Abstract
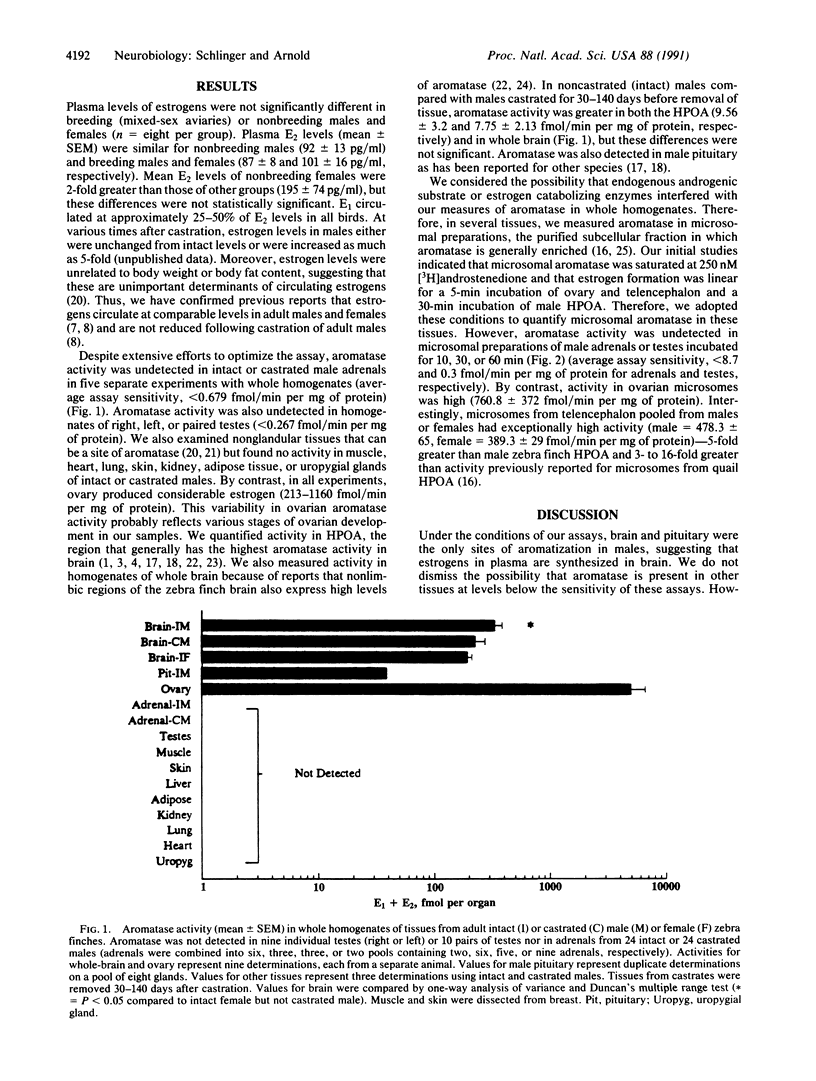
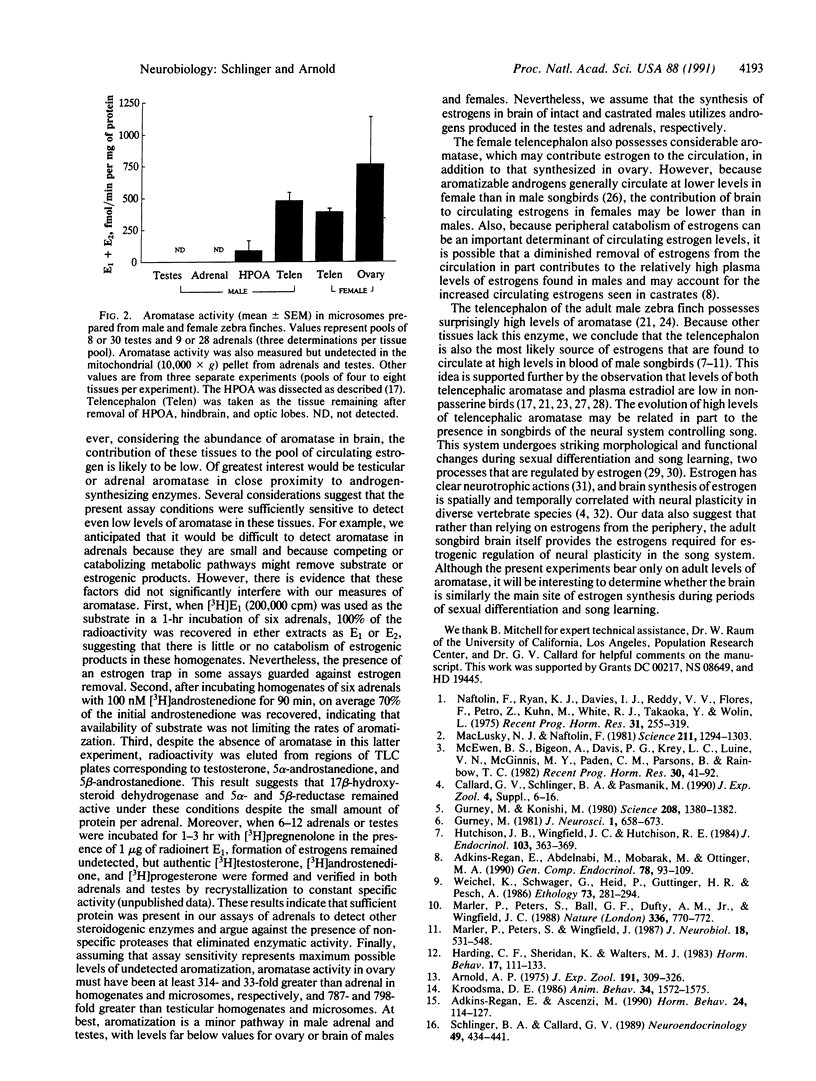
The neural system controlling song in passerine birds can undergo striking morphological and functional changes during both development and adulthood, and many of these changes are regulated by estrogenic hormones. Estrogens circulate at high levels in blood of male songbirds and persist after castration. We measured the activity of aromatase, the enzyme that converts androgens to estrogens, in various tissues from adult male and female zebra finches. As expected, aromatase activity was present in male hypothalamus/preoptic area and pituitary and female ovary, but aromatase was unusually active in whole telencephalon of males and females. By contrast, activity was undetected in testes, adrenals, or other tissues of males. These results suggest that brain is the source of circulating estrogens in the male zebra finch and that estrogen actions on the song system result from local rather than peripheral aromatization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins-Regan E., Abdelnabi M., Mobarak M., Ottinger M. A. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata). Gen Comp Endocrinol. 1990 Apr;78(1):93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E., Ascenzi M. Sexual differentiation of behavior in the zebra finch: effect of early gonadectomy or androgen treatment. Horm Behav. 1990 Mar;24(1):114–127. doi: 10.1016/0018-506x(90)90031-r. [DOI] [PubMed] [Google Scholar]
- Arnold A. P., Bottjer S. W., Brenowitz E. A., Nordeen E. J., Nordeen K. W. Sexual dimorphisms in the neural vocal control system in song birds: ontogeny and phylogeny. Brain Behav Evol. 1986;28(1-3):22–31. doi: 10.1159/000118689. [DOI] [PubMed] [Google Scholar]
- Arnold A. P. The effects of castration and androgen replacement on song, courtship, and aggression in zebra finches (Poephila guttata). J Exp Zool. 1975 Mar;191(3):309–326. doi: 10.1002/jez.1401910302. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callard G., Schlinger B., Pasmanik M. Nonmammalian vertebrate models in studies of brain-steroid interactions. J Exp Zool Suppl. 1990;4:6–16. doi: 10.1002/jez.1402560404. [DOI] [PubMed] [Google Scholar]
