Abstract
Background
Our study aimed to compare the outcomes of surgical treatment of tongue cancer patients in three different age groups.
Methods
From 2004 to 2013, we retrospectively analyzed the clinical data of 1,712 patients who were treated in the four institutions constituting the Chang Gung Memorial Hospitals (CGMH). We divided and studied the patients in three age groups: Group 1, younger (<65 years); Group 2, young old (65 to <75); and Group 3, older old patients (≥75 years).
Results
Multivariate analyses determined the unfavorable, independent prognostic factors of overall survival to be male sex, older age, advanced stage, advanced T, N classifications, and surgery plus chemotherapy. No significant differences were found in adjusted hazard ratios (HR) of death in early-stage disease (stage I–II) among Group 1 (HR 1.0), Group 2 (HR 1.43, 95% confidence interval (CI) [0.87–2.34], p = 0.158), and Group 3 (HR 1.22, 95% CI [0.49–3.03], p = 0.664) patients. However, amongst advanced-stage patients (stage (III–IV)), Group 3 (HR 2.53, 95% CI [1.46–4.38], p = 0.001) showed significantly worse survival than the other two groups after other variables were adjusted for. Fourteen out of 21 older old, advanced-staged patients finally died, and most of the mortalities were non-cancerogenic (9/14, 64.3%), and mostly occurred within one year (12/14, 85%) after cancer diagnosis. These non-cancer cause of death included underlying diseases in combination with infection, pneumonia, poor nutrition status, and trauma.
Conclusions
Our study showed that advanced T classification (T3–4), positive nodal metastasis (N1–3) and poorly differentiated tumor predicted poor survival for all patients. Outcome of early-stage patients (stage I–II) among three age groups were not significantly different. However, for advanced-stage patients (stage III–IV), the older old patients (≥75) had significantly worse survival than the other two patient groups. Therefore, for early-stage patients, age should not deny them to receive optimal treatments. However, older old patients (≥75) with advanced cancer should be comprehensively assessed by geriatric tools before surgical treatment and combined with intensive postoperative care to improve outcome, especially the unfavorable non-cancerogenic mortalities within one year after cancer diagnosis.
Keywords: Prognosis, Risk factors, Oral cancer, Oral cavity, Survival, CGRD (Chang Gung Research Database), Age, Old
Introduction
The tongue is the most common site of intraoral cancers in Taiwan and most other countries (Huang et al., 2008; Taiwan Ministry of Health and Welfare, 2016; Moore et al., 2000). The tongue cancer patients are predominantly male, and incidence of tongue cancer peaks at 45–65 years in male and 55–75 years of age in female (Taiwan Ministry of Health and Welfare, 2016). According to a recent national cancer registry’s annual report of Taiwan, incidence and mortality of head and neck cancers (ICD-O-3, C00-C14) rank sixth and fifth, respectively (Taiwan Ministry of Health and Welfare, 2016).
Taiwan, like the other developed countries, has gradually transformed into a society of the aged with those older than 65 years accounting for 12% of its population (Kowal et al., 2012; Taiwan National Development Council, 2014). This figure is likely to hit 20% in 2025, turning Taiwan into a super-ageing society (Taiwan National Development Council, 2014). The numbers of elderly patients with tongue cancer is expected to increase in the future. Nowadays, surgery is the therapeutic mainstay for early-stage tongue cancer, but it is often part of a multi-modal approach to treat advanced disease (Calabrese et al., 2011). However, many elderly patients may not be considered as candidates for aggressive multimodal treatments due to other ageing-associated comorbidities, general debility, and concerns regarding low tolerance to treatment and resulting toxicity (Siddiqui & Gwede, 2012; Zabrodsky et al., 2004).
Recent reports on the relationship between elderly patients with head and neck cancer and their prognosis have been conflicting (Airoldi et al., 2004; Bhattacharyya, 2003; Chang et al., 2013; Clayman et al., 1998; Italiano et al., 2008; Kruse et al., 2010; Luciani et al., 2010; Lusinchi et al., 1990; Ortholan et al., 2009; Sarini et al., 2001; Zabrodsky et al., 2004). Some concluded that older patients suffered a worse survival than younger patients (Bhattacharyya, 2003; Chang et al., 2013; Clayman et al., 1998). However, many others failed to show a significant difference between outcomes of old and young patients (Airoldi et al., 2004; Argiris et al., 2004; Lusinchi et al., 1990; Sarini et al., 2001). In many previously published reports (Airoldi et al., 2004; Bhattacharyya, 2003; Chang et al., 2013; Clayman et al., 1998; Italiano et al., 2008; Kruse et al., 2010; Lusinchi et al., 1990; Ortholan et al., 2009; Sarini et al., 2001; Zabrodsky et al., 2004), the cutoff age values (65, 70, 75, 80, or 85 years) and the definition of elderly patients were inconsistent. Besides, some previous reports included a small sample size or lacked cancer staging. In the present study, we intend to focus on the survival outcomes of older adults with homogenous tongue cancer receiving curative surgery in order to provide evidence for preoperative risk explanations and decision making for the surgeons or oncologists. The National Institute of Aging have classified the elderly patients into three age groups: 65–74 years as “young old,” 75–84 years as “older old,” and >85 years as “oldest old” (NIH, 1998).
Here, we compared treatment results of tongue cancer patients, stratifying by three age groups: Group 1, <65 years (younger population); Group 2, 65–<75 years (young old population) and Group 3, ≥75 years (older old population).
Materials and Methods
Data source
The data were obtained from the largest private hospital system in Taiwan, the Chang Gung Memorial Hospital (CGMH), using the Chang Gung Research Database (CGRD). The database combines original medical record from four medical institutes, Keelung CGMH, Linkou CGMH, Chiayi CGMH, and Kaohsiung CGMH. They are located in the northeast, northern, central, and southern regions of Taiwan, respectively. According to the Taiwanese national cancer registry’s report, this combined hospital system had treated ∼20% of head and neck cancer patients.
