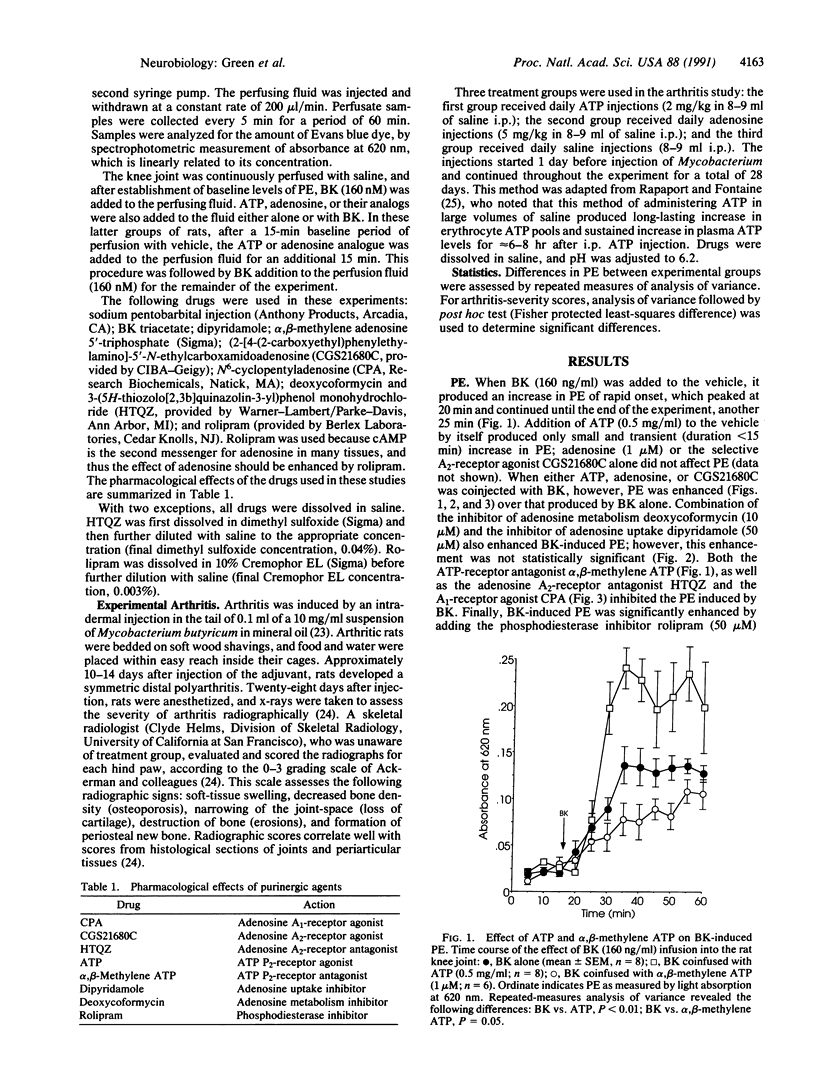
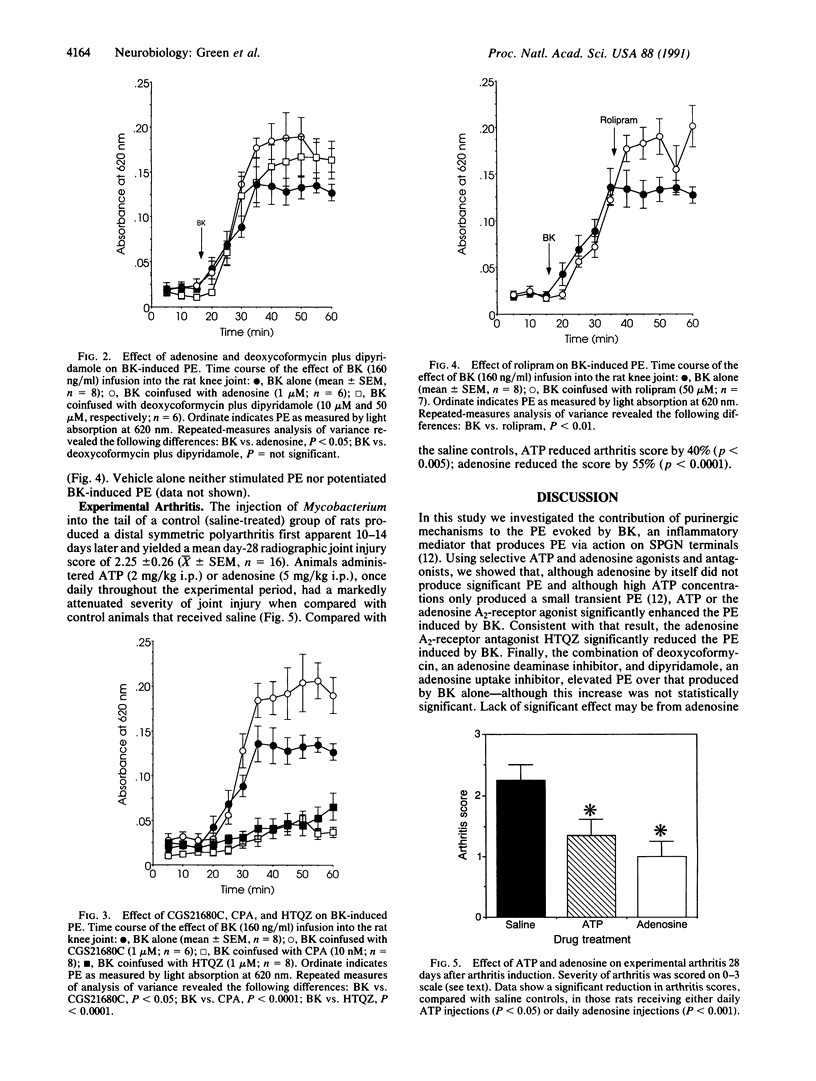
We assessed the contribution of ATP and adenosine (i) to a major sign of acute inflammation, plasma extravasation (PE), in the rat knee joint and (ii) to the severity of joint injury in adjuvant-induced experimental arthritis, a chronic inflammatory disease. PE induced by local infusion of bradykinin, which we have previously shown to depend on the sympathetic postganglionic neuron terminal, was markedly enhanced by coinfusion of either ATP or the adenosine A2-receptor agonist 2-[4-(2-carboxyethyl)phenylethylamino]-5'-N-ethylcarboxamidoadenos ine. Bradykinin-induced PE was inhibited by coinfusion of the ATP receptor antagonist adenosine 5'-[alpha,beta-methylene]triphosphate, the A2-receptor antagonist 3-(5H-thiozolo[2,3b]quinazolin-3-yl)phenol monohydrochloride, or the adenosine A1-receptor agonist N6-cyclopentyladenosine. The joint injury associated with experimental arthritis, which is reduced in severity in sympathectomized rats, was also markedly attenuated by daily administration of either ATP (40% reduction) or adenosine (55% reduction). These results demonstrate that the purines ATP and adenosine (acting at the A2 receptor), cotransmitters in the sympathetic postganglionic neuron terminal, enhance bradykinin-induced sympathetic postganglionic neuron terminal-dependent PE but inhibit the joint injury of arthritis. These opposing purinergic effects on PE and joint injury suggest that enhanced PE protects against joint injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman N. R., Rooks W. H., 2nd, Shott L., Genant H., Maloney P., West E. Effects of naproxen on connective tissue changes in the adjuvant arthritic rat. Arthritis Rheum. 1979 Dec;22(12):1365–1374. doi: 10.1002/art.1780221208. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Vascular control by purines with emphasis on the coronary system. Eur Heart J. 1989 Nov;10 (Suppl F):15–21. doi: 10.1093/eurheartj/10.suppl_f.15. