Abstract
Purpose
To assess the anatomical outcome of patients with exudative age-related macular degeneration resistant to ranibizumab treated with aflibercept.
Method
Prospective, interventional, case series, where we treated a group of patients deemed resistant to Ranibizumab after 6 months of persistence of intra- or subretinal fluid despite continuous treatment.
Results
The study included 17 patients, 3 males and 14 females. The average males age was 85 (range 83–87), and that of females was 79.64 (range 68–88).
At the start of the study, the central foveal thickness CFT average was 534.76 μm (range 252–999). At 1 month and after 1 injection of Aflibercept, the CFT average was 324.82 μm (range 222–585). At 4 months and after 3 consecutive injections of Aflibercept the CFT average was 294.76 μm (range 184–640). At 6 months the CFT average was 356 μm (range 206–609).
At the 5th visit only 8 out 17 (47%) patients required repeated injection either for persistent fluids or for recurrence. At the 6th and final visit only 4 out of 17 (23.5%) needed repeated injections, of them only one was treated on the visit before and treatment was given as very little response was observed from last injection, and all other 3 were not treated on the visit before.
Conclusion
Our results showed that aflibercept was able to dry the macula even in advanced case of wet AMD resistant to Ranibizumab.
Keywords: Ranibizumab, Aflibercept, Age-related macular degeneration
Introduction
Aflibercept (INN, USAN) is a biopharmaceutical drug invented by Regeneron Pharmaceuticals, approved in the United States and Europe for the treatment of wet macular degeneration under the trade name Eylea. It is an inhibitor of all types of vascular endothelial growth factor (VEGF) in addition to the Placental growth factor (PGF).1
Aflibercept is a recombinant fusion protein consisting of vascular endothelial growth factor (VEGF)-binding portions from the extracellular domains of human VEGF receptors 1 and 2 that are fused to the Fc portion of the human IgG1 immunoglobulin.2
In the VIEW studies, two similarly designed, phase-3 studies (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD [VIEW 1, VIEW 2]) of neovascular age-related macular degeneration (AMD) compared monthly and every-2-month dosing of intravitreal aflibercept injection (VEGF Trap-Eye; Regeneron, Tarrytown, NY, and Bayer HealthCare, Berlin, Germany) with monthly ranibizumab the most commonly used licensed Anti-VEGF treatment for exudative age-related macular degeneration (AMD), and aflibercept showed similar efficacy when used at fixed interval of 8 weeks. The studies were double-masked, multicentre, parallel-group, active-controlled and randomized trials that included 2419 Patients with active, submacular, choroidal neovascularization (CNV) lesions.3
Recently same group4 released the data of 96 weeks of follow-up of the VIEW studies, where patient following the initial regimen for the first year was switched to follow a PRN (pro re nata) regimen with mandatory treatment at 12 weeks interval. Results showed that proportions of eyes maintaining best corrected visual acuity BCVA across treatments were 94.4–96.1% at week 52 and 91.5–92.4% at week 96. Mean BCVA gains were 8.3–9.3 letters at week 52 and 6.6–7.9 letters at week 96. Proportions of eyes without retinal fluid decreased from week 52 (60.3–72.4%) to week 96 (44.6–54.4%), and more monthly treated (2q4) eyes were without fluid at weeks 52 and 96 than monthly ranibizumab (Rq4) eyes. The number of injections during weeks 52 through 96 was lower in the 2q4 and bimonthly treated (2q8) groups versus the Rq4 group.
Method
In this prospective, interventional, study, we treated a group of patients deemed resistant to Ranibizumab after 6 months of persistence of intra- or subretinal fluid (SRF) despite continuous treatment. All patients were initially consented to receive Ranibizumab and then re-consented for Aflibercept. All patients had permanent foveal damage with advanced submacular scarring. The main aim of the trial was to access the number of patients with dry macula at months 1, 4 and 6, and visual acuity change was a secondary outcome.
Patients were treated with 3 monthly injections of aflibercept, with no treatment on the fourth visit, if required on the fifth visit and if required and not being treated on the previous visit on the sixth visit.