We retrospectively reviewed the CGRD database from January 2004 to December 2013, and retrieved data of tongue cancer patients (n = 2, 487). We excluded patients with recurrent or secondary oral cancers, or those with other malignancies (n = 471). Patients with poor performance status (ECOG ≥ 3), end-stage renal disease, Child-Pugh C liver cirrhosis, or poor heart or lung function, or who were unfit for surgery, were also excluded to reduce confounding factors and bias. Finally, 1,712 patients with primary tongue cancer who received curative surgery were studied. The ethics review board of our institution approved the study (CGMH-IRB No. 104-4642B).
Surgery, adjuvant therapy, and follow-up
Patients were evaluated preoperatively according to the CGMH oral cavity cancer guidelines, which were modified from the NCCN guideline (Pfister et al., 2000; Pfister et al., 2013). Evaluations included patient history taking, physical examination, nasopharyngoscopy, complete blood count, blood biochemistry, chest X-ray, electrocardiography, abdominal sonography and panendoscopy, computed tomography or MRI of head and neck, and bone scan or FDG-PET. Cancer staging accorded with the American Joint Committee on Cancer staging classification (AJCC, 6th edition) (Edge & Compton, 2010).
All patients were treated based on the CGMH oral cavity cancer guidelines. Tumors were resected with at least 1 cm gross, safe margin in all patients. Level I–III cervical lymph node resections were performed in patients without lymph node metastases. Level I–V cervical lymph node resections or more extensive resections were done in patients with lymph node metastases. All patients who received free-flap reconstruction were admitted to the ICU after surgery, and were followed by intensive flap monitoring. Post-operative concurrent chemo-radiotherapy (CCRT) with 60–70 Gray (1.8–2.0 Gray per fraction) and Cisplatin-based regimen (weekly 30–40 mg/m2/wk × 6–7 weeks) was administered in patients with positive surgical margins or extracapsular extension of lymph nodes. In patients with other risk factors (such as T3/T4, N1, N2/N3, perineural invasion, or vascular tumor embolism), postoperative radiotherapy (RT) with 60–70 Gray (1.8–2.0 Gray per fraction) was administered. However, in older patients or patients with multiple comorbidities, RT or chemotherapy (CT) programs were cancelled following discussions with their families. We followed up the patients since their cancers’ diagnosis until death, cancer recurrence, or the last follow-up. All patients received regular postoperative follow-up.
Age definitions, outcomes, and covariates
The final dataset was divided into three groups: Group 1, younger population (< 65 years); Group 2, young old population (65 to <75 years); and Group 3, older old population (≥75 years). Patient characteristics included age, gender, cancer TNM staging, histological grade, treatment modalities (surgery, surgery with adjuvant RT or CT). The main outcome was overall survival rate.
Statistical analysis
Gender, cancer staging, histological grade, and treatment modalities were compared amongst the three Groups by the Pearson’s χ2 test. We estimated the survival rates during the entire follow-up period by the Kaplan–Meier method and compared survival rates amongst the three groups by the Log-rank test. The multivariate Cox proportional hazards analysis was performed for gender, age group, cancer stage, histological grade and treatment modalities. Statistical analyses used the statistical software R (version 3.1.3). For all tests, significance was defined at p < 0.05.
Results
Patient characteristics and treatments
Patients’ characteristics are presented in Table 1. The mean/median ages for Group 1, Group 2, and Group 3 were 48.7/49, 68.7/69, and 79.5/79 years, respectively. The proportion of female patients increased with age and were significantly different in three groups (p < 0.001). Cancer staging, T–N classification, and tumor differentiation were not significantly different among the three Groups. The ratio of patients receiving surgery alone without CT or RT increased with age and were significantly different amongst the three groups (p = 0.004). To clarify the difference in treatment patterns among the three groups, all patients were further divided into early (stage I–II) and advanced stages (stage III–IV) for comparison (Table 2). For early-stage patients, 86.7%, 85%, and 81% received surgery alone in Group 1, Group 2, and Group 3, respectively. The treatment patterns were not significantly different among the three Groups (p = 0.558). However, in patients with advanced-stage disease, adjuvant treatment (which were usually needed for control of the advanced stage) was given to fewer patients with increasing age. The proportion of advanced stage patients receiving adjuvant treatment were significantly different among the three groups (p < 0.001).
Table 1. Clinicopathological characteristics of 1,712 patients with oral tongue cancer receiving surgery stratified by three age groups.
