Abstract
Background
Aboriginal women have been identified as having poorer pregnancy outcomes than other Canadian women, but information on risk factors and outcomes has been acquired mostly from retrospective databases. We compared prenatal risk factors and birth outcomes of First Nations and Métis women with those of other participants in a prospective study.
Methods
During the 12-month period from July 1994 to June 1995, we invited expectant mothers in all obstetric practices affiliated with a single teaching hospital in Edmonton to participate. Women were recruited at their first prenatal visit and followed through delivery. Sociodemographic and clinical data were obtained by means of a patient questionnaire, and microbiological data were collected at 3 points during gestation: in the first and second trimesters and during labour. Our primary outcomes of interest were low birth weight (birth weight less than 2500 g), prematurity (birth at less than 37 weeks' gestation) and macrosomia (birth weight greater than 4000 g).
Results
Of the 2047 women consecutively enrolled, 1811 completed the study through delivery. Aboriginal women accounted for 70 (3.9%) of the subjects who completed the study (45 First Nations women and 25 Métis women). Known risk factors for adverse pregnancy outcome were more common among Aboriginal than among non-Aboriginal women, including previous premature infant (21% v. 11%), smoking during the current pregnancy (41% v. 13%), presence of bacterial vaginosis in midgestation (33% v. 13%) and poor nutrition as measured by meal consumption. Although Aboriginal women were less likely than non-Aboriginal women to have babies of low birth weight (odds ratio [OR] 1.46, 95% confidence interval [CI] 0.52–4.15) or who were born prematurely (OR 1.45, 95% CI 0.57–3.72) and more likely to have babies with macrosomia (OR 2.04, 95% CI 1.03–4.03), these differences were lower and statistically nonsignificant after adjustment for smoking, cervicovaginal infection and income (adjusted OR for low birth weight 0.85, 95% CI 0.19–3.78; for prematurity 0.90, 95% CI 0.21–3.89; and for macrosomia 2.12, 95% CI 0.84-5.36).
Interpretation
After adjustment for potential confounding factors, we found no statistically significant relation between Aboriginal status and birth outcome.
It is generally recognized that Aboriginal women experience poorer birth outcomes than other North American women, including higher rates of stillbirth,1 low-birth-weight infants1,2,3 and prematurity.2,3 Although significant efforts have been made to reduce Aboriginal infant mortality rates, these rates remain higher than for other infants in both Canada4 and the United States.5 Little is known about the reasons for differences in birth outcomes, although social, economic, medical and prenatal care factors have been suggested. Recent publications, based on retrospective analyses of large databases, have confirmed disparities in birth outcomes between Aboriginal and all other groups,3,6,7 but there is a paucity of prospective data. In addition, although the term “Aboriginal” refers to a heterogeneous population comprising First Nations people, Métis and Inuit, there are few comparisons between specific Aboriginal groups or of Aboriginal groups with the general population.
We report here the results of a prospective study in a general obstetric population, comparing birth outcomes and known pregnancy risk factors of Aboriginal women with those of non-Aboriginal Canadian women. In addition to well-recognized socioeconomic and reproductive risk factors, we investigated the prevalence of maternal cervicovaginal infections, which have been increasingly linked to prematurity.8,9
Methods
This study was approved by the ethics committee, Faculty of Medicine, University of Alberta.
The study was conducted at 3 private obstetric offices, one hospital-based office and the University of Alberta Hospital (UAH) in Edmonton. The first examination occurred at the initial visit (during the first trimester), the second took place in the obstetrician's office between 26 and 30 weeks' gestation, and the third in the hospital at time of labour and delivery. The UAH is the sole hospital used for all deliveries associated with the 4 offices in the study, and deliveries for women seen in other practices are not performed at UAH. All infants were born in the UAH labour and delivery suites. Microbiology was performed on site; Gram staining and bacterial culture were performed at the UAH laboratories, whereas serologic testing and chlamydial and mycoplasma culture were performed at the Provincial Laboratory of Public Health for Northern Alberta, also on the UAH campus.
