Abstract
Background
Patients with medullary sponge kidney (MSK) commonly encounter recurrent nephrolithiasis. The existing knowledge on safety of donors with MSK has not been studied.
Methods
We conducted a retrospective cohort study at a tertiary referral hospital to assess the outcomes of living kidney donors with MSK. All adults with MSK (N = 26) who underwent nephrectomy as living kidney donors between January 2000 and September 2014 were included. Non-donors with MSK (N = 78) were randomly selected by matching the year of birth and the comorbidity score with a ratio of 1:3 for comparison.
Results
The incident rates of symptomatic stone were 0.7, 0.4 and 4.9 events/100 patient-years in donors, recipients and non-donors, respectively. After adjusting for history of kidney stones and baseline estimated glomerular filtration rate (eGFR), the kidney stone-related event was significantly lower in donors than in non-donors (hazard ratio 0.14; 95% confidence interval 0.01–0.66). One recipient of MSK living donor had symptomatic stone at median follow-up time of 8.4 years (interquartile range 5.6–12.4 years). None of MSK donors had hypercalciuria, hypocitraturia or hyperoxaluria prior to kidney donation. At 5 years after the index surgery date, there was no significant difference in eGFR between donors and non-donors (76.1 versus 70.9 mL/min/1.73 m2, P = 0.12).
Conclusions
These findings are reassuring for the safety of MSK kidney donors with normal kidney function, low kidney stone risk and no significant comorbidity.
Keywords: end-stage renal disease, kidney stones, living kidney donor, medullary sponge kidney, outcomes
Introduction
The number of patients anticipating kidney transplantation in the USA has steadily risen over time, by 3000–4000 patients each year [1], with the gap between allograft supply and demand continuing to grow [2–5]. As of the year 2010, nearly 93 000 end-stage renal disease (ESRD) patients were listed on the transplant waiting list at the United Network for Organ Sharing (UNOS) in the USA [6, 7]. An increase in the number of living donors is needed to improve this trend [2–5].
Data on the long-term outcomes of living kidney donors is needed to ensure public confidence in the transplantation system [6]. Since kidney stones are very frequent problem in the general population with an estimated global prevalence of 10–15% [8–13], the safety of donors with a history of kidney stones is an ongoing donor concern. Although there is considerable variability in the criteria used to exclude potential donors, nephrocalcinosis, bilateral kidney stones and recurrent stones are generally accepted as absolute contraindications [14, 15].
Recently, Thomas et al. [16] conducted a population-based retrospective matched cohort study and demonstrated no difference in the rate of kidney stone-related events between donors and non-donors, which was reassuring for the safety of living kidney donation. However, the existing knowledge on safety of donors with medullary sponge kidney (MSK), who have higher risk of developing recurrent nephrolithiasis, has not been studied. Overall, patients with MSK usually have an excellent long-term prognosis [17]. In addition, a large cohort study of potential kidney donors found that radiographic findings of MSK were characterized in patients with asymptomatic stone disease [18]. However, there have been ongoing concerns that a few individuals with MSK with recurrent calcium phosphate and calcium oxalate stones, leading to recurrent urinary tract obstructions, can have higher risk of renal function decline [19]. MSK-related kidney stones and urinary tract infections were also reported as potential causes of ESRD [20].
The objective of this study is to assess the clinical and safety outcomes of living kidney donors with MSK.
Materials and methods
Study population
This was a single-center matched cohort study conducted at a tertiary referral hospital with a kidney transplant center. The Mayo Clinic Institution Review Board approved this study. We included all adult patients (age ≥18 years) with MSK who underwent nephrectomy as living kidney donors (donor group) at Mayo Clinic Hospital, Rochester, MN between January 2000 and September 2014. We excluded patients who did not provide research authorization. For comparison, we selected three control MSK patients who did not undergo nephrectomy (non-donor group) for each case and matched for the same year of birth and Charlson Comorbidities Index (CCI) scores [21], which included 19 comorbidities weighted 1–6 corresponding to disease severity, at the index surgery date (±1 score). We assigned the nephrectomy date of each case as an index surgery date of their matched control patients.
MSK diagnosis and kidney donor protocol for donor nephrolithiasis
Individuals with MSK were initially identified using ICD-9 code 753.17. Comprehensive medical record review by a board-certified physician (W.C.) was subsequently performed to validate the diagnosis of MSK. The definite diagnosis of MSK was based on intravenous pyelogram or computer tomography urogram.