- Gurney M. E. Hormonal control of cell form and number in the zebra finch song system. J Neurosci. 1981 Jun;1(6):658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M. E., Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980 Jun 20;208(4450):1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Harding C. F., Sheridan K., Walters M. J. Hormonal specificity and activation of sexual behavior in male zebra finches. Horm Behav. 1983 Mar;17(1):111–133. doi: 10.1016/0018-506x(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Hutchison J. B., Steimer T. Formation of behaviorally effective 17 beta-estradiol in the dove brain: steroid control of preoptic aromatase. Endocrinology. 1986 Jun;118(6):2180–2187. doi: 10.1210/endo-118-6-2180. [DOI] [PubMed] [Google Scholar]
- Hutchison J. B., Wingfield J. C., Hutchison R. E. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984 Dec;103(3):363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F., Goldman-Rakic P. S. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci U S A. 1986 Jan;83(2):513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F. Sexual differentiation of the central nervous system. Science. 1981 Mar 20;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Marler P., Peters S., Ball G. F., Dufty A. M., Jr, Wingfield J. C. The role of sex steroids in the acquisition and production of birdsong. Nature. 1988 Dec 22;336(6201):770–772. doi: 10.1038/336770a0. [DOI] [PubMed] [Google Scholar]
- Marler P., Peters S., Wingfield J. Correlations between song acquisition, song production, and plasma levels of testosterone and estradiol in sparrows. J Neurobiol. 1987 Nov;18(6):531–548. doi: 10.1002/neu.480180605. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Biegon A., Davis P. G., Krey L. C., Luine V. N., McGinnis M. Y., Paden C. M., Parsons B., Rainbow T. C. Steroid hormones: humoral signals which alter brain cell properties and functions. Recent Prog Horm Res. 1982;38:41–92. doi: 10.1016/b978-0-12-571138-8.50007-x. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Davies I. J., Reddy V. V., Flores F., Petro Z., Kuhn M., White R. J., Takaoka Y., Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- RYAN K. J. Biological aromatization of steroids. J Biol Chem. 1959 Feb;234(2):268–272. [PubMed] [Google Scholar]