Details of questionnaire development, pilot-testing and standardization have been reported previously.10 All pregnant women seen for a first visit before 20 weeks' gestation at each of the 4 obstetric offices associated with UAH were eligible for the study. We invited all eligible women over a 12-month period (from July 1994 through June 1995) to participate.
A sealed box was left in each obstetrician's office in which patients could deposit their completed questionnaires. The study nurse then contacted and interviewed each patient and reviewed the clinic charts to determine obstetric risk factors, as described previously.10 Among the variables identified in previous studies as being associated with birth outcomes and assessed in this study were maternal age, ethnicity, parity, marital status, welfare status, cigarette smoking, alcohol and other drug consumption, prior preterm delivery, and urinary and genital tract infections during the current pregnancy. Nutritional habits were determined from each woman's self-reporting of whether she customarily ate breakfast, lunch, supper and snacks, since meal consumption can be viewed as an indicator of adequate nutrition during pregnancy.11 Clinical variables such as underlying medical conditions, current medications, reproductive history and obstetric data were recorded. Ethnicity was determined by the following question: “To which ethnic or racial group(s) do you belong?” The number “1” was entered for each positive response, which allowed multiple answers. Ethnicity data were recorded as missing for individuals having inconsistent entries (e.g., Caucasian and First Nation). In our study the term “Aboriginal” covers First Nation, Métis and Inuit people, but there was only 1 Inuit woman in the sample; her data were not included in this analysis. Test-retest reliability of the questionnaire was ascertained by mailing repeat questionnaires to 50 of the enrolled patients to compare responses with those provided during their first visit.
Each patient enrolled in the study underwent 3 speculum examinations (in the first and second trimesters and during delivery), and microbiological investigations were performed on the specimens collected. Specimens for culture of Neisseira gonorrhoeae, Chlamydia trachomatis, group B Streptococcus, Mycoplasma hominis and Ureaplasma urealyticum and specimens for Gram staining for bacterial vaginosis, Trichomonas-like organisms and yeast were collected in the obstetrician's office at the initial visit and between 26 and 30 weeks' gestation and in the hospital during labour. Culture techniques for the isolation of bacteria, Chlamydia and mycoplasmas have been reported in detail previously.10 Bacterial vaginosis was diagnosed by Gram staining of vaginal swab specimens, graded according to the criteria described by Nugent and associates.12
All data were entered into a database, and accuracy of the data, as well as accuracy of data entry, was verified with sample checks. The distribution of all variables was checked for outliers; inconsistent data and data outside valid ranges were omitted. Descriptive statistics were calculated for each variable. For categorical data, 2 х 2 tables with the Fisher's exact test were used, and for data with more than 2 categories the χ2 statistic was used. The 2-sample t test was used to compare continuous variables. Potential confounders in the relation between ethnicity and birth outcomes were assessed as follows. Variables kept in the final logistic regression model were those that were first associated at least at the 15% level of statistical significance with both ethnicity and birth outcomes, that were not biologically perceived to be intermediary variables and that were not effect modifiers (according to the Breslow–Day test for homogeneity of the odds ratio [OR]). All p values were 2-sided, and a 0.05 level was used to assess statistical significance. The 95% confidence intervals [CIs] of the proportions were computed using the approximation to the normal distribution. The Wald statistic was used to compute the 95% confidence limits of the OR.