| Age < 65 n = 1, 476 (86.2%) | Age 65 to <75 n = 178 (10.4%) | Age ≥ 75 n = 58 (3.4%) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Mean age (SD) | 48.7 (8.5) | 68.7 (2.7) | 79.5 (3.9) | ||||
| Median (range) | 49 (21–64) | 69 (65–74) | 79 (75–92) | ||||
| Gender | <0.001 | ||||||
| Male | 1,323 | 89.6% | 145 | 81.5% | 36 | 62.1% | |
| Female | 153 | 10.4% | 33 | 18.5% | 22 | 37.9% | |
| Stage | 0.829 | ||||||
| I | 521 | 35.3% | 67 | 37.6% | 23 | 39.7% | |
| II | 346 | 23.4% | 46 | 25.8% | 14 | 24.1% | |
| III | 202 | 13.7% | 22 | 12.4% | 9 | 15.5% | |
| IV | 407 | 27.6% | 43 | 24.2% | 12 | 20.7% | |
| T classification | 0.151 | ||||||
| 1 | 558 | 37.8% | 74 | 41.6% | 25 | 43.1% | |
| 2 | 534 | 36.2% | 70 | 39.3% | 25 | 43.1% | |
| 3 | 138 | 9.3% | 13 | 7.3% | 5 | 8.6% | |
| 4 | 246 | 16.7% | 21 | 11.8% | 3 | 5.2% | |
| N classification | 0.716 | ||||||
| 0 | 1,025 | 69.4% | 134 | 75.3% | 43 | 74.1% | |
| 1 | 160 | 10.8% | 14 | 7.9% | 6 | 10.3% | |
| 2 | 288 | 19.5% | 30 | 16.9% | 9 | 15.5% | |
| 3 | 3 | 0.2% | 0 | 0.0% | 0 | 0.0% | |
| Histological grade | 0.847 | ||||||
| Well | 444 | 30.1% | 55 | 30.9% | 17 | 29.3% | |
| Moderately | 896 | 60.7% | 103 | 57.9% | 34 | 58.6% | |
| Poorly | 136 | 9.2% | 20 | 11.2% | 7 | 12.1% | |
| Treatment | 0.004 | ||||||
| Surgery alone | 868 | 58.8% | 120 | 67.4% | 44 | 75.9% | |
| Surgery + CT or RT | 608 | 41.2% | 58 | 32.6% | 14 | 24.1% | |
| RT alone | 180 | 12.2% | 23 | 12.9% | 8 | 13.8% | |
| CT alone | 58 | 3.9% | 8 | 4.5% | 2 | 3.4% | |
| CCRT | 370 | 25.1% | 27 | 15.2% | 4 | 6.9% | |
Notes.
- SD
- Standard deviation
- CT
- Chemotherapy
- RT
- Radiotherapy
- CCRT
- Concurrent chemo-radiotherapy
Table 2. Characteristics and treatments of early stage (Stage I–II) and advanced stage (Stage III–IV) patients stratified by three age groups.
| Stage I-II | P-value | Stage III-IV | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| <65 | 65 to <75 | ≥75 | <65 | 65 to <75 | ≥75 | |||
| Numbers | 867 (85.3%) | 113 (11.1%) | 37 (3.6%) | 609 (87.6%) | 65 (9.4%) | 21 (3.0%) | ||
| Mean age (±SD) | 48.6 (±8.6) | 68.8 (±2.6) | 80.0 (±4.3) | 48.8 (±8.3) | 68.6 (±2.9) | 78.6 (±2.8) | ||
| Median (range) | 49 (21–64) | 69 (65–74) | 79 (75–92) | 49 (27–64) | 69 (65–74) | 79 (75–85) | ||
| Gender | <0.001 | <0.001 | ||||||
| Male | 763 (88.0%) | 92 (81.4%) | 21 (56.8%) | 560 (92.0%) | 53 (81.5%) | 15 (71.4%) | ||
| Female | 104 (12.0%) | 21 (18.6%) | 16 (43.2%) | 49 (8.0%) | 12 (18.5%) | 6 (28.6%) | ||
| Treatment | 0.558 | <0.001 | ||||||
| Surgery alone | 752 (86.7%) | 96 (85.0%) | 34 (91.9%) | 116 (19.0%) | 24 (36.9%) | 10 (47.6%) | ||
| Surgery + CT or RT | 115 (13.3%) | 17 (15.0%) | 3 (8.1%) | 493 (81.0%) | 41 (63.1%) | 11 (52.4%) | ||
| RT alone | 81 (9.3%) | 9 (8.0%) | 3 (8.1%) | 99 (16.3%) | 14 (21.5%) | 5 (23.8%) | ||
| CT alone | 15 (1.7%) | 3 (2.7%) | 0 (0.0%) | 43 (7.1%) | 5 (7.7%) | 2 (9.5%) | ||
| CCRT | 19 (2.2%) | 5 (4.4%) | 0 (0.0%) | 351 (57.6%) | 22 (33.8%) | 4 (19.0%) | ||
Notes.
- SD
- Standard deviation
- CT
- Chemotherapy
- RT
- Radiotherapy
- CCRT
- Concurrent chemo-radiotherapy
The median follow-up time was 40.0 months (range 0.3–98.2 months).
Survival
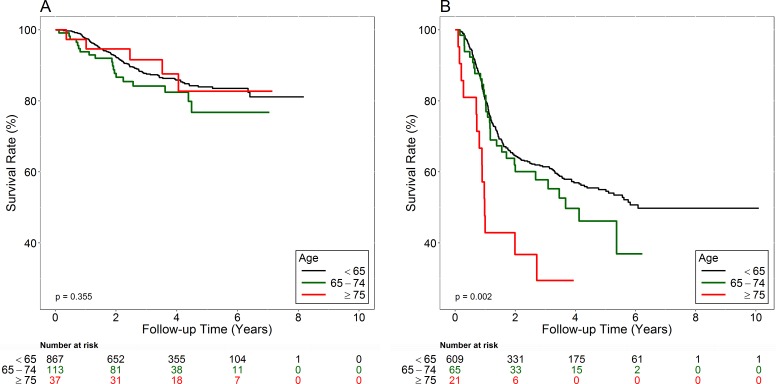
The medial follow-up times of all and surviving patients are 2.88 and 3.66 years, respectively. Figure 1A shows the early-stage patients’ overall survival curves. Survival rates were not significantly different amongst Group 1, Group 2, and Group 3 patients (Log-rank test, p = 0.355). The 5-year survival rates were 83.9% in Group 1; 76.8% in Group 2; and 82.7% in Group 3.
Figure 1. Overall survival curves of early-stage (A) and advanced-stage (B) patients.
