Abstract
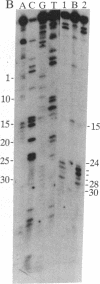
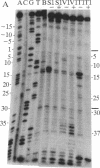
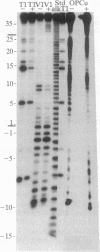
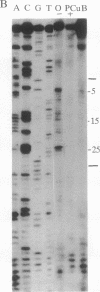
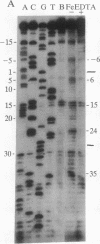
The ferritin iron regulatory element (IRE), a conserved sequence of 28 nucleotides in a hairpin loop, is a conserved mRNA-specific translational regulatory element; flanking the IRE are regions of varying sequence, which form 9-17 base pairs close to the 5' cap. P-90 is a ferritin mRNA-specific translation regulatory protein purified from animal liver and reticulocytes. To study the P-90-RNA interaction, protein nucleases (RNase S1 and T1) and chemical nucleases FeEDTA and/or 1,10-phenanthroline-Cu were used as probes of an oligonucleotide (n = 55), containing the IRE and flanking regions (FL), and natural ferritin mRNA. Footprints and "toeprints" showed that P-90 binding was confined to the stem and loop of the IRE itself. However, P-90 altered the structure of the flanking region by increasing base stacking or helicity (RNase V1 sensitivity). Comparison of the reactivity of the IRE and flanking regions in natural mRNA and the 55-mer showed that long-range interactions included protecting bulges, single-stranded, and stacked regions from protein nucleases as well as stabilizing the P-90-RNA interaction. Structural integration of the IRE with the base-paired flanking regions was indicated by common features of reactivity (periodic hypersensitivity to FeEDTA) and changes in the FL region caused by P-90. The increased secondary structure of the IRE flanking regions caused by P-90 binding to the IRE provides a likely mechanism for blocking initiation of ferritin mRNA translation, since the combined structure (IRE + FL) is so close (8-17 nucleotides) to the cap.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughman S. W., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. J Biol Chem. 1988 Dec 15;263(35):19048–19052. [PubMed] [Google Scholar]
- Celander D. W., Cech T. R. Iron(II)-ethylenediaminetetraacetic acid catalyzed cleavage of RNA and DNA oligonucleotides: similar reactivity toward single- and double-stranded forms. Biochemistry. 1990 Feb 13;29(6):1355–1361. doi: 10.1021/bi00458a001. [DOI] [PubMed] [Google Scholar]
- Celander D. W., Cech T. R. Visualizing the higher order folding of a catalytic RNA molecule. Science. 1991 Jan 25;251(4992):401–407. doi: 10.1126/science.1989074. [DOI] [PubMed] [Google Scholar]
- Churchill M. E., Tullius T. D., Klug A. Mode of interaction of the zinc finger protein TFIIIA with a 5S RNA gene of Xenopus. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5528–5532. doi: 10.1073/pnas.87.14.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckman I. C., Draper D. E. S4-alpha mRNA translation regulation complex. II. Secondary structures of the RNA regulatory site in the presence and absence of S4. J Mol Biol. 1987 Jul 20;196(2):323–332. doi: 10.1016/0022-2836(87)90693-0. [DOI] [PubMed] [Google Scholar]
- Dickey L. F., Wang Y. H., Shull G. E., Wortman I. A., 3rd, Theil E. C. The importance of the 3'-untranslated region in the translational control of ferritin mRNA. J Biol Chem. 1988 Mar 5;263(7):3071–3074. [PubMed] [Google Scholar]
- Didsbury J. R., Theil E. C., Kaufman R. E., Dickey L. F. Multiple red cell ferritin mRNAs, which code for an abundant protein in the embryonic cell type, analyzed by cDNA sequence and by primer extension of the 5'-untranslated regions. J Biol Chem. 1986 Jan 15;261(2):949–955. [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Klausner R. D. The inhibition of the iron responsive element RNA-protein interaction by heme does not mimic in vivo iron regulation. J Biol Chem. 1990 Aug 5;265(22):12786–12789. [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Traut R., Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Leibold E. A., Laudano A., Yu Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990 Apr 11;18(7):1819–1824. doi: 10.1093/nar/18.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Patino M. M., Gaffield L., Walden W. E., Thach R. E. Derepression of ferritin messenger RNA translation by hemin in vitro. Science. 1990 Jan 5;247(4938):74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- Lowman H. B., Draper D. E. On the recognition of helical RNA by cobra venom V1 nuclease. J Biol Chem. 1986 Apr 25;261(12):5396–5403. [PubMed] [Google Scholar]