Data were collected at every visit, including BCVA using Log of the minimum angle of resolution (Log MAR) and optical coherence tomography OCT scan. Any ocular or non-ocular adverse event was recorded.
The only inclusion criterion was the no response to ranibizumab despite continuous treatment in the last 6 months before switching. Exclusion criteria included continuous small improvement to ranibizumab with residual IRF or SRF, good response in case of recurrence. All patients attended their 6 visits, were seen, scanned, and treated by the same doctor.
All patients had no other macular abnormalities, including epiretinal membrane, vitreomacular traction, taut posterior hyaloid or any other retina-hyaloid interface abnormalities.
All patients had unilateral wet AMD disease, with only dry macular changes in the fellow eye. And all patients were pseudophakic in the treated eye except the female patient aged 68 who was phakic.
All patients were given the choice of leaving the study at every visit where they were confirming the willingness to continue to participate.
Results
Our study included 17 patients, 3 males and 14 females. The average males age was 85 (range 83–87), and that of females was 79.64 (range 68–88) (Photo 1).
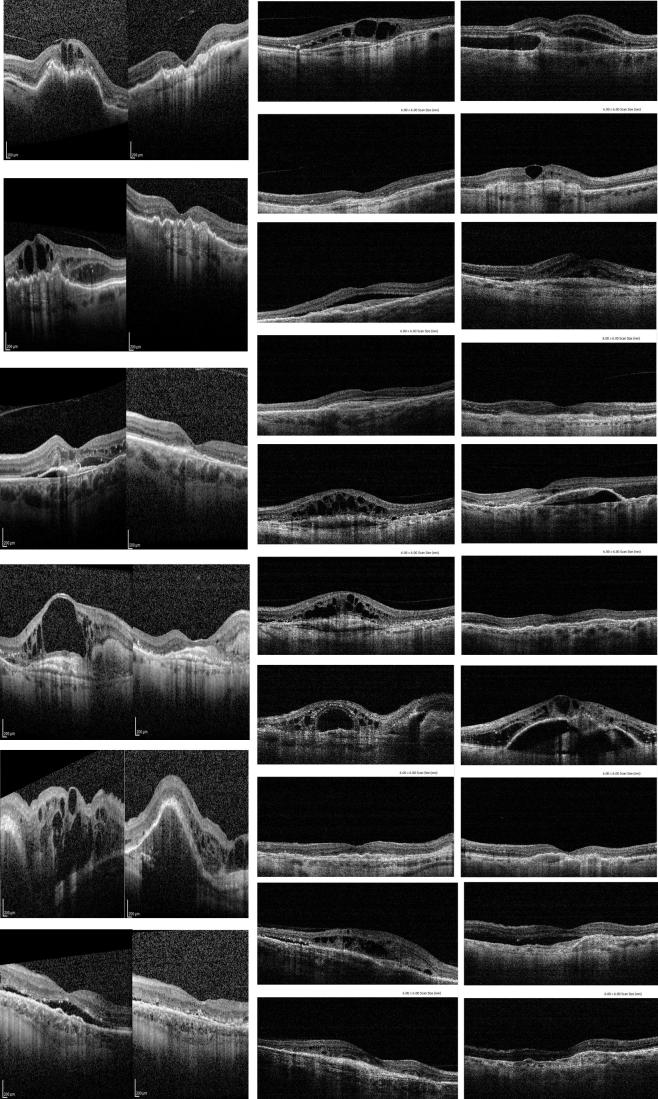
Photo 1.
This collection of patients before and after 3 injections of aflibercept illustrates clearly the major anatomical improvement is almost all patients. Picture on the left or on the top in coupled pictures in before, picture on the right or the bottom is the after treatment.
The time since diagnosis of exudative AMD was quite variable ranging from 18 to 67 months. Patients received treatment very frequently to the day of starting our study with an average number of injections of 16.47 (range of 8–29) with average number of visits of 25.29 (range 14–39) over an average period of 37.64 months (range of 18–67).