Results
A total of 2047 (92%) of the 2237 women approached for the study agreed to participate and returned questionnaires. Of these, 1811 (81% of the total) completed the study through delivery. This group included 25 Métis and 45 First Nations women (3.9%). That proportion is similar to 1996 census data for Edmonton, which indicated that 4.3% of the female population was Aboriginal. The remainder of the study participants reflected the racial and ethnic composition of Edmonton.13
Table 1 compares sociodemographic variables and microbiologic characteristics of Aboriginal and non-Aboriginal women enrolled in the study. This table accounts for a total of 1975 women, and for 1811 of these we had data on at least 1 of the 3 birth outcomes of interest. Aboriginal women were less likely to be married and more likely to report incomes below the 1996 official poverty line of $12 000 per year (both p < 0.001). However, Aboriginal women were not a uniform group with respect to their risk factors (data not shown). Specifically, Métis women were more often married (46% v. 16%, p = 0.01) and less often living below the poverty line (15% v. 43%, p = 0.06) than the First Nations participants. Métis women were also older than First Nations women at the time of entry into the study (mean age 27.6 v. 24.7 years; p = 0.05).
Table 1
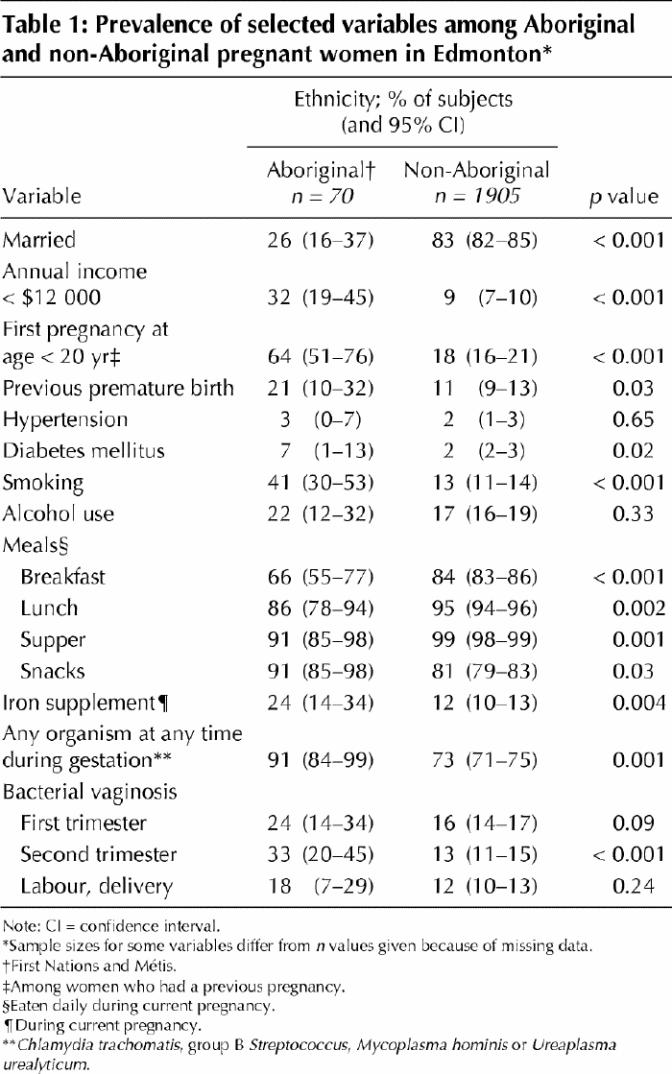
Some risk factors for pregnancy outcome were common to both Aboriginal groups. For example, cigarette smoking and alcohol use during the current pregnancy were similar for Métis and First Nations women (36% v. 44% for cigarette smoking, 20% v. 23% for alcohol use), although these risk factors were more prevalent within the Aboriginal group as a whole than among non-Aboriginal women (41% v. 13% for cigarette smoking [p < 0.001], 22% v. 17% for alcohol use [p = 0.33]). Diabetes was more common among Aboriginal women (7% v. 2%, p = 0.02). The differences in prevalence of heart, kidney and thyroid disease between Aboriginal and other participants were not statistically significant.
