Abstract
Background
Despite the many advantages it offers, the percentage of dialysis patients that receive home dialysis [peritoneal dialysis (PD) or home haemodialysis (HHD)] in the Netherlands has declined over the last decade. Pre-dialysis education could stimulate the use of home dialysis. This article presents the results of the pre-dialysis programme GUIDE, with regard to the following question: Does the implementation of a structured pre-dialysis programme with a home-focused approach increase the number of pre-dialysis patients that choose and receive home dialysis?
Methods
The GUIDE process starts when a patient has an eGFR of 15 mL/min/1.73 m2. The process begins with a home visit from a case manager and the completion of questionnaires by the patient, the case manager and the nephrologist. A multidisciplinary meeting (MDM) is held to determine a specific patient profile (or treatment recommendation). This is followed by patient education, a second MDM and finally the selection of the treatment by the patient and the nephrologist. This retrospective observational study describes the selection process of all patients that received a treatment recommendation between 12 September 2013 and 18 December 2014 at Meander Medical Centre. Data were collected by file research and analysis of questionnaires.
Results
One hundred and two patients were included. They started the process at a mean eGFR of 12.3 mL/min/1.73 m2. Home dialysis was recommended for 62.8% of the patients who were advised to have dialysis treatment. Of the patients that opted for dialysis, 34.2% chose PD and 8.2% chose HHD; 22.9% started home dialysis as their first therapy, compared with 17.6% in the months before implementation of GUIDE. Finally, 32.1% of the patients that received dialysis therapy received home dialysis. In the months before GUIDE, an average of just 19.5% of the patients that received dialysis received home dialysis.
Conclusions
In comparison to historical data, the pre-dialysis programme GUIDE increases the number of patients that choose and receive home dialysis.
Keywords: end-stage renal disease, home dialysis, modality selection, peritoneal dialysis, pre-dialysis education
Introduction
On January 1st 2015, 16 316 patients received renal replacement therapy (RRT) for end-stage renal disease (ESRD) in the Netherlands [1]. Available treatment modalities are kidney transplantation (in some cases pre-emptive), peritoneal dialysis (PD) at home, in-centre haemodialysis (CHD), home haemodialysis (HHD) and conservative treatment without RRT (supportive care).
Numerous studies show the advantages of home dialysis therapy (HHD or PD) [2]. PD has similar or even better clinical outcomes compared with HD [3, 4]. Frequent (daily or five to six times a week) HD is especially suitable as a home treatment and has more favourable outcomes than conventional CHD, typically performed three times a week [5]. Quality of life [6] and treatment satisfaction [7] are greater for home dialysis, and home dialysis offers patients more flexibility, independence and responsibility [8]. Finally, home dialysis is more cost effective than in-centre dialysis [9].
Despite these advantages, the percentage of patients treated with home dialysis in the Netherlands has declined over the last decade: on 31 December 2013, only 14.2% of all dialysis patients were on PD, compared with 28.3% on 31 December 2003 [10]. Meanwhile, the percentage of patients on HHD increased only slightly, from 2.0% in 2003 to 3.3% in 2013 [11]. This decline in the proportion of PD has been observed in most developed countries. Various factors could be responsible for the decline, including financial influences and the incentive to use the capacity of available HD facilities [12].
Oreopoulos et al. [2] proposed a change in approach: to present patients with the choice between dialysis at home (PD or HD) or dialysis in the hospital, rather than to choose between PD or HD. Goovaerts et al. [13] used a similar system in their pre-dialysis education programme: when absolute contraindications for PD were present, the patient could still choose between various HD modalities. Only the patients that were not eligible for any self-care therapy were directly referred for CHD. Pre-dialysis education on treatment modalities is essential to increase the percentage of patients treated with home dialysis [13–15]. Furthermore, patient involvement through shared decision-making is advocated to ensure that the dialysis modality decision meets the clinical as well as the psychosocial needs of the patient [16]. Factors that influence decision-making are the timing of education and the reluctance to change treatments after starting dialysis, despite potential advantages [17]. Moreover, the implementation of a home visit in the pre-dialysis phase is associated with a higher probability of home dialysis [18].