As a part of our standard donor protocol, we obtained a urinary supersaturation profile for all donors with tiny, medullary or papillary calcifications, not constituting kidney stones based on radiologic findings. Urinary concentrations (24 h) of supersaturation were measured in the Mayo Clinic Renal Testing Laboratory. Urine oxalate was measured by oxalate oxidase. Supersaturation was calculated using the EQUIL2 program [4]. Individuals with bilateral medullary or papillary calcinosis and <50 years old, especially with an abnormal supersaturation profile, were categorized as relative contraindications. History of multiple stones or history of a single stone and current metabolic abnormalities predisposing to recurrence were classified as relative contraindications to kidney donation. History of cystine stones or struvite stones was considered as contraindication to donation. During the study period, there was no specific protocol for an evaluation of the potential living kidney donor with MSK.
Data collection
Clinical characteristics, demographic information and laboratory data were collected using manual and automated retrieval from institutional electronic medical records. Data collected included age, sex, race, body mass index (BMI), CCI score, baseline serum creatinine and history of kidney stones before the index surgery date. Baseline serum creatinine was defined as the minimum serum creatinine value within 6 months prior to the index surgery date. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [22].
Clinical outcomes
The primary clinical outcome was kidney stone-related events, defined as the occurrence of symptomatic or passing kidney stones after the index surgery date. The secondary outcomes were the kidney function, ESRD requiring dialysis and mortality after the index surgery date. We determined these outcomes with comprehensive medical record review. In addition to institutional registration and electronic medical record, we verified all patients' vital status using the Social Security Death Index Registry (http://www.genealogybank.com/gbnk/ssdi/). Social Security Death Index data were updated to the latest version published by the Social Security Administration as of February 2014.
Statistical analysis
In the event of missing data, data were not imputed. Continuous variables were reported as medians [interquartile range (IQR)] or means ± SD and were compared using the Wilcoxon rank-sum test or the Student's t-test, as appropriate for non-parametric and parametric data, respectively. Categorical variables were reported as counts with percentages and were compared using the χ2 test. The incidence rate of kidney stone-related event was reported as the number of events per 100 patient-years. Matching was performed in R statistical software (MatchIt package, version 2.4–21) [23]. Outcome data for kidney stone-related events between the donor and non-donor group were presented using the Kaplan–Meier plot and were compared using the log-rank test. We performed multivariate Cox proportional hazard analyses to assess the difference in kidney stone-related events, adjusting for history of kidney stone prior to the index transplant date and eGFR. We performed linear regression analysis to assess the association between eGFR and time in years after the index date. To exclude the effects of postoperative acute kidney injury, we did not include serum creatinine within 7 days after each index surgery date. The two-sided P value of <0.05 was considered statistically significant. Unless specified, analysis was performed using JMP statistical software (version 9.0, SAS Institute Inc., Cary, NC).
Results
Patient characteristics
We identified 746 patients with MSK. A total of 26 living kidney donors with MSK and 78 non-donors with MSK were included in this study. All 78 non-donors with MSK had never been evaluated for kidney donation. Baseline characteristics showed similar mean age of 44 ± 14 years in donor and non-donor cohorts, P = 0.90. The majority of donors and non-donors with MSK were females (69 versus 65%, P = 0.72). BMI was comparable between donor and non-donor cohorts (26.1 ± 2.9 versus 26.2 ± 4.4 kg/m2, P = 0.91). There was no difference in baseline comorbidities between donors and non-donors with MSK [CCI 0 (0–0) versus 0 (0–1), P = 0.32]. Baseline kidney functions were normal in both donors and non-donors with a mean creatinine (Cr) of 1.0 ± 0.1 versus 1.0 ± 0.5 mg/dL, P = 0.15, and eGFR of 80 ± 14 versus 79 ± 22 mL/min/1.73 m2, P = 0.81. Both donors and non-donors with MSK had comparable history of kidney stones prior to the index date [13 (50%) versus 43 (55%), P = 0.65] (Table 1).
Table 1.
Baseline characteristics of donors and non-donors with MSK
| Characteristics | Non-donor (n = 78) | Donor (n = 26) | P-value |
|---|---|---|---|
| Age (years) | 44 ± 14 | 44 ± 14 | 0.90 |
| Male sex | 27 (35) | 8 (31) | 0.72 |
| Caucasian | 73 (94) | 21 (81) | 0.08 |
| BMI (kg/m2) | 26.2 ± 4.4 | 26.1 ± 2.9 | 0.91 |
| Charlson scorea | 0 (0–1) | 0 (0–0) | 0.32 |
| Diabetes mellitus | 1 (1) | 0 (0) | 0.56 |
| Baseline Cr (mg/dL) | 1.0 ± 0.5 | 1.0 ± 0.1 | 0.15 |
| Baseline GFR (mL/min/1.73 m2) | 79 ± 22 | 80 ± 14 | 0.81 |
| History of kidney stone | 43 (55) | 13 (50) | 0.65 |
Continuous data are presented as mean ± SD; categorical data are presented as N (%) if not indicated. aMedian (range) are reported.