Aboriginal women were more likely to have had one or more pregnancies before the study (81% v. 69%, p = 0.02). They were also significantly more likely to have had their first pregnancy before the age of 20 years (64% v. 18%, p < 0.001) (Table 1). Among women who had had a previous pregnancy, 21% of the Aboriginal women (32% of Métis women and 16% of First Nations women) had delivered at least one previous premature infant, whereas only 11% of non-Aboriginal women had done so (p = 0.03) (Table 1).
Mean baseline body weights were not markedly different between Métis and First Nations subjects (61.9 v. 62.0 kg). However, First Nations women gained 1.3 kg more during their pregnancy than Métis mothers, although the difference did not reach statistical significance. The mean birth weight of First Nations infants was also higher than the birth weight of other infants (3633 v. 3386 g, p = 0.01).
At each sampling point during gestation and labour, Aboriginal women were more likely to have positive test results for any of C. trachomatis, group B Streptococcus, M. hominis, U. urealyticum and bacterial vaginosis. Bacterial vaginosis was significantly more likely to be present in the second trimester among Aboriginal than among non-Aboriginal study participants (33% v. 13%, p < 0.001), or when combined, in at least 1 of the first 2 trimesters among Aboriginal than among non-Aboriginal study participants (41% v. 23%, p = 0.004).
First Nations infants were more likely than study infants in all other ethnic groups to have macrosomia (birth weight greater than 4000 g) (p = 0.004), and this association remained statistically significant (p = 0.03) after adjustment in multivariate analyses for all the above variables (OR 4.5, 95% CI 1.6–12.5).
Among selected variables presented in Table 2, family income under $12 000 was associated with low birth weight and inversely associated with macrosomia. Neither marital status nor age at first pregnancy appeared to be associated with birth outcomes. Low birth weight was significantly higher among women who smoked at least 1 cigarette daily than among nonsmokers (11% v. 5%, p < 0.001). Macrosomia was less common among smokers (7% v. 12%, p = 0.03). Low birth weight was more common among women with any cervicovaginal infection than among women who had no such infections (6% v. 3%, p = 0.02).
Table 2
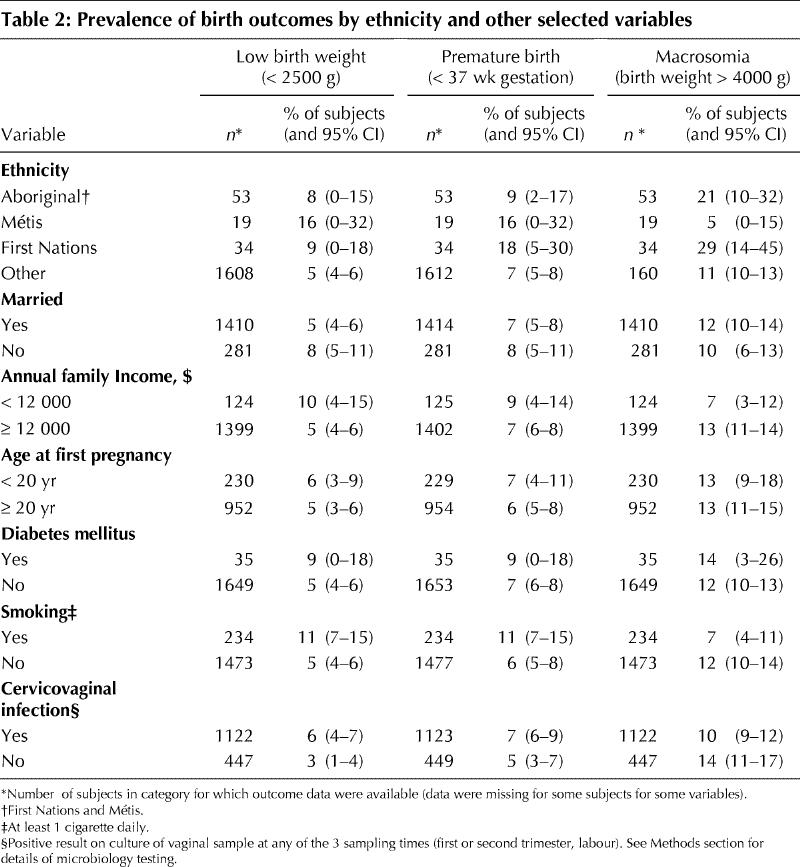
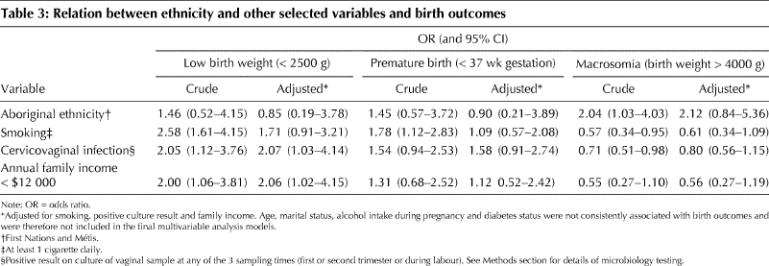
Table 3 shows the relations between ethnicity and other selected variables and birth outcomes. Adjustments were made for smoking, positive cervicovaginal culture result and low income. Age, marital status, diabetes, history of a previous premature birth and alcohol intake were associated with some birth outcomes but not ethnicity or vice versa and therefore were not included in the final multivariable analysis models. After adjustment, Aboriginal ethnicity was not associated with low birth weight, prematurity or macrosomia. Smoking was positively associated with low birth weight and prematurity but this relation disappeared after adjustment. Positive cervicovaginal culture result and income under $12 000 were both associated with low birth weight, an association that remained statistically significant after adjustment.