Figure 1B shows the advanced-stage patients’ overall survival curves. Group 3 had the worst prognosis (Log-rank test, p = 0.002). The 5-year survival rate was 55.0% in Group 1, 46.1% in Group 2, and 29.4% in Group 3. Table 3 shows the multivariate analysis data used to compare the hazard ratios (HR) of death between different genders, the three Groups, stages (I and II vs III and IV), T classifications (T1–2 vs T3–4), N classifications (N0 vs N1–3), tumor differentiation (well to moderately differentiated vs poorly differentiated), and treatments (Surgery vs Surgery + RT vs Surgery + CT vs Surgery + CCRT). For all patients, HR for death was 1.39 times greater for the male than for the female patients (95% CI [1.00–1.93]; p = 0.048). After adjusting for other factors, Group 2 (HR 1.38, 95% CI [1.02–1.87], p = 0.037) and Group 3 (HR 1.91, 95% CI [1.2–3.06], p = 0.007) had greater likelihood of death than Group 1. Advanced-stage (stage III–IV) patients had the worse survival (HR 1.64, 95% CI [1.07–2.50], p = 0.022) than patients with early-stage diseases (stage I–II). Adjusted HR for death were1.42 times (95% CI [1.10–1.84], p = 0.008) and 2.41 times (95% CI [1.7–3.42], p < 0.001) for the advanced T (T3–4) and N (N1–3) classifications than for the early T (T1–2) and N0. Poorly differentiated tumors also predicted poor survival rates than well differentiated and moderately differentiated tumors (HR 1.51, 95% CI [1.16–1.97], p = 0.003). Surgery plus CT alone led to worse survival rates than surgery alone (HR 1.97, 95% CI [1.33–2.90], p = 0.001). Table 4 shows HR of death for early-stage (stage I and II) patients. The adjusted HR for death was 1.64 times greater for the male than for the female patients (95% CI [0.92–2.94], p = 0.095). The adjusted HR for death was greater for the Group 2 (HR 1.43, 95% CI [0.87–2.34], p = 0.158) and Group 3 (HR 1.22, 95% CI [0.49–3.03], p = 0.664) than for Group 1, but the differences were not statistically significant. Table 5 shows the HR of death for advanced-stage (stage III and IV) patients. The adjusted HR for death was 1.16 times greater for the male than for the female patients (95% CI [0.78–1.72], p = 0.454). The adjusted HR for death was significantly greater for Group 3 (HR 2.53, 95% CI [1.46–4.38], p = 0.001) than for Group 1, but the difference was not significant for Group 2 (HR 1.19, 95% CI [0.81–1.75], p = 0.372) as compared with Group 1.
Table 3. Multivariate analyses of risk factors regarding overall survival of all patients (n = 1, 712) using Cox Proportional Hazard Model.
| Covariate | HR | CI(95%) | P-value | |
|---|---|---|---|---|
| Gender (ref: female) | ||||
| Male | 1.39 | 1.00 | 1.93 | 0.048 |
| Age (ref: <65) | ||||
| 65–<75 | 1.38 | 1.02 | 1.87 | 0.037 |
| ≥75 | 1.92 | 1.20 | 3.06 | 0.007 |
| Stage (ref: I+II) | ||||
| III+IV | 1.64 | 1.07 | 2.50 | 0.022 |
| T classification (ref: T1, T2) | ||||
| T3, T4 | 1.42 | 1.10 | 1.84 | 0.008 |
| N classification (ref: No) | ||||
| N1, N2, N3 | 2.41 | 1.70 | 3.42 | <0.001 |
| Histological grade (ref: WD, MD) | ||||
| PD | 1.51 | 1.16 | 1.97 | 0.003 |
| Treatment (ref: surgery alone) | ||||
| Surgery + RT alone | 1.07 | 0.77 | 1.48 | 0.683 |
| Surgery + CT alone | 1.97 | 1.33 | 2.90 | 0.001 |
| Surgery + CCRT | 0.93 | 0.69 | 1.26 | 0.645 |
Notes.
- HR
- Hazard ratio
- CI
- Confidence interval
- WD
- Well differentiated
- MD
- Moderately differentiated
- PD
- Poorly differentiated
- RT
- Radiotherapy
- CT
- Chemotherapy
- CCRT
- Concurrent chemo-radiotherapy
Table 4. Multivariate analyses of risk factors regarding overall survival of early-stage (I–II) patients (n = 1, 017) using Cox Proportional Hazard Model.
| Covariate | HR | CI(95%) | P-value | |
|---|---|---|---|---|
| Gender (ref: female) | ||||
| Male | 1.64 | 0.92 | 2.94 | 0.095 |
| Age (ref: <65) | ||||
| 65–<75 | 1.43 | 0.87 | 2.34 | 0.158 |
| ≥75 | 1.22 | 0.49 | 3.03 | 0.664 |
| Histological grade (ref: WD, MD) | ||||
| PD | 1.81 | 1.00 | 3.28 | 0.051 |
| Treatment (ref: surgery alone) | ||||
| Surgery + RT alone | 1.95 | 1.19 | 3.22 | 0.009 |
| Surgery + CT alone | 3.63 | 1.68 | 7.86 | 0.001 |
| Surgery + CCRT | 4.04 | 2.03 | 8.01 | <0.001 |
Notes.
- HR
- Hazard ratio
- CI
- Confidence interval
- WD
- Well differentiated
- MD
- Moderately differentiated
- PD
- Poorly differentiated
- RT
- Radiotherapy
- CT
- Chemotherapy
- CCRT
- Concurrent chemo-radiotherapy
Table 5. Multivariate analyses of risk factors regarding overall survival of advanced stage (III–IV) patients (n = 695) using Cox Proportional Hazard Model.
| Covariate | HR | CI(95%) | P-value | |
|---|---|---|---|---|
| Gender (ref: female) | ||||
| Male | 1.16 | 0.78 | 1.72 | 0.454 |
| Age (ref: <65) | ||||
| 65–<75 | 1.19 | 0.81 | 1.75 | 0.372 |
| ≥75 | 2.53 | 1.46 | 4.38 | 0.001 |
| Histological grade (ref: WD, MD) | ||||
| PD | 1.46 | 1.08 | 1.97 | 0.013 |
| Treatment (ref: surgery alone) | ||||
| Surgery + RT alone | 0.65 | 0.44 | 0.95 | 0.028 |
| Surgery + CT alone | 1.62 | 1.06 | 2.47 | 0.026 |
| Surgery + CCRT | 0.89 | 0.67 | 1.19 | 0.424 |
Notes.