This article presents the results of the pre-dialysis programme GUIDE, which is based on these principles and aspires to increase the use of home dialysis. We aim to answer the following question: Does the implementation of a structured pre-dialysis programme with a home-focused approach increase the number of pre-dialysis patients that choose home dialysis, and the number of patients that eventually receive home dialysis?
Materials and methods
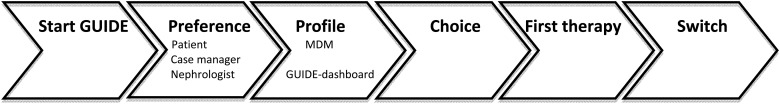
Gezonde nieren (Dutch for healthy kidneys) is a concept for prevention and treatment of chronic kidney damage in the Netherlands [19]. As part of this programme, we have standardised the pre-dialysis process (now named GUIDE) (Figure 1) to start education and preparation for RRT at an eGFR of 15 mL/min/1.73 m2 (or 20 mL/min/1.73 m2 in patients with rapid deterioration of renal function). The process commences with a home visit from a social worker (the case manager), during which the first education is given and the suitability for home dialysis is assessed. Subsequently, the patient, case manager and nephrologist each fill out questionnaires. The patient questionnaire contains questions about the patient's social support system, daily activities, level of independence in activities of daily living (ADL), the aspects of life that the patient values most and the preferences and expectations with regard to RRT. The medical questionnaire comprises the categories Transplantation, PD and HD, which contain questions about relative and absolute contraindications for each therapy and, finally, the nephrologist's treatment preference. The case manager's questionnaire covers the suitability of the home, the social environment and the balance between burden and capacity and ends with the case manager's judgment of whether or not home dialysis would be suitable.
Fig. 1.
Treatment selection steps in the GUIDE programme.
Patients that chose conservative treatment or pre-emptive transplantation at the start of the programme did not receive a home visit and did not fill out the patient questionnaire; their preference was deduced from their treatment choice.
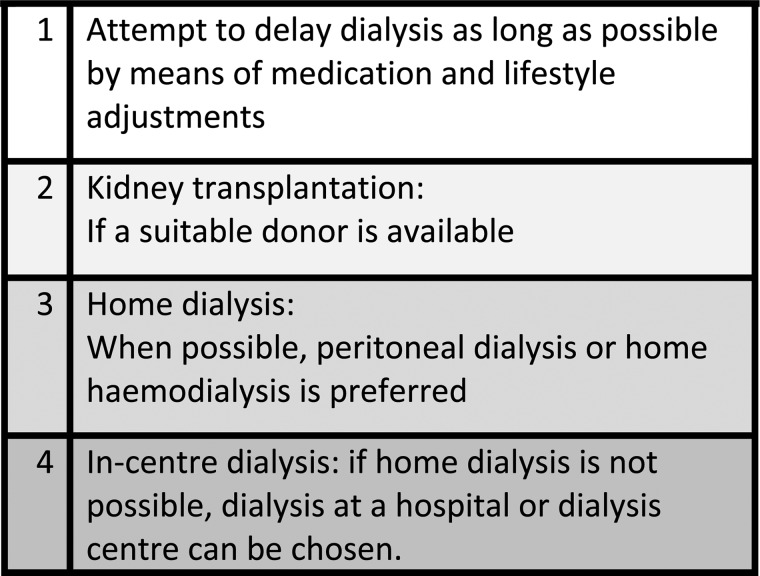
In a multidisciplinary meeting (MDM), the most suitable treatment (the treatment recommendation, or profile) for the particular patient is chosen, while taking into account the sequence of the programme's treatment preference (Figure 2). This sequence implies that, when deemed possible for a particular patient, we recommend transplantation over dialysis and home dialysis over in-centre dialysis. An automated GUIDE dashboard, which generates a profile using an algorithm based on answers to the questionnaires, has been developed as an aid during the MDM.
Fig. 2.
Sequence in treatment preference.