Table 3
Interpretation
We found a higher prevalence of smoking, poor nutrition, low income, a previous premature infant and bacterial vaginosis among Aboriginal women than among non-Aboriginal women. Although Aboriginal women and specific subgroups of Aboriginal women (First Nations and Métis women) were more likely to have babies that weighed less than 2500 g (low birth weight) or more than 4000 g (macrosomia) or were born at less than 37 weeks' gestation (premature), these differences were statistically nonsignificant.
Disparities in birth outcomes have been recognized for Aboriginal groups in North America and Australia.1,2,3,4,5,6,7,14,15,16 Differences between Aboriginal and non-Aboriginal birth outcomes in Australia have received considerable investigative attention, and factors such as maternal malnutrition, smoking and hypertension15,16 have been implicated in poor outcomes. In contrast to the present study, information on Aboriginal births in North America has been obtained largely from analyses of secondary databases. For example, Baldwin and associates6 found a significantly higher rate of low birth weight among American Indians and Alaska Natives in an analysis of a large national database for the period 1989 to 1991. Many factors, including inadequate prenatal care,6,17 socioeconomic disparities2,17,18 and short interpregnancy intervals,19 have been implicated in the incidence of low birth weight in studies involving North American native people. Johnson and colleagues7 assessed the role of poverty by comparing band-registered First Nations people in Alberta with a non-First Nations group with low socioeconomic status. Both groups had poorer birth outcomes than other women in the province, and both had higher rates of behavioural risk factors such as smoking and alcohol use. The behavioural risk profiles of our Aboriginal cohorts were similar to those reported in other Canadian centres.20,21 Although not studied previously in Aboriginal women, genital tract infections have been increasingly recognized as important risk factors in premature birth.8,9,22,23 The prevalence of positive cultures for bacterial vaginosis in the first and second trimesters was higher among Aboriginal than non-Aboriginal women, which is consistent with studies reporting that the stage of bacterial vaginosis infection in pregnancy is important to pregnancy outcome.24,25 Our findings that First Nations infants had higher mean birth weights than children of other women and that more Aboriginal infants presented with macrosomia than non-Aboriginal infants are consistent with other Canadian reports.26,27,28,29
Our study had a few limitations, notably the relatively small numbers of Aboriginal participants and the even smaller number of Métis and First Nations women with certain specific outcomes. These small sample sizes limited the use of multivariable analyses, as the number of individuals decreased with the number of variables in the models because of missing data. Therefore, some of the nonsignificant associations might be due to a lack of statistical power.