- HR
- Hazard ratio
- CI
- Confidence interval
- WD
- Well differentiated
- MD
- Moderately differentiated
- PD
- Poorly differentiated
- RT
- Radiotherapy
- CT
- Chemotherapy
- CCRT
- Concurrent chemo-radiotherapy
Causes of death in Group 3 patients with advanced disease
Fourteen of the 21 advanced-staged, Group 3 patients died. Causes of death are listed in Table 6. Nearly all mortalities (12 out of 14, 85.7%) occurred within 1 year after cancer diagnosis. The causes of death were classified into cancer recurrence (four patients, 28.6%), non-cancerogenic cause of death (nine patients, 64.3%), and unknown (one patient, 7.1%). The non-cancer cause of death are the primary causes of death in this age group, including underlying diseases in combination with infection, pneumonia, poor nutrition status, and trauma. Sixteen patients (16/21, 76.2%) were indicated to receive adjuvant therapy; however, 11 patients (11/16, 68.8%) completed the therapy. The surviving patients all completed the planned oncological treatments (either surgery alone, or surgery and adjuvant treatments).
Table 6. Causes of mortality of very old patients with stage III–IV tongue cancer (n = 21).
| No | Sex | Age | Survival time (days) | Adjuvant (needed/done) | Cause of death | Details |
|---|---|---|---|---|---|---|
| 1 | F | 79 | 38 | Y/N | Non-cancer | Sepsis, acute renal failure, pneumonia, malnutrition, type II DM (die on post-op day 10) |
| 2 | M | 76 | 53 | Y/N | Non-cancer | Pneumonia, respiratory failure |
| 3 | M | 81 | 76 | Y/N | Non-cancer | Severe hyponatremia caused by syndrome of inappropriate antidiuretic hormone secretion (SIADH) |
| 4 | F | 77 | 102 | Y/N | Non-cancer | Pneumonia, DM, HT |
| 5 | M | 85 | 257 | N/N | Non-cancer | Pneumonia, poor renal function, COPD, DM, HT, anemia, |
| 6 | M | 76 | 264 | Y/Y | Cancer | Multiple bone metastasis, poor intake, hospice |
| 7 | M | 78 | 294 | Y/Y | Unknown | Medical record of death in other hospital |
| 8 | M | 76 | 323 | Y/Y | Cancer | Neck local recurrence and pneumonia |
| 9 | M | 79 | 326 | Y/N | Cancer | Cancer recurrence, cachexia, COPD, DM, major depression |
| 10 | F | 79 | 329 | Y/Y | Cancer | Lung metastasis, neck metastasis and trachea invasion with bleeding |
| 11 | M | 81 | 354 | N/N | Non-cancer | Pneumonia, sepsis, type II DM, renal failure |
| 12 | M | 82 | 365 | N/N | Non-cancer | Atrial fibrillation and flutter |
| 13 | F | 79 | 727 | Y/Y | Non-cancer | Pneumonia |
| 14 | M | 75 | 992 | Y/Y | Non-cancer | Fall down and femoral fracture, sepsis, poor nutrition, hypokalemia |
| 15 | F | 82 | 486 | N/N | Alive | |
| 16 | M | 75 | 658 | N/N | Alive | |
| 17 | M | 78 | 862 | Y/Y | Alive | |
| 18 | M | 76 | 1,022 | Y/Y | Alive | |
| 19 | F | 82 | 1,056 | Y/Y | Alive | |
| 20 | M | 76 | 1,279 | Y/Y | Alive | |
| 21 | M | 79 | 1,442 | Y/Y | Alive |
Notes.
- HR
- Hazard ratio
- DM
- Diabetes mellitus
- HT
- Hypertension
- COPD
- Chronic obstructive pulmonary disease
Survival time: from day of diagnosis to death or last follow-up dates.
Discussion
Our study showed that advanced T classification (T3–4), positive nodal metastasis (N1–3) and poorly differentiated tumor predicted poor survival for all patients, which were compatible with previous studies (Aksu et al., 2006; Goto et al., 2005; Liao et al., 2008). The male patients showed significantly poor survival than the female patients for all patients, but showed no significant difference after dividing all patients into early and advanced stages. This may be due to the relatively smaller sample size of female (1,504 men vs 208 women) in our study cohort. Previously published literature on the outcomes of the surgical treatment of the tongue cancer in different age groups has been controversial. (Sarini et al., 2001) reported the treatment outcomes of older patients (≥75 years) with head and neck squamous cell carcinoma did not differ significantly from younger patients’ outcomes. Davidson, Root & Trock (2001) reported a large series (n = 749) of the tongue cancer patients enrolled in the Surveillance, Epidemiology, and End Results (SEER) database concluded that an increasing age predicted the worse disease-specific survival. Chang et al. (2013) reported old patients (>65 years) with oral cavity cancer had lower survival rate than young patients (<45 years). However, no details of cancer staging were reported in these study (Chang et al., 2013; Davidson, Root & Trock, 2001). Jones et al. (1998) and Clayman et al. (1998) also reported that older patients with head and neck cancer had worse survival that younger patients. Nonetheless, oral cavity cancer patients comprised less than 25% to 60% of their patients.
In our study, we included 1,712 homogeneous tongue cancer patients (1,476 younger, 178 young old and 58 older old patients) with clear pathological staging after radical surgery, and compared their overall survival rate with younger patients all treated under the standard guidelines. Our study clearly showed that elderly patients are likely to face the worst survival rate amongst the tongue cancer patients after having been treated by radical surgery. After adjusting for other variables, young old and older old patients were more likely to die than younger patients. No significant difference in adjusted HR of death was found for early-stage patients (stage I–II) amongst the younger, young old, or older old patients which implied that age should not deny older people to receive optimal treatment. However, for advanced-stage disease (stage III–IV), the older old patients showed significantly worse survival than the other two groups after adjusting for other variables.