After the MDM, a specialised pre-dialysis nurse provides education tailored to the patient's profile. All patients receive general renal replacement information.
If the patient's profile includes transplantation, they receive information from a transplant nurse. If it includes PD, they receive information from a PD nurse. CHD is discussed briefly, and is only further explained if the patient does not choose PD. If the patient's profile includes HHD, the principles of HD are discussed. Furthermore, the training that the patient (and if possible, the partner or other family members) receives before the start of home dialysis is discussed. If there are no family members who are willing or able to contribute, passive HHD (or passive PD) with the help of home care is discussed. If the profile only includes CHD, no information is provided on other modalities.
The education is provided in a single session, which is repeated if the patient wishes. Written brochures and educational videos are also provided. Meetings with other patients are offered and arranged if requested by the patient or their family.
The patient's response to this educational session is discussed in a second MDM. Following the second MDM, the patient and nephrologist choose a treatment modality during the next visit to the outpatient clinic.
This retrospective observational file research study examined all patients that received a treatment recommendation in the GUIDE programme between 12 September 2013 and 18 December 2014 at Meander Medical Centre. This includes all patients that started preparation for dialysis or transplantation, as well as all patients that were registered during this period because of their choice for conservative treatment.
Patient characteristics (gender, age, BMI, educational level, primary cause of renal disease [20] and Charlson comorbidity index [21, 22]) were collected by means of record investigation and analysis of the questionnaires filled out during the GUIDE process. Furthermore, eGFR values at the start of the GUIDE process and at the start of RRT (calculated by the MDRD formula and expressed as ml/min/1.73 m2), information on the timeline of the patients' GUIDE process and the outcome of the steps (Figure 1) taken before 15 January 2015 were collected. The outcome of each step was compared with the patients' previous steps.
Information on the modality distribution before the implementation of GUIDE was collected through analysis of the hospital's registry on patients who started dialysis. The period of May 2012 to August 2013 (16 months, the same duration as the research period) was chosen to compare with the research period.
Statistics
Patient characteristics are presented as mean and SD for normally distributed continuous variables and as median, interquartile range (IQR), minimum and maximum for abnormally distributed variables. The distribution of patients between the treatment modalities or between grouped variables is presented as the number of patients and percentage of the total.
The patient characteristics, timeline and eGFR values of the group that chose home dialysis were compared with those of the group that chose in-centre dialysis. For normally distributed continuous variables (e.g. age or eGFR), the t-test for independent samples was used. For nominal variables (e.g. acute decline of renal function), the χ2-test was used, and for ordinal variables (e.g. educational level) and not normally distributed continuous variables (e.g. time between steps in the process), the Mann–Whitney U-test was used. A P-value ≤0.05 was considered significant.
All statistical analyses were performed using SPSS Statistics Advanced 22 (IBM, Armonk, NY, USA).
Results
Patient characteristics
Between 12 September 2013 and 18 December 2014, 102 consecutive patients at Meander Medical Centre, a large, non-academic teaching hospital in Amersfoort, The Netherlands, received a treatment recommendation in the GUIDE programme. Their mean age was 68.6 years and 44.1% were female. Patient characteristics are summarised in Table 1. When comparing the patients that chose home dialysis with those that chose in-centre dialysis, no significant differences were found in patient characteristics.
Table 1.