Overall, the present study demonstrates that, despite sharing known risk factors for adverse pregnancy outcomes, Aboriginal women are not a homogeneous maternity group, and birth outcomes were different between First Nations and Métis women in the study. However, Aboriginal ethnicity itself was not independently associated with low birth weight, prematurity or macrosomia. These findings should be considered in programs addressing pregnancy outcomes in Canadian Aboriginal populations.
β See related articles pages 577, 597
Acknowledgments
This work was supported by a grant from the Children's Hospital Foundation, Alberta.
Footnotes
This article has been peer reviewed.
Contributors: Wanda Wenman designed the study, participated in laboratory supervision and data interpretation, and drafted the paper. Michel Joffres participated in formulating the questionnaire, supervised the statistical methods, performed the data analysis and participated in revising the paper. Ivanna Tataryn participated in formulating the questionnaire, collecting the data and revising the paper.
Competing interests: None declared.
Correspondence to: Dr. Wanda M. Wenman, Section of Pediatric Infectious Diseases, University of California Davis Medical Center, 2516 Stockton Blvd., Sacramento CA 95817; fax 916 734-7890; wmwenman@ucdavis.edu
References
- 1.Edouard L, Gillis D, Habbick B. Pregnancy outcome among native Indians in Saskatchewan. CMAJ 1991;144(12):1623-5. [PMC free article] [PubMed]
- 2.Grossman DC, Krieger JW, Sugarman JR, Forquera RA. Health status of urban American Indians and Alaska Natives. JAMA 1994;271:845-50. [PubMed]
- 3.Abel EL, Kruger M, Burd L. Effects of maternal and paternal age on Caucasian and Native American preterm births and birth weights. Am J Perinatol 2002; 19:49-54. [DOI] [PubMed]
- 4.Royal Commission on Aboriginal Peoples. Looking forward, looking back: report of the Royal Commission on Aboriginal peoples. Vol 1. Ottawa: The Commission; 1996.
- 5.Trends in Indian Health. Rockville (MD): Indian Health Service; 1997.
- 6.Baldwin LM, Grossman DC, Casey S, Hollow W, Sugarman JR, Freeman WL, et al. Perinatal and infant health among rural and urban American Indians/Alaska Natives. Am J Public Health 2002;92(9):1491-7. [DOI] [PMC free article] [PubMed]
- 7.Johnson D, Jin Y, Truman C. Influence of Aboriginal and socioeconomic status on birth outcome and maternal morbidity. J Obstet Gynaecol Can 2002; 24: 633-40. [DOI] [PubMed]
- 8.Andrews W, Goldenberg R, Hauth J. Preterm labor: emerging role of genital tract infections. Infect Agents Dis 1995;4:196-211. [PubMed]
- 9.Hillier S, Nugent R, Eschenbach D, Krohn M, Gibbs R, Martin D, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 1995;33:1737-42. [DOI] [PubMed]
- 10.Wenman WM, Tataryn IV, Joffres MR, Pearson R, Grace MGA, Albritton WL, et al. Demographic, clinical and microbiological characteristics of maternity patients: a Canadian clinical cohort study. Can J Infect Dis 2002; 13 (5): 311-8. [DOI] [PMC free article] [PubMed]
- 11.Siega-Riz AM, Herrmann TS, Savitz DA, Thorp JM. Frequency of eating during pregnancy and its effect on preterm delivery. Am J Epidemiol 2001; 153: 647-52. [DOI] [PubMed]
- 12.Nugent RP, Krohn M, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991;29:297-301. [DOI] [PMC free article] [PubMed]
- 13.Statistics for Edmonton (Census Metropolitan Area), Alberta. Ottawa: Statistics Canada; 1996. Available: www.statcan.ca/english/census96 (accessed 2002 May).