Italiano et al. (2008) reported on 316 head and neck squamous cell carcinoma patients aged >80 years receiving radiotherapy (N = 180; 57.0%), surgery (N = 97; 30.7%) and no treatment (N = 39; 12.3%). They reported that outcomes of patients with stage I/II was similar to that of younger patients, but those with stage III/IV showed poor survival. These results are in agreement with our results. In our series, younger, young old, and older old patients received similar treatment modalities (Table 2, 81.0–86.7% patients underwent surgery alone without CT or RT) and had comparably optimal survival rate in early-stage tongue cancer. Our data represented the first evidence that old age ≥75 years should not be a reason to deny patients of early-staged tongue cancer to receive curative surgery.
For advanced-stage patients, older old patients had worst prognosis as compared with the other two age groups. Fourteen out of 21 older old, advanced-staged patients finally died and most of the mortalities occurred within 1 year after cancer diagnosis (12/14, 85.7%). The causes of deaths were mostly non-cancerogenic (9/14, 64.3%) including underlying diseases in combination with infection, pneumonia, poor nutrition status, and fall-related injury.
Reid et al. (2001) concluded that comorbidities also predict survival in the older people with head and neck cancer. Previous studies have also emphasized the importance of careful assessment of comorbidities, physical status, and patients’ psychological profiling before operation (Grenman et al., 2010; Kruse et al., 2010). Many studies have indicated regular physical activity is essential for the elderly cancer patients to aid in the process of recovery, improve fitness and prevent falls (Cho et al., 2015; Genden et al., 2005; Keogh et al., 2015; Lee et al., 2016; Pinto & Ciccolo, 2010; Rock et al., 2012). Besides, our results (Table 2) showed that the proportion of the patients who received postoperative CCRT was significantly low in the elderly patients. Adjuvant RT or CT after surgery was indicated for eighteen of 21 older old, advanced-staged patients, and was received by 11 of those patients (61%). The reason for not receiving adjuvant therapy were advanced age (n = 4), comorbidities (n = 2), and early death (n = 1). Thus, suboptimal treatments might increase the risk of cancer recurrence and disease metastasis in cases with advanced disease.
The following measures possibly could improve the survival rate of the elderly patients with tongue cancer: (1) thoroughly evaluating patients pre-operationally and controlling the underlying disease (2) using geriatric assessment tools to predict mortality and assist treatment decision-making process (Extermann & Hurria, 2007; Italiano et al., 2008); (3) screening the cancer intensively to diagnose cancer as early as possible (Reid, 2013); (4) ensuring that patients receive post-operational rehabilitation for cancer-related deconditioning as soon as possible (Saotome, Klein & Faux, 2015); (5) increasing nutrition supplementation and preventing choking and aspiration pneumonia (Farhangfar et al., 2014); and (6) Modification of environmental hazards and performing physical activities to prevent falls which is common in older cancer patients (Cho et al., 2015; Keogh et al., 2015; Lee et al., 2016; Rock et al., 2012; Sattar et al., 2016; Ungar & Rafanelli, 2015).
Future research by incorporating these factors or measures should be considered in order to improve survivals in those patients.
Conclusion
Our study showed that advanced T classification (T3–4), positive nodal metastasis (N1–3) and poorly differentiated tumor predicted poor survival for all patients. For early-stage patients (stage I–II), the overall survival rate among the younger age, young old, and older old patients were not significantly different. However, for advanced-stage patients (stage III–IV), the older old patients (≥75) had significantly worse survival than the other two patient groups. Based on the present study, we suggest that age should not deny early stage patients to receive optimal oncological treatment. However, older old patients (≥75) with advanced cancer should be comprehensively assessed by geriatric tools before surgical treatment combined with intensive postoperative care to improve survival.
Acknowledgments
The authors would like to thank Center of Excellence for Chang Gung Research Datalink (CORPG6D0161-2, CORPG6D0251-2) for the comments and assistance in data analysis.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ming-Shao Tsai conceived and designed the experiments, performed the experiments, wrote the paper.
Chia-Hsuan Lai conceived and designed the experiments, performed the experiments.
Chuan-Pin Lee analyzed the data, prepared figures and/or tables.
Yao-Hsu Yang analyzed the data.
Pau-Chung Chen, Re-Ming A. Yeh and Wen-Cheng Chen reviewed drafts of the paper.
Chung-Jan Kang, Geng-He Chang, Yao-Te Tsai, Chih-Yen Chien and Ku-Hao Fang contributed reagents/materials/analysis tools.
Chang-Hsien Lu wrote the paper, reviewed drafts of the paper.
Chi-Kuang Young contributed reagents/materials/analysis tools, wrote the paper.
Chin-Jui Liu prepared figures and/or tables.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The ethics review board of our institution approved the study (CGMH-IRB No. 104-4642B).
Data Availability
The following information was supplied regarding data availability:
Mortality in tongue cancer patients treated by curative surgery: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/U0RZHZ.