Patient characteristics
| Variable | Results |
|---|---|
| Number of patients | 102 |
| Females | 45 (44.1) |
| Age in years, mean (SD) (n = 102) | 68.6 (15.1) |
| BMI, mean (SD) (n = 98) | 27.8 (5.8) |
| Educational level (n = 102) | |
| None | 1 (1.0) |
| Primary education | 15 (14.7) |
| Secondary education | 16 (15.7) |
| Secondary vocational education (MBO) | 17 (16.7) |
| Higher professional education (HBO) | 14 (13.7) |
| University or higher education | 2 (2.0) |
| Unknown | 37 (36.3) |
| Primary cause of renal disease (n = 102) | |
| Glomerulonephritis/-sclerosis | 9 (8.8) |
| Pyelonephritis | 9 (8.8) |
| Polycystic kidneys, adult type | 5 (4.9) |
| Hypertension | 22 (21.6) |
| Renal vascular disease | 12 (11.8) |
| Diabetes mellitus | 22 (21.6) |
| Miscellaneous | 18 (17.6) |
| Unknown | 5 (4.9) |
| Charlson Comorbidity Index, mean (SD) (n = 102) | 3.8 (1.5) |
| Charlson Comorbidity Index, age-adjusted score, mean (SD) (n = 102) | 6.2 (2.4) |
| Low score (≤3) | 17 (16.7) |
| Moderate score (4.5) | 21 (20.6) |
| High score (6.7) | 26 (25.5) |
| Very high score (≥8) | 38 (37.3) |
All variables are presented as n (%), unless otherwise specified.
Timeline
The mean eGFR at the start of the GUIDE process was 12.3 mL/min/1.73 m2; 16.7% of the patients started the GUIDE process during a period of acute decline of renal function, at a significantly lower mean eGFR of 10.2 mL/min/1.73 m2 (t-test for independent samples, P = 0.012) (Supplementary data, Table S1). The median time between the start of the process and the treatment choice was 8 weeks, excluding six patients who started the process after starting therapy. The first therapy (dialysis or pre-emptive transplantation) started after a median of 10 weeks. Between 60.5 and 77.6% of patients had a sufficient amount of time for preparation between treatment choice and start of the first therapy (arbitrarily defined as ≥12 weeks to prepare PD or HD access). The mean eGFR at the start of GUIDE was 10.1 mL/min/1.73 m2 in the group with an insufficient amount of time, significantly lower than the mean of 12.7 mL/min/1.73 m2 in the group with a sufficient amount of time (t-test for independent samples, P = 0.005).
No significant differences were found between the timeline of the patients that chose home dialysis and the timeline of patients that chose in-centre dialysis. Nor was there a significant difference in the number of acute patients: 22.6% of the patients that chose home dialysis and 14.3% of the patients that chose in-centre dialysis started GUIDE in a period of acute deterioration (χ2-test, P = 0.360).
Unexpectedly, a significantly lower eGFR at the start of GUIDE was observed in the group that chose home dialysis (11.0 mL/min versus 12.7 mL/min/1.73 m2 in the group that chose in-centre dialysis, P = 0.028).
Treatment selection steps
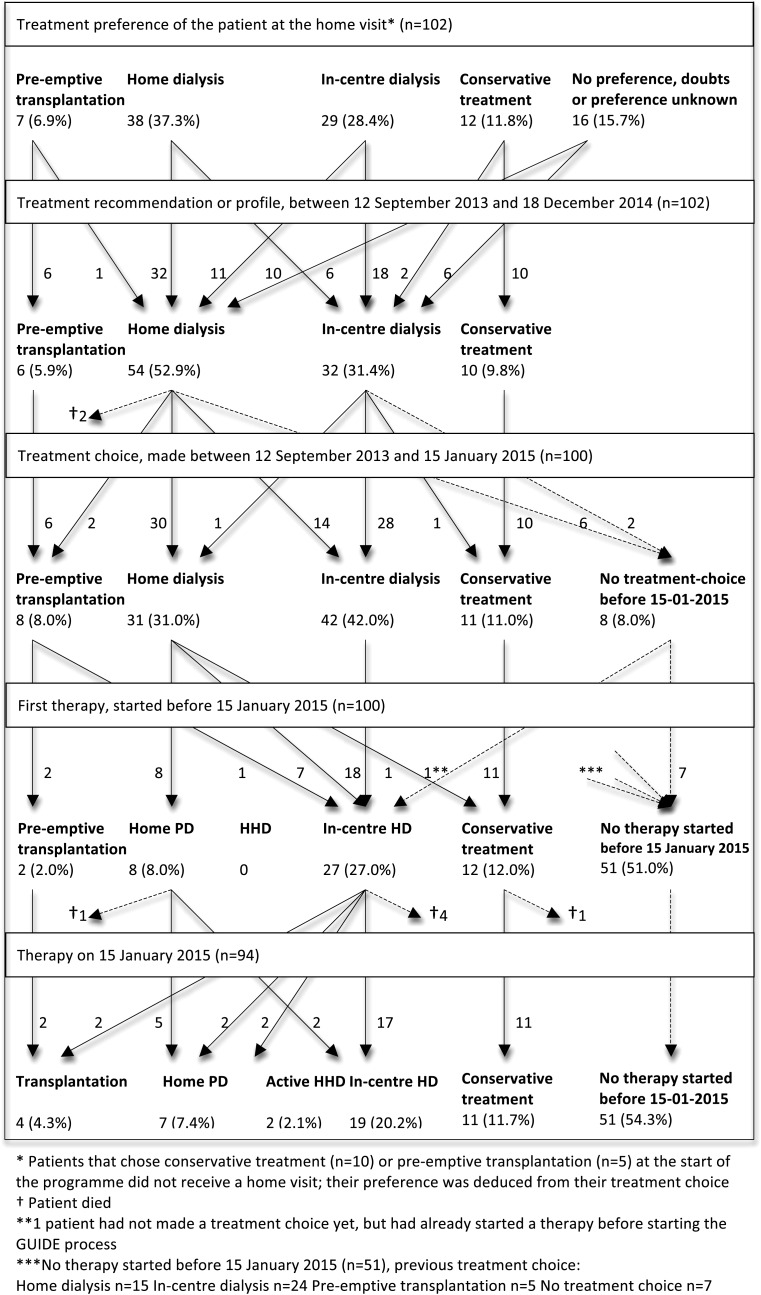
Figure 3 shows the patient flow through the treatment selection steps.