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Levine J. D. Neural control of vascular permeability: interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989 Jul;62(1):48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- Collis M. G. The vasodilator role of adenosine. Pharmacol Ther. 1989;41(1-2):143–162. doi: 10.1016/0163-7258(89)90104-6. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Daguma L., Nichols D., Hutchison A. J., Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990 Apr;85(4):1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:29–46. [PubMed] [Google Scholar]
- De Mey J., Burnstock G., Vanhoutte P. M. Modulation of the evoked release of noradrenaline in canine saphenous vein via presynaptic receptors for adenosine but not ATP. Eur J Pharmacol. 1979 May 15;55(4):401–405. doi: 10.1016/0014-2999(79)90115-8. [DOI] [PubMed] [Google Scholar]
- Des Rosiers C., Nees S. Functional evidence for the presence of adenosine A2-receptors in cultured coronary endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):94–98. doi: 10.1007/BF00177757. [DOI] [PubMed] [Google Scholar]
- Engel D. The influence of the sympathetic nervous system on capillary permeability. Res Exp Med (Berl) 1978 Jul 24;173(1):1–8. doi: 10.1007/BF01851368. [DOI] [PubMed] [Google Scholar]
- Engel D. The influence of the sympathetic nervous system on capillary permeability. J Physiol. 1941 Jan 14;99(2):161–181. doi: 10.1113/jphysiol.1941.sp003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Sollevi A. Cardiovascular effects of adenosine. Clin Physiol. 1986 Feb;6(1):1–21. doi: 10.1111/j.1475-097x.1986.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Gonzales R., Goldyne M. E., Taiwo Y. O., Levine J. D. Production of hyperalgesic prostaglandins by sympathetic postganglionic neurons. J Neurochem. 1989 Nov;53(5):1595–1598. doi: 10.1111/j.1471-4159.1989.tb08557.x. [DOI] [PubMed] [Google Scholar]
- Green K. L. Role of endogenous catecholamines in the anti-inflammatory activity of alpha-adrenoceptor blocking agents. Br J Pharmacol. 1974 May;51(1):45–53. doi: 10.1111/j.1476-5381.1974.tb09630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Adenosine inhibits ischemia-reperfusion-induced leukocyte adherence and extravasation. Am J Physiol. 1989 Nov;257(5 Pt 2):H1334–H1339. doi: 10.1152/ajpheart.1989.257.5.H1334. [DOI] [PubMed] [Google Scholar]