At the start of the study, the central foveal thickness (CFT) average was 534.76 μm (range 252–999). At 1 month and after 1 injection of Aflibercept, the CFT average was 324.82 μm (range 222–585). At 4 months and after 3 consecutive injection of Aflibercept the CFT average was 294.76 μm (range 184–640). At 6 months the CFT average was 356 μm (range 206–609).
At the 5th visit only 8 out 17 (47%) patients required repeated injection either for persistent fluids or for recurrence. At the 6th and final visit only 4 out of 17 (23.5%) needed repeated injections, of them only one was treated on the visit before and treatment was given as very little response was observed from last injection, and all other 3 were not treated on the visit before.
Immediate response was observed in all patients after first injection of Aflibercept (Graph 1).
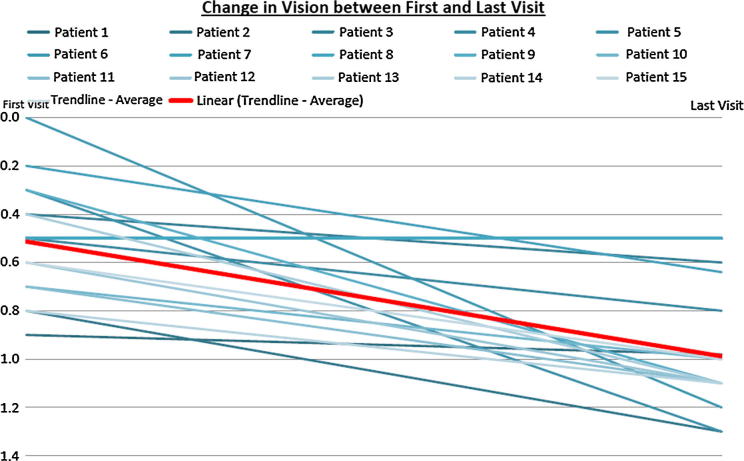
Graph 1.
This graph shows the visual acuity decline in all patients from day of diagnosis to the day of switch to aflibercept. It shows clear stead deterioration of vision in all patients.
Best corrected visual acuity BCVA, at the beginning of the study had an average of 0.998, and at month 1 the average was 1.01, at month 4 and after 3 consecutive injections of Aflibercept the average of BCVA was 0.952 and at month 6 it was 0.933 (Graph 2).
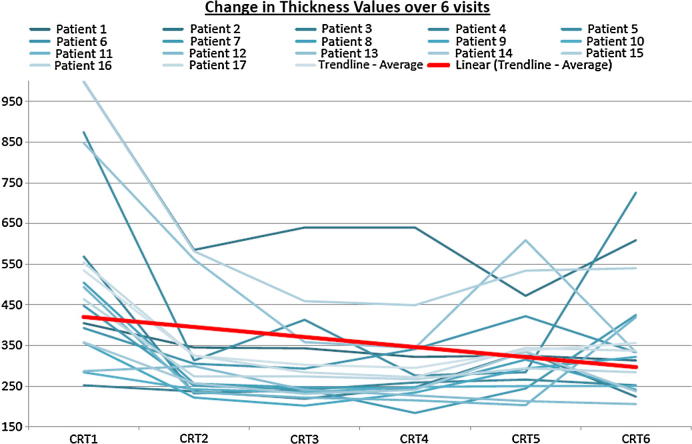
Graph 2.
This graph shows the central foveal thickness in all patients during the 6 months of treatment. It shows immediate response to first injection in all patients. Final thickness is better than the initial in all patients.
The anatomical improvement did not correlate with functional improvement in all patients, a fact that we anticipated and all patients were cautioned before entering the study. All patients were back to PRN regimen after the sixth visit (Graph 3).