Fig. 3.
Patient flow diagram of the first 102 patients in the GUIDE programme.
Preference. During the home visit, 37.3% of patients expressed a preference for home dialysis (PD or HD), 28.4% preferred in-centre dialysis and 11.8% preferred conservative treatment, while 6.9% preferred to receive a pre-emptive transplant from an available living donor; 10.8% expressed no preference and in 4.9%, the treatment preference was unknown (Supplementary data, Table S2). Eighty-seven patients received a home visit. The case manager considered home dialysis suitable for 40.2% of the patients, while there were doubts about the suitability in 24.5% of cases. Home dialysis was considered unsuitable for 17.6% of patients, and for 2.9% of patients, the case manager's preference was unknown. At the start of the GUIDE process, the nephrologist deemed 44.1% of patients suitable for transplantation. For 10.8%, pre-emptive transplantation was considered possible (available living donor), and for the other 33.3%, dialysis was expected to be necessary before a transplantation could be performed. PD was the dialysis modality the nephrologist preferred most frequently, in 42.2% of cases, followed by CHD (26.5%) and HHD (7.8%). Conservative treatment was preferred in 12.7%.
For 10 patients (11.9%) there was a difference in the case manager's and the nephrologist's treatment preference; in 7 of those cases, the case manager's preference determined the treatment recommendation in the MDM. The case manager's preference was adopted in the MDM in 62.7%, while in 14.5%, the case manager was in doubt but the nephrologist's preference was not adopted. Therefore, the case manager's judgement determined the treatment recommendation in at least 77.1% of patients.
Recommendation. In the MDM, the treatment recommendation for each patient was discussed (Supplementary data, Table S3). For 6 patients, pre-emptive transplantation was recommended, while for 10 patients, conservative treatment was recommended. For the other 86 patients dialysis was recommended: 54 patients (62.8%) received a home profile (PD or HHD), and for 26 patients this was to be followed by transplantation. Thirty-two patients (37.2%) received a centre profile (CHD), and for 13 patients this was to be followed by transplantation.
Choice. Following education provided by a pre-dialysis nurse, 90.2% of patients chose a treatment modality before 15 January 2015 (Supplementary data, Table S4). In 80.4% of cases this choice was consistent with the recommended treatment. However, 14 patients were advised to have home dialysis but chose in-centre dialysis: 9 of these cases because the patient preferred in-centre dialysis and 5 because of relative contraindications discussed in the MDM that determined the patient's choice. In total, 42.5% of the 73 patients that chose dialysis therapy chose a form of home dialysis.
