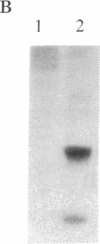
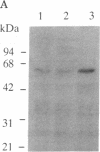
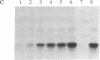
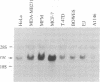
Abstract
A partial cDNA was isolated that encoded a protein kinase, termed rac (related to the A and C kinases). This cDNA was subsequently used to screen libraries derived from the human cell lines MCF-7 and WI38 and led to the isolation of full-length cDNA clones. DNA sequence analysis identified an open reading frame of 1440 base pairs encoding a protein of 480 amino acids (Mr, 55,716). This result was supported by the synthesis of a Mr 58,000 protein in an in vitro translation system that used RNA transcribed from cloned cDNAs with SP6 RNA polymerase. The predicted protein contains consensus sequences characteristic of a protein kinase catalytic domain and shows 73% and 68% similarity to protein kinase C and the cAMP-dependent protein kinase, respectively. Northern (RNA) analysis revealed a single mRNA transcript of 3.2 kilobases that varied up to 300-fold between different cell lines. Specific antisera directed towards the carboxyl terminal of the rac protein kinase were prepared and used to identify that phosphorylated several substrates in immunoprecipitates prepared with the rac-specific antisera.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adavani S. R., Schwarz M., Showers M. O., Maurer R. A., Hemmings B. A. Multiple mRNA species code for the catalytic subunit of the cAMP-dependent protein kinase from LLC-PK1 cells. Evidence for two forms of the catalytic subunit. Eur J Biochem. 1987 Sep 1;167(2):221–226. doi: 10.1111/j.1432-1033.1987.tb13326.x. [DOI] [PubMed] [Google Scholar]
- Ballou L. M., Siegmann M., Thomas G. S6 kinase in quiescent Swiss mouse 3T3 cells is activated by phosphorylation in response to serum treatment. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7154–7158. doi: 10.1073/pnas.85.19.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe S. J., Oyen O., Sandberg M., Frøysa A., Hansson V., Jahnsen T. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis--representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990 Mar;4(3):465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., DeBlasi A., Stone W. C., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989 Oct 13;246(4927):235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Brenner S. Phosphotransferase sequence homology. Nature. 1987 Sep 3;329(6134):21–21. doi: 10.1038/329021a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hanks S. K. Homology probing: identification of cDNA clones encoding members of the protein-serine kinase family. Proc Natl Acad Sci U S A. 1987 Jan;84(2):388–392. doi: 10.1073/pnas.84.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Jenö P., Jäggi N., Luther H., Siegmann M., Thomas G. Purification and characterization of a 40 S ribosomal protein S6 kinase from vanadate-stimulated Swiss 3T3 cells. J Biol Chem. 1989 Jan 15;264(2):1293–1297. [PubMed] [Google Scholar]
- Khew-Goodall Y., Hemmings B. A. Tissue-specific expression of mRNAs encoding alpha- and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett. 1988 Oct 10;238(2):265–268. doi: 10.1016/0014-5793(88)80493-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Isolation of a yeast protein kinase gene by screening with a mammalian protein kinase cDNA. DNA. 1988 Sep;7(7):469–474. doi: 10.1089/dna.1.1988.7.469. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D., Fischer E. H., Demaille J. G., Krebs E. G. Identification of an inhibitory region of the heat-stable protein inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4379–4383. doi: 10.1073/pnas.82.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J., Hemmings B. A. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987 Nov 17;26(23):7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- Takio K., Wade R. D., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Guanosine cyclic 3',5'-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984 Aug 28;23(18):4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]