- Gözsy B., Kátó L. Role of norepinephrine and 5-hydroxytryptamine in the delayed phase of the inflammatory reaction in rats. Int Arch Allergy Appl Immunol. 1966;30(6):553–560. doi: 10.1159/000229842. [DOI] [PubMed] [Google Scholar]
- Helme R. D., Andrews P. V. The effect of nerve lesions on the inflammatory response to injury. J Neurosci Res. 1985;13(3):453–459. doi: 10.1002/jnr.490130311. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Fye K., Heller P., Basbaum A. I., Whiting-O'Keefe Q. Clinical response to regional intravenous guanethidine in patients with rheumatoid arthritis. J Rheumatol. 1986 Dec;13(6):1040–1043. [PubMed] [Google Scholar]
- Levine J. D., Moskowitz M. A., Basbaum A. I. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985 Aug;135(2 Suppl):843s–847s. [PubMed] [Google Scholar]
- Li Y. O., Fredholm B. B. Adenosine analogues stimulate cyclic AMP formation in rabbit cerebral microvessels via adenosine A2-receptors. Acta Physiol Scand. 1985 Jun;124(2):253–259. doi: 10.1111/j.1748-1716.1985.tb07659.x. [DOI] [PubMed] [Google Scholar]
- Linde B., Chisolm G., Rosell S. The influence of sympathetic activity and histamine on the blood-tissue exchange of solutes in canine adipose tissue. Acta Physiol Scand. 1974 Oct;92(2):145–155. doi: 10.1111/j.1748-1716.1974.tb05731.x. [DOI] [PubMed] [Google Scholar]
- McCormack D. G., Clarke B., Barnes P. J. Characterization of adenosine receptors in human pulmonary arteries. Am J Physiol. 1989 Jan;256(1 Pt 2):H41–H46. doi: 10.1152/ajpheart.1989.256.1.H41. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Fontaine J. Anticancer activities of adenine nucleotides in mice are mediated through expansion of erythrocyte ATP pools. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1662–1666. doi: 10.1073/pnas.86.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Su C. Neurogenic release of purine compounds in blood vessels. J Pharmacol Exp Ther. 1975 Oct;195(1):159–166. [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]