First therapy. Subsequently, 37 patients started therapy in the research period (Supplementary data, Table S5), at a mean eGFR of 8.1 mL/min/1.73 m2. Most of them (73.0%) started CHD, 21.6% started PD and two patients (5.4%) underwent pre-emptive transplantation. In 75.7% of cases, the first started therapy was the chosen therapy, but seven patients that chose home dialysis started CHD first. The reasons for this were the start of the GUIDE process after starting CHD (n = 3, mostly because of acute renal failure), limited capacity of HHD training (n = 3) and the need to start CHD because of a rapid decline in renal function (n = 1).
Therapy switch. Eight patients made a therapy switch during the research period: two started HHD, two started PD, two started CHD and two received a transplant. At the end of the research period, 32.1% of the patients that received dialysis therapy received a form of home dialysis.
Distribution of dialysis modalities before the implementation of GUIDE. In the 16 months before the implementation of GUIDE, 51 patients started dialysis. Forty-two patients (82.4%) started CHD as their first therapy and nine patients (17.6%) started PD. At the time, HHD was not yet available at our hospital. During this period, an average of 142.3 patients received dialysis each month, of which 114.5 (80.5%) received CHD and 27.8 (19.5%) received PD.
Discussion
In response to the declining home dialysis rates in the Netherlands, the standardisation and home-focused approach of the GUIDE process, with its home visit, questionnaires, multidisciplinary character and education by a specialised pre-dialysis nurse, aims to optimize the availability and desirability of home dialysis.
Consistent with its objective, the GUIDE programme seems to stimulate the use of home dialysis in a single-centre setting when compared with a historical control group. Of the patients that were advised to have dialysis during the MDM, 62.8% were advised to have home dialysis. Of the patients that chose dialysis, 34.2% eventually chose PD and 8.2% chose HHD; 22.9% of the patients that started dialysis started home dialysis as their first therapy, compared with 17.6% of the patients in the period before the implementation of GUIDE. At the end of the study, 32.1% of the patients that received dialysis therapy received home dialysis, compared with 19.5% before the implementation of GUIDE. These findings are in line with data from Goovaerts et al. [13] that showed an increased use of self-care RRT modalities after exposure to a structured pre-dialysis education programme.
The case manager plays an important part in the programme, as her conclusions based on the home visit greatly influence the MDM recommendation; her treatment preference determined the MDM recommendation more often than the nephrologist's.
In our opinion, the home visit is of great importance to the programme, because it allows someone other than the nephrologist (with a long-standing treatment relation to the patient) to objectively evaluate the patient's home and social situation. When a patient is referred to GUIDE, the nephrologist only provides information on the course of the information process and avoids recommending a specific treatment modality (other than pre-emptive transplantation). A treatment recommendation or profile is only provided after multidisciplinary discussion. In this way, we aim to help the patient, after completion of the process, choose the best suitable therapy.
We found that most patients (80.4%) followed their MDM recommendation. Further research by means of interviews could clarify the reasons why 14 patients that received a home dialysis recommendation chose in-centre dialysis. If their decision was caused by fear or lack of knowledge, this may be prevented in the future by further improvement of the education provided. A total of 75.7% of the patients that started therapy, started with the therapy of their choice. This discrepancy can be clarified for three patients, who had already received CHD before starting the GUIDE process. There was a (at least in part temporary) loss of three HHD patients, which could be prevented in the future by enhancing the capacity of HHD training.
In contrast to the common belief that an acute start of dialysis leads to more use and continuation of in-centre dialysis, we found no significant difference in the number of acute patients between the group that chose home dialysis and the group that chose in-centre dialysis, even though some patients that chose home dialysis had to start with in-centre dialysis at first.