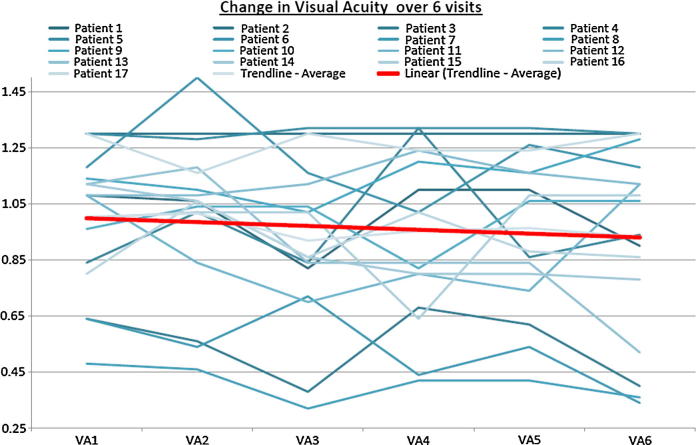
Graph 3.
This graph illustrates the visual acuity changes during the study. It confirms the stable visual acuity with very mild gain in some patients but no deterioration.
No cases of endophthalmitis ocular or systemic side effects were observed.
Discussion
In 2007, ranibizumab was licensed to treat the exudative form of AMD, following 2 pivotal trials, Anchor and Marina. Many regimens were advocated thereafter, namely monthly treatment similar to the trials, PRN regimens, treat and extend and even 3 monthly.
In the Trials of Ranibizumab in the Treatment of Neovascular AMD (MARINA) and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR), 95% of ranibizumab-treated patients maintained visual acuity compared to 62% of sham or photodynamic therapy (PDT) groups (p < 0.01) at 1 year. Furthermore, up to 40% demonstrated an improvement in vision of at least three lines.5
It is still unknown why more than half of patients do not improve after anti-VEGF therapy and about 10% of patients do not respond at all to treatment. Tachyphylaxis or loss of drug effectiveness after repetitive injections of ranibizumab was recently recognized. Genetic factors are another important parameter that should be considered.
Tranos et al. conducted a review of the literature for the possible causes of failure after treatment with anti-VEGF agents.6 They conducted a comprehensive search of the literature published until 2012 to identify studies evaluating the effects of anti-VEGF therapeutic agents in the management of AMD. These included randomized controlled studies, prospective and retrospective case-control studies, and case series. Studies with other clinical subtypes of AMD were not excluded. English and non-English language articles with abstracts translated into English were retrieved too. These searches were supplemented by manually searching the reference lists of all major review articles.
In their review, Tranos et al. found that poor response to anti-VEGF may be due in nearly 50% of cases to misdiagnosis of AMD, such as polypoidal choroidal vasculopathy (PCV) that accounts for the majority of the underlying pathology. Tachyphylaxis also appeared to play a significant role and they believed that a small number of AMD patients may be genetically predisposed to show resistance to anti-VEGF treatment.
In 2011, Manoj 7 published a retrospective study between January 2007 and December 2008 to evaluate eyes with AMD that responded poorly to anti-VEGF therapy and to investigate reasons for treatment failure. In 24 eyes (46.2%) of the patients with poor response to treatment, he identified cases of PCV (19 eyes), retinal angiomatous proliferation (RAP) (four eyes), and vitelliform lesion (one eye). After excluding the misdiagnosed cases, 14 eyes (26.9%) were identified, which were nonresponders and eleven eyes (22.2%) which fulfilled the criteria for diagnosing Tachyphylaxis. Six eyes (11.5%) developed complications such as retinal pigment epithelium (RPE) tear, scarring, RPE atrophy, and eventually poor visual outcome.
Immunization against Ranibizumab was recently identified in 17% of patients treated with Ranibizumab, using ELISA, in a study conducted by Leveziel et al.8 This study showed that no immunization was detected among naïve patients and it increased with the number of injections. For patients with 10 or fewer previous IVTs, immunization against Ranibizumab was detected in 4/36 patients (11.1%) whereas immunization was observed in 10/46 patients (21.7%) with more intravitreal injections (IVTs).
In an approach to try to understand the pathology of resistant cases, Shin JY et al.9 described OCT characteristics of wet AMD patients refractory to IVTs (Ranibizumab, Bevacizumab) and their responses to alternative anti-VEGF agents or photodynamic therapy (PDT).