References
- Airoldi et al. (2004).Airoldi M, Cortesina G, Giordano C, Pedani F, Gabriele AM, Marchionatti S, Bumma C. Postoperative adjuvant chemoradiotherapy in older patients with head and neck cancer. Archives of Otolaryngology—Head & Neck Surgery. 2004;130:161–166. doi: 10.1001/archotol.130.2.161. [DOI] [PubMed] [Google Scholar]
- Aksu et al. (2006).Aksu G, Karadeniz A, Saynak M, Fayda M, Kadehci Z, Kocaelli H. Treatment results and prognostic factors in oral tongue cancer: analysis of 80 patients. International Journal of Oral and Maxillofacial Surgery. 2006;35:506–513. doi: 10.1016/j.ijom.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Argiris et al. (2004).Argiris A, Li Y, Murphy BA, Langer CJ, Forastiere AA. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. Journal of Clinical Oncology. 2004;22:262–268. doi: 10.1200/JCO.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya (2003).Bhattacharyya N. A matched survival analysis for squamous cell carcinoma of the head and neck in the elderly. The Laryngoscope. 2003;113:368–372. doi: 10.1097/00005537-200302000-00030. [DOI] [PubMed] [Google Scholar]
- Calabrese et al. (2011).Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA, Santoro L, Navach V, Preda L, Alterio D, Ansarin M, Chiesa F. Compartmental tongue surgery: long term oncologic results in the treatment of tongue cancer. Oral Oncology. 2011;47:174–179. doi: 10.1016/j.oraloncology.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Chang et al. (2013).Chang T-S, Chang C-M, Ho H-C, Su Y-C, Chen L-F, Chou P, Lee C-C. Impact of young age on the prognosis for oral cancer: a population-based study in Taiwan. PLoS ONE. 2013;8:e75855. doi: 10.1371/journal.pone.0075855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho et al. (2015).Cho J, Smith ML, Ahn S, Kim K, Appiah B, Ory MG. Effects of an evidence-based falls risk-reduction program on physical activity and falls efficacy among oldest-old adults. Frontiers in Public Health. 2015;2 doi: 10.3389/fpubh.2014.00182. Article 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayman et al. (1998).Clayman GL, Eicher SA, Sicard MW, Razmpa E, Goepfert H. Surgical outcomes in head and neck cancer patients 80 years of age and older. Head & Neck. 1998;20:216–223. doi: 10.1002/(SICI)1097-0347(199805)20:3<216::AID-HED6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Davidson, Root & Trock (2001).Davidson BJ, Root WA, Trock BJ. Age and survival from squamous cell carcinoma of the oral tongue. Head & Neck. 2001;23:273–279. doi: 10.1002/hed.1030. [DOI] [PubMed] [Google Scholar]
- Edge & Compton (2010).Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Extermann & Hurria (2007).Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. Journal of Clinical Oncology. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- Farhangfar et al. (2014).Farhangfar A, Makarewicz M, Ghosh S, Jha N, Scrimger R, Gramlich L, Baracos V. Nutrition impact symptoms in a population cohort of head and neck cancer patients: multivariate regression analysis of symptoms on oral intake, weight loss and survival. Oral Oncology. 2014;50:877–883. doi: 10.1016/j.oraloncology.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Genden et al. (2005).Genden EM, Rinaldo A, Shaha AR, Clayman GL, Werner JA, Suarez C, Ferlito A. Treatment considerations for head and neck cancer in the elderly. The Journal of Laryngology & Otology. 2005;119:169–174. doi: 10.1258/0022215053561521. [DOI] [PubMed] [Google Scholar]
- Goto et al. (2005).Goto M, Hasegawa Y, Terada A, Hyodo I, Hanai N, Ijichi K, Yamada H, Fujimoto Y, Ogawa T. Prognostic significance of late cervical metastasis and distant failure in patients with stage I and II oral tongue cancers. Oral Oncology. 2005;41:62–69. doi: 10.1016/j.oraloncology.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Grenman et al. (2010).Grenman R, Chevalier D, Gregoire V, Myers E, Rogers S. Treatment of head and neck cancer in the elderly: European Consensus (panel 6) at the EUFOS Congress in Vienna 2007. European Archives of Oto-Rhino-Laryngology. 2010;267:1619–1621. doi: 10.1007/s00405-010-1263-6. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2008).Huang SF, Kang CJ, Lin CY, Fan KH, Yen TC, Wang HM, Chen IH, Liao CT, Cheng AJ, Chang JT. Neck treatment of patients with early stage oral tongue cancer: comparison between observation, supraomohyoid dissection, and extended dissection. Cancer. 2008;112:1066–1075. doi: 10.1002/cncr.23278. [DOI] [PubMed] [Google Scholar]
- Italiano et al. (2008).Italiano A, Ortholan C, Dassonville O, Poissonnet G, Thariat J, Benezery K, Vallicioni J, Peyrade F, Marcy PY, Bensadoun RJ. Head and neck squamous cell carcinoma in patients aged > or = 80 years: patterns of care and survival. Cancer. 2008;113:3160–3168. doi: 10.1002/cncr.23931. [DOI] [PubMed] [Google Scholar]
- Jones et al. (1998).Jones AS, Beasley N, Houghton D, Husband DJ. The effects of age on survival and other parameters in squamous cell carcinoma of the oral cavity, pharynx and larynx. Clinical Otolaryngology and Allied Sciences. 1998;23:51–56. doi: 10.1046/j.1365-2273.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- Keogh et al. (2015).Keogh JW, Olsen A, Climstein M, Sargeant S, Jones L. Benefits and barriers of cancer practitioners discussing physical activity with their cancer patients. Journal of Cancer Education. 2015 doi: 10.1007/s13187-015-0893-1. Epub ahead of print Aug 12 2015. [DOI] [PubMed] [Google Scholar]
