Abstract
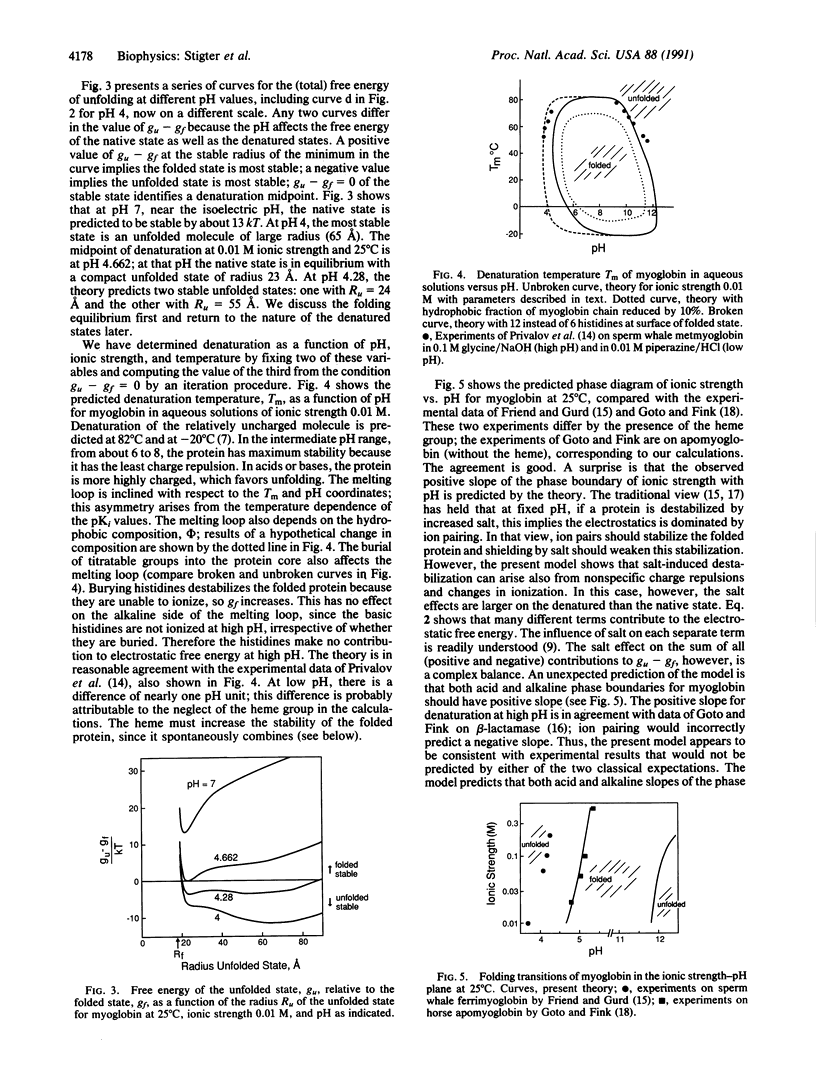
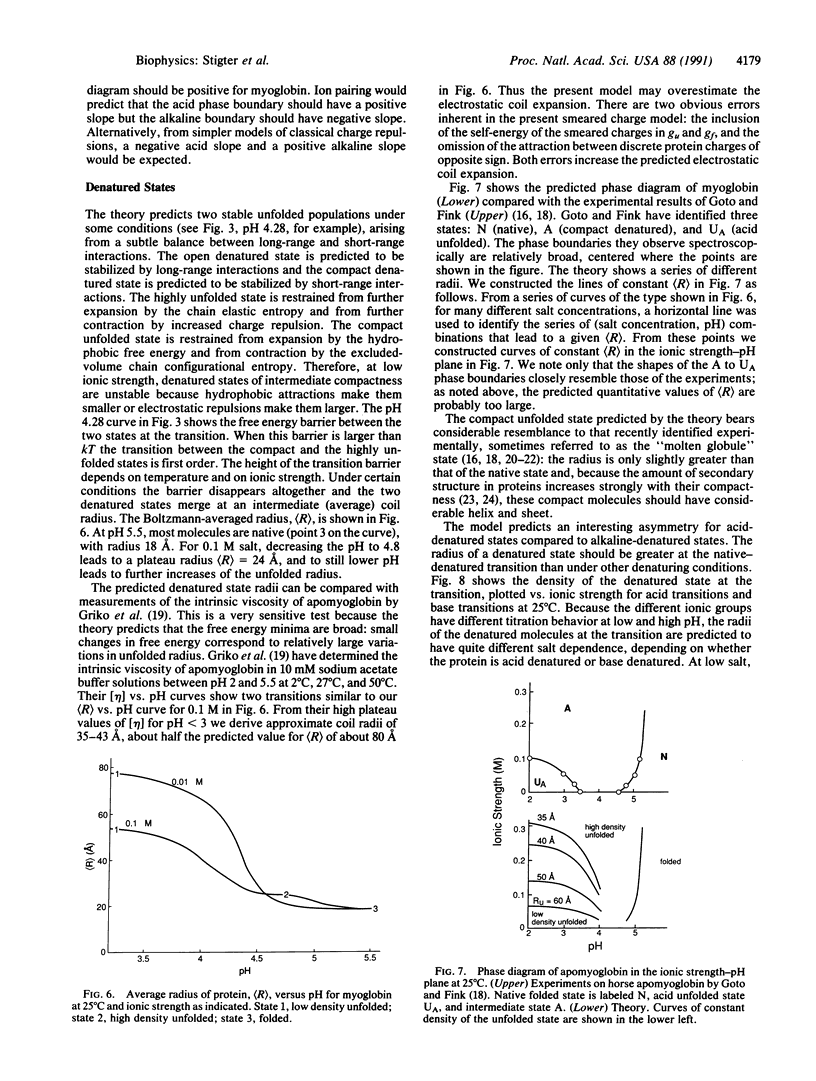
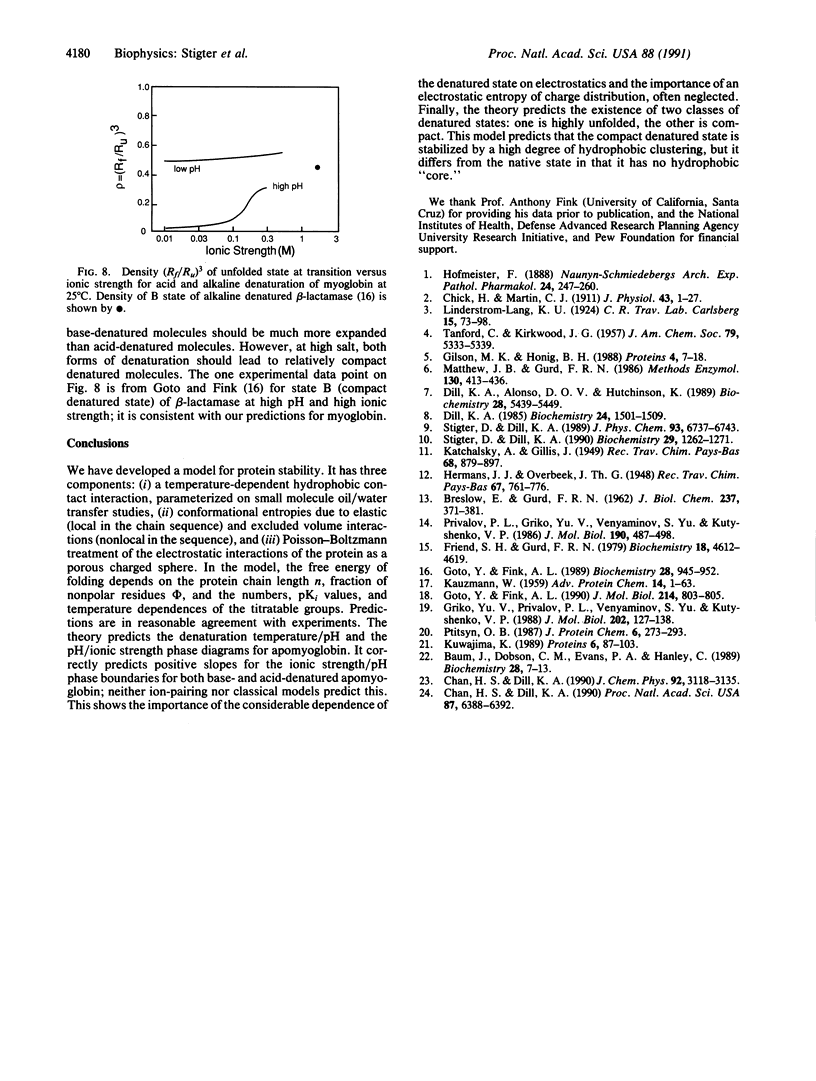
Globular proteins can be denatured by changing pH and ionic strength. Much recent evidence has led to the surprising conclusion that there are two acid-denatured states: one highly unfolded and the other more compact, sometimes called the "molten globule." Here we describe a molecular theory for electrostatic stability of globular proteins based on the properties of the constituent amino acids: oil/water partition coefficients, pK values of the titratable groups, and their temperature dependences. Predicted denaturation temperatures vs. pH are in good agreement with experiments of other workers on myoglobin. The theory also predicts two populations of denatured species, one open and the other more compact, with densities in the range found experimentally for molten globular states. In addition, it predicts a phase diagram (stability vs. pH, ionic strength) in good agreement with experiments of Goto and Fink [Goto, Y. & Fink, A. L. (1989) Biochemistry 28, 945-952; and Goto, Y. & Fink, A. L. (1990) J. Mol. Biol. 214, 803-805]. The well-known salt destabilization of myoglobin has been generally considered evidence for ion pairing, but the present theory, based on smeared charge repulsion, explains the salt destabilization at low pH without ion pairing. In addition, for myoglobin the theory predicts salt stabilization at high pH, as observed for beta-lactamase by Goto and Fink.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESLOW E., GURD F. R. Reactivity of sperm whale metmyoglobin towards hydrogen ions and p-nitrophenyl acetate. J Biol Chem. 1962 Feb;237:371–381. [PubMed] [Google Scholar]