Kidney stone-related events in donor and non-donors with MSK
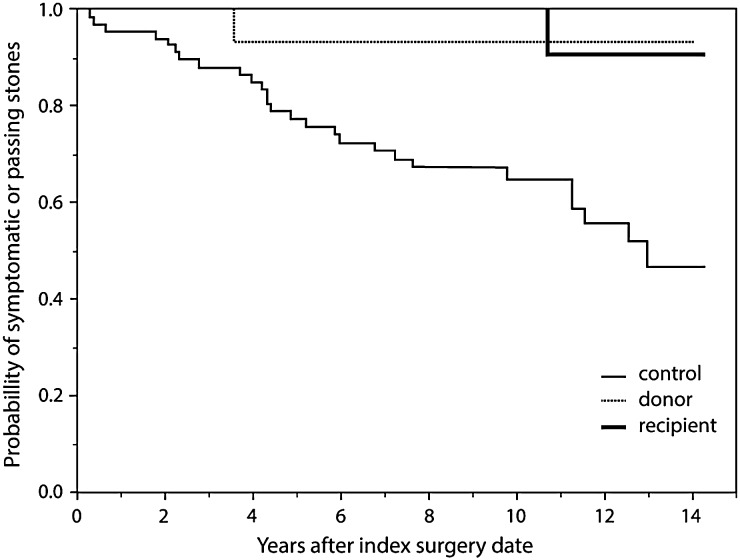
The incident rates of symptomatic stone were 0.7 and 4.9 events/100 patient-years in donors and non-donors, respectively (Figure 1). We further investigated the kidney stone-related events in 26 recipients of living kidney donor with MSK from our cohort and found the incident rate to be 0.4 events/100 patient-years. After adjusting for history of kidney stone prior to the index transplant date and baseline eGFR, the kidney stone-related event was significantly lower in donors than in non-donors (hazard ratio 0.14; 95% confidence interval 0.01–0.66). One recipient of MSK living donor had symptomatic stone at median follow-up time of 8.4 years (IQR 5.6–12.4 years).
Fig. 1.
Kaplan–Meier curves for stone-related events. Hazard ratio 0.14 (0.01–0.66), P = 0.04, after adjusting for history of stone before the index surgery date and baseline GFR.
In the MSK donor group, urine supersaturation profiles were further reviewed. None of MSK donors had hypercalciuria (173 ± 49 mg/specimen; reference range 20–275 mg/specimen), hypocitraturia (622 ± 296 mg/24 h; reference range ≥385 mg/24 h) or hyperoxaluria (0.28 ± 0.09 mmol/specimen; reference range 0.11–0.46 mmol/specimen) prior to kidney donation.
Renal function and mortality after kidney donations
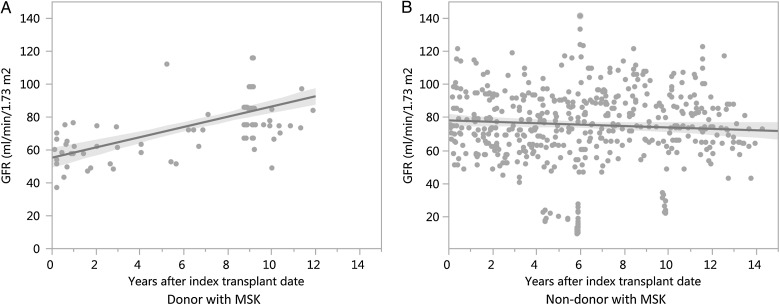
After nephrectomy, donors' eGFR decreased to 55.33 ± 5 mL/min/1.73 m2 at 7 days after the index date. The eGFR of donors subsequently increased significantly at a rate of +3.11 mL/min/1.73 m2 per year, P < 0.001 (Figure 2). At 5 years after the index transplant date, there was no significant difference in eGFR between donors and non-donors (76.1 versus 70.9 mL/min/1.73 m2, P = 0.12). None of the donors required dialysis at median follow-up time of 8.6 years (IQR 0.8–9.8 years). One non-donor developed ESRD requiring dialysis at median follow-up time of 8.9 years (IQR 5.7–12.4 years). No donors had died at median follow-up time of 12.7 years (IQR 8.6–13.9 years). Three non-donors had died at median follow-up time of 12.6 years (IQR 8.6–13.6 years). Outcomes with median follow-up times of donors and non-donors with MSK are shown in Table 2.