- Kowal et al. (2012).Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Ridaura RL, Maximova T, Arokiasamy P, Phaswana-Mafuya N, Williams S. Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE) International Journal of Epidemiology. 2012;41:1639–1649. doi: 10.1093/ije/dys210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse et al. (2010).Kruse AL, Bredell M, Luebbers HT, Gratz KW. Head and neck cancer in the elderly: a retrospective study over 10 years (1999–2008) Head and Neck Oncology. 2010;2 doi: 10.1186/1758-3284-2-25. Article 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2016).Lee CE, Warden SJ, Szuck B, Lau Y. A preliminary study on the efficacy of a community-based physical activity intervention on physical function-related risk factors for falls among breast cancer survivors. American Journal of Physical Medicine & Rehabilitation. 2016;95:561–570. doi: 10.1097/PHM.0000000000000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. (2008).Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, Lin CH, Chen IH, Huang SF, Cheng AJ, Yen TC. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Annals of Surgical Oncology. 2008;15:915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- Luciani et al. (2010).Luciani A, Ascione G, Bertuzzi C, Marussi D, Codecà C, Di Maria G, Caldiera SE, Floriani I, Zonato S, Ferrari D. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. Journal of Clinical Oncology. 2010;28:2046–2050. doi: 10.1200/JCO.2009.25.9978. [DOI] [PubMed] [Google Scholar]
- Lusinchi et al. (1990).Lusinchi A, Bourhis J, Wibault P, Le Ridant A, Eschwege F. Radiation therapy for head and neck cancers in the elderly. International Journal of Radiation Oncology, Biology, Physics. 1990;18:819–823. doi: 10.1016/0360-3016(90)90403-7. [DOI] [PubMed] [Google Scholar]
- Moore et al. (2000).Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of tongue cancer: a review of global incidence. Oral Diseases. 2000;6:75–84. doi: 10.1111/j.1601-0825.2000.tb00105.x. [DOI] [PubMed] [Google Scholar]
- NIH (1998).NIH . Aging and old age as risk factors for multiple primary tumors. National Institute of Health; Bethesda: 1998. [Google Scholar]
- Ortholan et al. (2009).Ortholan C, Lusinchi A, Italiano A, Bensadoun RJ, Auperin A, Poissonnet G, Bozec A, Arriagada R, Temam S, Benezery K, Thariat J, Tao Y, Janot F, Mamelle G, Vallicioni J, Follana P, Peyrade F, Sudaka A, Bourhis J, Dassonville O. Oral cavity squamous cell carcinoma in 260 patients aged 80 years or more. Radiotherapy and Oncology. 2009;93:516–523. doi: 10.1016/j.radonc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Pfister et al. (2013).Pfister DG, Ang K-K, Brizel DM, Burtness BA, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW. Head and neck cancers, version 2.2013. Journal of the National Comprehensive Cancer Network. 2013;11:917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- Pfister et al. (2000).Pfister D, Ang K, Brockstein B, Colevas A, Ellenhorn J, Goepfert H, Hicks Jr W, Hong W, Kies M, Lydiatt W. NCCN practice guidelines for head and neck cancers. Oncology. 2000;14:163–194. [PubMed] [Google Scholar]
- Pinto & Ciccolo (2010).Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Physical Activity and Cancer. 2010;186:367–387. doi: 10.1007/978-3-642-04231-7_16. [DOI] [PubMed] [Google Scholar]
- Reid (2013).Reid BC. Visual screening for oral cancer may reduce oral cancer mortality in high-risk adult populations through early diagnosis and treatment. Journal of Evidence Based Dental Practice. 2013;13:174–176. doi: 10.1016/j.jebdp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Reid et al. (2001).Reid BC, Alberg AJ, Klassen AC, Samet JM, Rozier RG, Garcia I, Winn DM. Comorbidity and survival of elderly head and neck carcinoma patients. Cancer. 2001;92:2109–2116. doi: 10.1002/1097-0142(20011015)92:8<2109::AID-CNCR1552>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Rock et al. (2012).Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians. 2012;62:242–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- Saotome, Klein & Faux (2015).Saotome T, Klein L, Faux S. Cancer rehabilitation: a barometer for survival? Supportive Care in Cancer. 2015;23:3033–3041. doi: 10.1007/s00520-015-2673-1. [DOI] [PubMed] [Google Scholar]
- Sarini et al. (2001).Sarini J, Fournier C, Lefebvre J-L, Bonafos G, Van JT, Coche-Dequéant B. Head and neck squamous cell carcinoma in elderly patients: a long-term retrospective review of 273 cases. Archives of Otolaryngology—Head & Neck Surgery. 2001;127:1089–1092. doi: 10.1001/archotol.127.9.1089. [DOI] [PubMed] [Google Scholar]
- Sattar et al. (2016).Sattar S, Alibhai SM, Spoelstra SL, Fazelzad R, Puts MT. Falls in older adults with cancer: a systematic review of prevalence, injurious falls, and impact on cancer treatment. Supportive Care in Cancer. 2016;24:4459–4469. doi: 10.1007/s00520-016-3342-8. [DOI] [PubMed] [Google Scholar]
- Siddiqui & Gwede (2012).Siddiqui F, Gwede CK. Head and neck cancer in the elderly population. Seminars in Radiation Oncology. 2012;22:321–333. doi: 10.1016/j.semradonc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Taiwan Ministry of Health and Welfare (2016).Taiwan Ministry of Health and Welfare Ministry of Health and Welfare, Taipei City, Taiwanhttp://www.hpa.gov.tw/BHPNet/Web/Stat/Statistics.aspx Cancer registry annual report of Taiwan 2012. 2016
- Taiwan National Development Council (2014).Taiwan National Development Council Population projection of Taiwan 2014–2061. 2014. National Development Council, Taiwan. http://www.ndc.gov.tw/Content_List.aspx?n=84223C65B6F94D72 .
- Ungar & Rafanelli (2015).Ungar A, Rafanelli M. My older patient with cancer reports falls: what should I do? Journal of Geriatric Oncology. 2015;6:419–423. doi: 10.1016/j.jgo.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Zabrodsky et al. (2004).Zabrodsky M, Calabrese L, Tosoni A, Ansarin M, Giugliano G, Bruschini R, Tradati N, De Paoli F, Tredici P, Betka J. Major surgery in elderly head and neck cancer patients: immediate and long-term surgical results and complication rates. Surgical Oncology. 2004;13:249–255. doi: 10.1016/j.suronc.2004.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
Mortality in tongue cancer patients treated by curative surgery: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/U0RZHZ.