- Baum J., Dobson C. M., Evans P. A., Hanley C. Characterization of a partly folded protein by NMR methods: studies on the molten globule state of guinea pig alpha-lactalbumin. Biochemistry. 1989 Jan 10;28(1):7–13. doi: 10.1021/bi00427a002. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Dill K. A. Origins of structure in globular proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6388–6392. doi: 10.1073/pnas.87.16.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick H. On the "heat-coagulation" of proteins: Part II. The action of hot water upon egg-albumen and the influence of acid and salts upon reaction velocity. J Physiol. 1911 Sep 11;43(1):1–27. doi: 10.1113/jphysiol.1911.sp001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill K. A., Alonso D. O., Hutchinson K. Thermal stabilities of globular proteins. Biochemistry. 1989 Jun 27;28(13):5439–5449. doi: 10.1021/bi00439a019. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Theory for the folding and stability of globular proteins. Biochemistry. 1985 Mar 12;24(6):1501–1509. doi: 10.1021/bi00327a032. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Gurd F. R. Electrostatic stabilization in myoglobin. pH dependence of summed electrostatic contributions. Biochemistry. 1979 Oct 16;18(21):4612–4619. doi: 10.1021/bi00588a023. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. Calculation of the total electrostatic energy of a macromolecular system: solvation energies, binding energies, and conformational analysis. Proteins. 1988;4(1):7–18. doi: 10.1002/prot.340040104. [DOI] [PubMed] [Google Scholar]
- Goto Y., Fink A. L. Conformational states of beta-lactamase: molten-globule states at acidic and alkaline pH with high salt. Biochemistry. 1989 Feb 7;28(3):945–952. doi: 10.1021/bi00429a004. [DOI] [PubMed] [Google Scholar]
- Goto Y., Fink A. L. Phase diagram for acidic conformational states of apomyoglobin. J Mol Biol. 1990 Aug 20;214(4):803–805. doi: 10.1016/0022-2836(90)90334-I. [DOI] [PubMed] [Google Scholar]
- Griko Y. V., Privalov P. L., Venyaminov S. Y., Kutyshenko V. P. Thermodynamic study of the apomyoglobin structure. J Mol Biol. 1988 Jul 5;202(1):127–138. doi: 10.1016/0022-2836(88)90525-6. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 1989;6(2):87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Gurd F. R. Calculation of electrostatic interactions in proteins. Methods Enzymol. 1986;130:413–436. doi: 10.1016/0076-6879(86)30019-3. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Griko YuV, Venyaminov SYu, Kutyshenko V. P. Cold denaturation of myoglobin. J Mol Biol. 1986 Aug 5;190(3):487–498. doi: 10.1016/0022-2836(86)90017-3. [DOI] [PubMed] [Google Scholar]
- Stigter D., Dill K. A. Charge effects on folded and unfolded proteins. Biochemistry. 1990 Feb 6;29(5):1262–1271. doi: 10.1021/bi00457a023. [DOI] [PubMed] [Google Scholar]