Fig. 2.
The eGFR (CKD-EPI) over time after the index surgery date was increased in donors (A) at the rate of +3.11 mL/min/1.73 m2 per year and decreased in non-donors (B) at the rate of −0.43 mL/min/1.73 m2 per year.
Table 2.
Outcomes with median follow-up times of donors and non-donors with MSK
| Outcome | Control | Donor |
|---|---|---|
| Symptomatic or passing stone | ||
| Median follow-up time (IQR), years | 7.4 (3.4–11.7) | 8.4 (0.8–9.4) |
| Events | 27 | 1 |
| The need for long-term dialysis | ||
| Median follow-up time (IQR), years | 8.9 (5.7–12.4) | 8.6 (0.8–9.8) |
| Events | 1 | 0 |
| Death | ||
| Median follow-up time (IQR), years | 12.6 (8.6–13.6) | 12.7 (8.6–13.9) |
| Events | 3 | 0 |
Discussion
The findings of our study demonstrate that kidney stone-related events are 7-fold decreased in donors with MSK after kidney donation compared with non-donors with MSK. There was no significant difference in eGFR between donors and non-donors at 5 years after the index surgery date. The risks of ESRD and mortality are also not significantly increased after donation. These findings are reassuring for the safety of healthy MSK kidney donors.
Since MSK is a congenital condition affecting bilateral kidneys in most cases [24, 25], it was not a surprise that donors with MSK developed fewer kidney stone-related events after nephrectomy compared with non-donors with MSK, who had two kidneys. A recent population-based retrospective matched cohort study reassured the safety of living kidney donation by showing no difference in the rate of kidney stone-related events between donors and non-donors [16]. However, the data on stone events after donor nephrectomy in MSK donors were unknown, which potentially excluded these potential kidney donors from living donation [26]. Our study showed that donors with MSK had lower kidney stone-related events after nephrectomy compared with non-donors with MSK. Moreover, the incident rate of kidney stone-related events in recipients of living kidney donor with MSK was very low at 0.4 events/100 patient-years. Urine supersaturation profiles for donors with MSK showed no hypercalciuria, hypocitraturia or hyperoxaluria, demonstrating low risks for kidney stone formation [27]. The findings of urinary supersaturation study likely explained the low number of kidney stone-related events after nephrectomy in donors with MSK in our study.
Although long-term studies have shown no significant progressive loss of eGFR over time after living kidney donation [28, 29], it was unclear in donors with MSK since they had increased risk of kidney stones compared with other healthy donors. Our study showed that compensation in the remaining kidney returned the eGFR to ∼95% of baseline at 5 years. In addition, we found that the risks of ESRD and mortality in donors with MSK were also not significantly increased after donation compared with non-donors with MSK.
There are several limitations in our study. First, this report is a retrospective study and inherently subjective to biases of a retrospective study. Second, the data on kidney stone-related events were collected by medical record review. Our institution performed living kidney transplant for patients from many states and overseas during the study period and donor contacts were limited. Therefore, we may have missed the events that occurred after their last follow-up visits. However, we used the survival analysis to assess the outcomes of stone-related events. Moreover, median follow-up times in both donor and non-donor cohorts were comparable. Lastly, the data on kidney stone type from stone composition study were limited, since the stone-related events mostly occurred during follow-up visits. However, previous studies have shown a conclusive finding that patients with MSK have increased risk of calcium phosphate and calcium oxalate stones [30].
In summary, the findings of this study reveal good outcomes of donors with MSK. After nephrectomy, MSK donors with low kidney stone risk have few kidney stone-related events after nephrectomy. The risks of renal function decline, ESRD and mortality are not significantly increased after donation. These findings are reassuring for the safety of selected healthy MSK kidney donors with baseline normal kidney function, low kidney stone risk and no significant comorbidity.
Conflicts of interest statement
None declared.