In his retrospective review of 267 neovascular AMD patients treated with intravitreal anti-VEGF injections, 20 patients (7.5%) were refractory to anti-VEGF injections (stationary or increased retinal exudation despite three or more monthly injections). He grouped them into either the extensive intraretinal fluid group (9 patients) or the subretinal fluid only group (11 patients) according to OCT findings. In the IRF group, response rates to subsequent treatment were 0% (0/7) for Bevacizumab, 50% (3/6) for Ranibizumab and 50% (3/6) for PDT ± anti-VEGF. Three out of four Bevacizumab-refractory patients showed response to Ranibizumab as a secondary treatment. In the SRF group, response rates were lower with 0% (0/7) for Bevacizumab, 22.2% (2/9) for Ranibizumab and 28.6% (2/7) for PDT ± anti-VEGF. One out of four Bevacizumab-refractory patients responded to Ranibizumab.
In anti-VEGF-refractory neovascular AMD, patients with extensive IRF refractory to Bevacizumab can be responsive to Ranibizumab while patients with SRF may be refractory to both, suggesting a different pathophysiology and intraocular pharmacokinetics.
With the release of Aflibercept with 100 times affinity to VEGF10, many authors introduced this new drug in cases with no response to Ranibizumab, with variable results.
In a retrospective review at Ophthalmic Consultants of Boston, Cho et al.11 evaluated the anatomical and visual effect of intravitreal Aflibercept 2.0 mg in cases of exudative age-related macular degeneration (AMD) with persistent fluid on optical coherence tomography (OCT) despite regular Ranibizumab 0.5 mg and/or Bevacizumab 1.25 mg treatment at 1 and 6 months.
They included a total of 353 eyes with exudative AMD that were switched to Aflibercept during the study period. Of these, 28 eyes in 28 patients had persistent fluid after an average of 20 regular Ranibizumab/Bevacizumab injections (range 7–37). At 1 month, 89% (25 eyes) showed anatomical improvement and 18% (five eyes) were dry after a single Aflibercept injection. Central subfoveal thickness improved from 295 to 272 μm (p < 0.001) after one Aflibercept injection. After an average of 4.4 Aflibercept injections (range 3–6) over 6 months, the central subfoveal thickness remained improved (274 μm, p = 0.008); 64% (18 eyes) showed anatomical improvement and a quarter of eyes (25%, seven eyes) were dry.
Visual acuity did not improve at 1 month (Log MAR 0.54, p = 0.64) or 6 months (Log MAR 0.57, p = 0.49). Despite no improvement in visual acuity, a significant proportion of cases responded anatomically to Aflibercept 2.0 mg.
Bakall et al.12 conducted a retrospective chart review looking for Ranibizumab/ Bevacizumab resistant cases and identified 36 eyes from 31 patients.
There were 13 male and 18 female patients. The number of prior injections with either Bevacizumab or Ranibizumab ranged from 6 to 74. After 3 monthly injections of Aflibercept, there was a reduction in either subretinal or intraretinal fluid in 18 of 36 (50.0%) of the treated eyes; the amount of fluid remained stable in 15 eyes (41.7%) and worsened in 3 eyes (8.3%). A significant average decrease was observed for the central macular thickness after 3 injections of 65 μm, with no significant change in visual acuity.
In addition to Aflibercept, the 2 mg dose of Ranibizumab was the subject of a trial in patients recalcitrant to ranibizumab 0.5 mg dose.13 This Phase I-II trial of 88 patients with recalcitrant neovascular AMD treated as needed for every 4 (cohort A) or 6 weeks (cohort B) following three monthly doses. 79 patients completed the 12-month endpoint and were given 11.6 (cohort A) and 8.6 (cohort B) mean treatments. Mean best corrected visual acuity gains of 4.1 letters following three monthly doses were sustained for 12 months for both cohorts. Anatomical improvements were sustained for 12 months for cohort A, but not for cohort B; cohort B demonstrated a gradual increase in mean central retinal thickness (p = 0.03).
The interesting fact in this trial is that visual and anatomical gains achieved with 2.0 mg Ranibizumab in recalcitrant neovascular AMD were sustained for 1 year only with monthly treatment. In comparison, anatomical gains were reduced with less than monthly treatment.