- McPheeters D. S., Stormo G. D., Gold L. Autogenous regulatory site on the bacteriophage T4 gene 32 messenger RNA. J Mol Biol. 1988 Jun 5;201(3):517–535. doi: 10.1016/0022-2836(88)90634-1. [DOI] [PubMed] [Google Scholar]
- Mertz J. R., Theil E. C. Subunit dimers in sheep spleen apoferritin. The effect on iron storage. J Biol Chem. 1983 Oct 10;258(19):11719–11726. [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann C., Ehresmann B. Messenger RNA structure and gene regulation at the translational level in Escherichia coli: the case of threonine:tRNAThr ligase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7892–7896. doi: 10.1073/pnas.85.21.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Proudhon D., Briat J. F., Lescure A. M. Iron induction of ferritin synthesis in soybean cell suspensions. Plant Physiol. 1989 Jun;90(2):586–590. doi: 10.1104/pp.90.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland M., Briat J. F., Gagnon J., Laulhere J. P., Massenet O., Theil E. C. Evidence for conservation of ferritin sequences among plants and animals and for a transit peptide in soybean. J Biol Chem. 1990 Oct 25;265(30):18339–18344. [PubMed] [Google Scholar]
- Romaniuk P. J., Lowary P., Wu H. N., Stormo G., Uhlenbeck O. C. RNA binding site of R17 coat protein. Biochemistry. 1987 Mar 24;26(6):1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., de Stevenson I. L., Wong H. H. Defining the binding site of Xenopus transcription factor IIIA on 5S RNA using truncated and chimeric 5S RNA molecules. Nucleic Acids Res. 1987 Mar 25;15(6):2737–2755. doi: 10.1093/nar/15.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger S., Müllner E. W., Kühn L. C. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990 Mar 11;18(5):1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Haile D. J., Harford J. B., Klausner R. D. The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5768–5772. doi: 10.1073/pnas.86.15.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer F. V., Theil E. C. The effect of iron on the synthesis and amount of ferritin in red blood cells during ontogeny. J Biol Chem. 1981 Feb 25;256(4):1711–1715. [PubMed] [Google Scholar]
- Selby M. J., Peterlin B. M. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990 Aug 24;62(4):769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Chen C. H. Chemical nucleases: new reagents in molecular biology. Annu Rev Biochem. 1990;59:207–236. doi: 10.1146/annurev.bi.59.070190.001231. [DOI] [PubMed] [Google Scholar]
- Swenson G. R., Patino M. M., Beck M. M., Gaffield L., Walden W. E. Characteristics of the interaction of the ferritin repressor protein with the iron-responsive element. Biol Met. 1991;4(1):48–55. doi: 10.1007/BF01135557. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Draper D. E. Unusual mRNA pseudoknot structure is recognized by a protein translational repressor. Cell. 1989 May 19;57(4):531–536. doi: 10.1016/0092-8674(89)90123-2. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Tuerk C., Eddy S., Parma D., Gold L. Autogenous translational operator recognized by bacteriophage T4 DNA polymerase. J Mol Biol. 1990 Jun 20;213(4):749–761. doi: 10.1016/S0022-2836(05)80261-X. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Walden W. E., Thach R. E. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry. 1986 Apr 22;25(8):2033–2041. doi: 10.1021/bi00356a030. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Lin P. N., Sczekan S. R., McKenzie R. A., Theil E. C. Ferritin mRNA probed, near the iron regulatory region, with protein and chemical (1,10-phenanthroline-Cu) nucleases. A possible role for base-paired flanking regions. Biol Met. 1991;4(1):56–61. doi: 10.1007/BF01135558. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Sczekan S. R., Theil E. C. Structure of the 5' untranslated regulatory region of ferritin mRNA studied in solution. Nucleic Acids Res. 1990 Aug 11;18(15):4463–4468. doi: 10.1093/nar/18.15.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks K. M., Ampe C., Schultz S. C., Steitz T. A., Crothers D. M. Fragments of the HIV-1 Tat protein specifically bind TAR RNA. Science. 1990 Sep 14;249(4974):1281–1285. doi: 10.1126/science.2205002. [DOI] [PubMed] [Google Scholar]
- Witherell G. W., Uhlenbeck O. C. Specific RNA binding by Q beta coat protein. Biochemistry. 1989 Jan 10;28(1):71–76. doi: 10.1021/bi00427a011. [DOI] [PubMed] [Google Scholar]
- Witherell G. W., Wu H. N., Uhlenbeck O. C. Cooperative binding of R17 coat protein to RNA. Biochemistry. 1990 Dec 18;29(50):11051–11057. doi: 10.1021/bi00502a006. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]