- Schlinger B. A., Callard G. V. A comparison of aromatase, 5 alpha-, and 5 beta- reductase activities in the brain and pituitary of male and female quail (C. c. japonica). J Exp Zool. 1987 May;242(2):171–180. doi: 10.1002/jez.1402420208. [DOI] [PubMed] [Google Scholar]
- Schlinger B. A., Callard G. V. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology. 1989 Apr;49(4):434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- Schlinger B. A., Fivizzani A. J., Callard G. V. Aromatase, 5 alpha- and 5 beta-reductase in brain, pituitary and skin of the sex-role reversed Wilson's phalarope. J Endocrinol. 1989 Aug;122(2):573–581. doi: 10.1677/joe.0.1220573. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Balthazart J. Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res. 1987 Sep 29;422(1):137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res. 1976 Apr 23;106(2):407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- Vockel A., Pröve E., Balthazart J. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J Neurobiol. 1990 Jul;21(5):808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- Vockel A., Pröve E., Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990 Mar 19;511(2):291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- Watson J. T., Abdelnabi M., Wersinger S., Ottinger M. A., Adkins-Regan E. Circulating estradiol and the activation of male and female copulatory behavior in Japanese quail (Coturnix japonica). Gen Comp Endocrinol. 1990 Feb;77(2):229–238. doi: 10.1016/0016-6480(90)90307-8. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Leshin M., George F. W. The Sebright bantam chicken and the genetic control of extraglandular aromatase. Endocr Rev. 1987 Nov;8(4):363–376. doi: 10.1210/edrv-8-4-363. [DOI] [PubMed] [Google Scholar]