References
- 1.Gaston RS, Danovitch GM, Adams PL et al. . The report of a national conference on the wait list for kidney transplantation. Am J Transplant 2003; 3: 775–785 [DOI] [PubMed] [Google Scholar]
- 2.Hou S. Expanding the kidney donor pool: ethical and medical considerations. Kidney Int 2000; 58: 1820–1836 [DOI] [PubMed] [Google Scholar]
- 3.Ellison MD, McBride MA, Taranto SE et al. . Living kidney donors in need of kidney transplants: a report from the organ procurement and transplantation network. Transplantation 2002; 74: 1349–1351 [DOI] [PubMed] [Google Scholar]
- 4.Port FK, Dykstra DM, Merion RM et al. . Trends and results for organ donation and transplantation in the United States, 2004. Am J Transplant 2005; 5 (4 Pt 2): 843–849 [DOI] [PubMed] [Google Scholar]
- 5.Delmonico FL, Sheehy E, Marks WH et al. . Organ donation and utilization in the United States, 2004. Am J Transplant 2005; 5 (4 Pt 2): 862–873 [DOI] [PubMed] [Google Scholar]
- 6.Leichtman AB, Cohen D, Keith D et al. . Kidney and pancreas transplantation in the United States, 1997–2006: the HRSA Breakthrough Collaboratives and the 58 DSA Challenge. Am J Transplant 2008; 8 (4 Pt 2): 946–957 [DOI] [PubMed] [Google Scholar]
- 7.Axelrod DA, McCullough KP, Brewer ED et al. . Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am J Transplant 2010; 10 (4 Pt 2): 987–1002 [DOI] [PubMed] [Google Scholar]
- 8.Goldfarb DS. Increasing prevalence of kidney stones in the United States. Kidney Int 2003; 63: 1951–1952 [DOI] [PubMed] [Google Scholar]
- 9.Long LO, Park S. Update on nephrolithiasis management. Minerva Urol Nefrol 2007; 59: 317–325 [PubMed] [Google Scholar]
- 10.Lopez M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol 2010; 25: 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamatelou KK, Francis ME, Jones CA et al. . Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63: 1817–1823 [DOI] [PubMed] [Google Scholar]
- 12.Rule AD, Lieske JC, Li X et al. . The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol 2014; 25: 2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheungpasitporn W, Rossetti S, Friend K et al. . Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta-analysis. J Nephrol 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasiske BL, Ravenscraft M, Ramos EL et al. . The evaluation of living renal transplant donors: clinical practice guidelines. Ad Hoc Clinical Practice Guidelines Subcommittee of the Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol 1996; 7: 2288–2313 [DOI] [PubMed] [Google Scholar]
- 15.Fischer SA, Avery RK. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant 2009; 9 (Suppl 4): S7–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SM, Lam NN, Welk BK et al. . Risk of kidney stones with surgical intervention in living kidney donors. Am J Transplant 2013; 13: 2935–2944 [DOI] [PubMed] [Google Scholar]
- 17.Feest TG. Medullary sponge kidney: abnormalities of renal tubular and glomerular function, and their relationship to clinical features. Proc Eur Dial Transplant Assoc 1977; 14: 511–517 [PubMed] [Google Scholar]
- 18.Lorenz EC, Lieske JC, Vrtiska TJ et al. . Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant 2011; 26: 2695–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungers P, Joly D, Barbey F et al. . ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis 2004; 44: 799–805 [PubMed] [Google Scholar]
- 20.Magee CC, Mah H, Tinckam K et al. . Successful living donor kidney transplantation across HLA and ABO incompatibilities. Nephrol Dial Transplant 2007; 22: 602–604 [DOI] [PubMed] [Google Scholar]
- 21.Charlson M, Szatrowski TP, Peterson J et al. . Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 22.Kilbride HS, Stevens PE, Eaglestone G et al. . Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 2013; 61: 57–66 [DOI] [PubMed] [Google Scholar]
- 23.Ho D, Imai K, King G et al. . MatchIt: nonparametric preprocessing for parametric casual inference. J Stat Softw 2011; 42: 1–28 [Google Scholar]
- 24.Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney Int 1994; 46: 951–964 [DOI] [PubMed] [Google Scholar]
- 25.Cheungpasitporn W, Erickson SB. Medullary sponge kidneys and the use of dual-energy computed tomography. Urol Ann 2015; 7: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsburgh J, Thomas K, Smith J et al. . Incidental stones in living kidney donors: Bench ureteroscopy maximising the donor pool. J Endourol 2009; A237: Abstract 23. [Google Scholar]
- 27.Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab 2012; 97: 1847–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gossmann J, Wilhelm A, Kachel HG et al. . Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant 2005; 5: 2417–2424 [DOI] [PubMed] [Google Scholar]
- 29.Anderson CF, Velosa JA, Frohnert PP et al. . The risks of unilateral nephrectomy: status of kidney donors 10 to 20 years postoperatively. Mayo Clin Proc 1985; 60: 367–374 [DOI] [PubMed] [Google Scholar]
- 30.Gambaro G, Danza FM, Fabris A. Medullary sponge kidney. Curr Opin Nephrol Hypertens 2013; 22: 421–426 [DOI] [PubMed] [Google Scholar]