Conclusion
In our study, all patients previously non-responding to Ranibizumab had immediate response to aflibercept shown as reduction in macular thickness mainly from the first injection.
The strength of this study comes from the prospective design, the absence of selection bias of patients as all patients with no response to ranibizumab were included regardless of their visual acuity, macular status or the amount of fluid in the macula.
The weakness of this study comes from the small number of patients treated, the short follow-up time and the fact that no Indocyanine green angiography (ICG) was performed to rule out polypoidal lesions although one case was suspicious of PCV, however we had a considerable anatomical improvement, although not complete, in that case.
Aflibercept was able to dry all maculaes even in very advanced cases deemed untreatable and is recommended as a treatment option in cases of no-response to ranibizumab.
Conflict of interest
The authors declare that there are no conflict of interests.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Fraser H.M., Wilson H., Silvestri A., Morris K.D., Wiegand S.J. The role of vascular endothelial growth factor and estradiol in the regulation of endometrial angiogenesis and cell proliferation in the marmoset. Endocrinology. 2008;149(9):4413–4420. doi: 10.1210/en.2008-0325. [DOI] [PubMed] [Google Scholar]
- 2.Ziv-Aflibercept. FDA Drug Approvals Database. Food and Drug Administration. August 3, 2012. Retrieved 2013-10-16.
- 3.Schmidt-Erfurth U. VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. Epub 2012 Oct 17. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi: 10.1016/j.ophtha.2013.08.011. Epub 2013 Sep 29. [DOI] [PubMed] [Google Scholar]
- 5.Presta L.G., Chen H., O’Connor S.J. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumours and other disorders. Cancer Res. 1997;57(20):4593–4599. [PubMed] [Google Scholar]
- 6.Tranos Paris, Vacalis Athanasios, Asteriadis Solon. Resistance to antivascular endothelial growth factor treatment in age-related macular degeneratio. Drug Des Devel Ther. 2013;7:485–490. doi: 10.2147/DDDT.S43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manoj S.M. Why does anti VEGF treatment fail in age related macular degeneration (AMD) Kerala J Ophthalmol. 2011 [Google Scholar]
- 8.Leveziel N., Pelat T., Watier H. Detection of antiranibizumab antibodies among patients with exudative age-related macular degeneration. Ophthalmologica. 2014;232(1):53–56. doi: 10.1159/000360186. Epub 2014 May 20. [DOI] [PubMed] [Google Scholar]
- 9.Shin J.Y., Woo S.J., Ahn J. Anti-VEGF-refractory exudative age-related macular degeneration: differential response according to features on optical coherence tomography. Korean J Ophthalmol. 2013;27(6):425–432. doi: 10.3341/kjo.2013.27.6.425. Epub 2013 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart Michael W., Rosenfeld Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab. Bevacizumab and aflibercept (vascular endothelial growth factor trap-EYE) Retina. 2012;32(3):434–457. doi: 10.1097/IAE.0B013E31822C290F. [DOI] [PubMed] [Google Scholar]
- 11.Cho H., Shah C.P., Weber M. Aflibercept for exudative AMD with persistent fluid on Ranibizumab and/or Bevacizumab. Br J Ophthalmol. 2013;97(8):1032–1035. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 12.Bakall B., Folk J.C., Boldt H.C. Aflibercept therapy for exudative age-related macular degeneration resistant to Bevacizumab and Ranibizumab. Am J Ophthalmol. 2013;156(1):15–22. doi: 10.1016/j.ajo.2013.02.017. 22.e1, Epub 2013 May 22. [DOI] [PubMed] [Google Scholar]
- 13.Wykoff C.C., Brown D.M., Chen E. SAVE (Super-dose anti-VEGF) trial: 2.0 mg ranibizumab for recalcitrant neovascular age-related macular degeneration: 1-year results. Ophthalmic Surg Lasers Imaging. Retina. 2013;44(2):121–126. doi: 10.3928/23258160-20130313-04. [DOI] [PubMed] [Google Scholar]