- 14.Coory M. An investigation into the disparity between Australian aboriginal and Caucasian perinatal mortality rates. Ann Epidemiol 1995;5:393-9. [DOI] [PubMed]
- 15.Sayers S, Powers J. Risk factors for aboriginal low birthweight, intrauterine growth retardation and preterm birth in the Darwin Health Region. Aust N Z J Public Health 1997;5:524-30. [DOI] [PubMed]
- 16.Chan A, Keane RJ, Robinson JS. The contribution of maternal smoking to preterm birth, small for gestational age and low birthweight among Aboriginal and non-Aboriginal births in South Australia. Med J Aust 2001;174:389-93. [DOI] [PubMed]
- 17.Grossman DC, Baldwin LM, Casey S, Nixon B, Hollow W, Hart LG. Disparities in infant health among American Indians and Alaska natives in US metropolitan areas. Pediatrics 2002;109:627-33. [DOI] [PubMed]
- 18.Rhoades ER, Brenneman G, Lyle J, Handler A. Mortality of American Indian and Alaska native infants. Annu Rev Public Health 1992;13:269-85. [DOI] [PubMed]
- 19.Khoshnood B, Lee KS, Wall S, Hsich HL, Mittendorf R. Short interpregnancy interals and the risk of adverse birth outcomes among five racial/ethnic groups in the United States. Am J Edidemiol 1998;148:798-805. [DOI] [PubMed]
- 20.Martens PJ. Being born in Manitoba: a look at prenatal health issues. In: Brownell M, Martens P, Kozyrskyj A, Fergusson P, Lerfald J, Mayer T, et al, editors. Assessing the health of children in Manitoba: a population-based study. Winnipeg: Manitoba Centre for Health Policy and Evaluation; 2001. p. 37-65.
- 21.Muhajarine N, D'Arcy C, Edouard L. Prevalence and predictors of health risk behaviours during early pregnancy: Saskatoon Pregnancy and Health Study. Can J Public Health 1997;88:375-9. [DOI] [PMC free article] [PubMed]
- 22.Hack M, Merkatz IR. Preterm delivery and low birth weight — a dire legacy. N Engl J Med 1995;333:1772-4. [DOI] [PubMed]
- 23.Goldenberg R, Andrews W, Yuan A, MacKay H, St Louis M. Sexually transmitted diseases and adverse outcomes of pregnancy. Clin Perinatol 1997;24:23-41. [PubMed]
- 24.Kurki T, Sivonen A, Renkonen O, Savia E, Ylikorkala O. Bacterial vaginosis in early pregnancy and pregnancy outcome. ObstetGynecol 1992;80:173-7. [PubMed]
- 25.Riduan J, Hillier SL, Utomo B, Wiknjosastro G, Linnan M, Kandun N. Bacterial vaginosis and prematurity in Indonesia: association in early and late pregnancy. Am J Obstet Gynecol 1993;169:175-8. [DOI] [PubMed]
- 26.Thomson M. Heavy birth weight of native Indians of British Columbia. Can J Public Health 1990;81:443-6. [PubMed]
- 27.Caulfield LE, Harris SB, Whalen EA, Sugamori ME. Maternal nutritional status, diabetes and risk of macrosomia among Native Canadian women. Early Hum Dev 1998;50:293-303. [DOI] [PubMed]
- 28.Rodrigues S, Robinson EJ, Kramer MS, Gray-Donald K. High rates of infant macrosomia: a comparison of Canadian native and non-native population. J Nutr 2000;130:806-12. [DOI] [PubMed]
- 29.Okun N, Verma A, Mitchell BF, Flowerdew G. Relative importance of maternal constitutional factors and glucose intolerance of pregnancy in the development of newborn macrosomia. J Matern Fetal Med 1997;6:285-90. [DOI] [PubMed]