Most of the patients (60.5–77.6%) had a sufficient amount of time to prepare for dialysis (which we arbitrarily defined as 12 weeks after the completion of GUIDE; for most patients this was sufficient time to perform access surgery). Since the group with an insufficient amount of time started GUIDE at a significantly lower eGFR, the group might be reduced by earlier start of the process, at an eGFR <15 ml/min/1.73 m2 or <20 mL/min/1.73 m2 with rapid deterioration. This limit for referral was already agreed upon when the GUIDE programme was introduced, but since the mean eGFR was 12.3 mL/min/1.73 m2, this criterion was not yet met.
The strength of this study is that the study population consists of the entire pre-dialysis population in one hospital within the study period, which makes the distribution among treatment modalities reliable. Based on the patient characteristics, the study population seems to represent the average pre-dialysis population in The Netherlands. When compared with European population data about patients at the start of dialysis, the mean age and BMI were somewhat higher in our study (respectively, 68.6 versus 64.1 years in the 2012 ERA-EDTA Registry [23] and 27.8 versus 25.3 kg/m2 in the NECOSAD cohort [24]), while the distribution of primary causes of renal disease was comparable [23]. The difference in age can be explained by the fact that our study included (mostly older) patients that chose conservative treatment.
The study is limited by its retrospective nature and by the limited period of follow-up. Ideally, all patients would have been followed until they started a therapy, chose conservative treatment or died, in order to gain a more reliable distribution of treatments. Furthermore, because the control group was historical, not all variables could be compared. Because this is a relatively small study, no definite statements can be made on subgroups, such as the patients that started the GUIDE process in a period of acute decline of renal function or the patients that chose conservative treatment. Further research could provide insight into the course of their pre-dialysis process. Moreover, while no significant differences in patient characteristics were found between the patients that chose home dialysis and in-centre dialysis, this could be due to the relatively small number of patients.
To conclude, compared with historical data, the standardised and home-focused pre-dialysis programme GUIDE, with its home visit, seems to successfully increase the number of patients that choose and receive home dialysis.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Conflict of interest statement
E.C.H. is a shareholder in Gezonde Nieren and received honoraria from Amgen.
Supplementary Material
Acknowledgements
We would like to thank Marion Swager, Ingrid Moraal and Petra van de Linde for their contribution to the construction of the questionnaires and for their constructive advice. We would also like to thank Dr W. Smit, of Jeroen Bosch Hospital, for her advice regarding the medical questionnaire. Furthermore, we would like to thank Stefanie Kersten and Leon Hooijer for their contribution to the development of the GUIDE dashboard. Finally, we would like to thank everyone who has contributed to the GUIDE programme at our hospital: the nephrologists, case managers, pre-dialysis nurses, dieticians and secretaries.
References
- 1.Renine. (Title translated from Dutch) Number of patients on January 1st, 2000 until 2015. Dialysis patients and patients with a functioning donor kidney. https://www.renine.nl/static?id=prev_years&var=dg&style=line&render=png (17 June 2015, date last accessed)
- 2.Oreopoulos DG, Thodis E, Passadakis P et al. . Home dialysis as a first option: a new paradigm. Int Urol Nephrol 2009; 41: 595–605 [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, Chiu YW, Kalantar-Zadeh K et al. . Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171: 110–118 [DOI] [PubMed] [Google Scholar]
- 4.Heaf JG, Wehberg S. Relative survival of peritoneal dialysis and haemodialysis patients: effect on cohort and mode of dialysis initiation. PLoS One 2014; 9: e901–e919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frequent Hemodialysis Network (FHN) Trial Group, Chertow GM, Levin NW et al. . In-center hemodialysis six times per week versus three times per week. N Engl J Med 2011; 364: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron JI, Whiteside C, Katz J et al. . Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis 2000; 35: 629–637 [DOI] [PubMed] [Google Scholar]
- 7.Fadem SZ, Walker DR, Abbott G et al. . Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol 2011; 6: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran J, Kraus M. Starting a home hemodialysis program. Semin Dial 2007; 20: 35–39 [DOI] [PubMed] [Google Scholar]
- 9.Walker R, Marshall M, Morton RL et al. . The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: a systematic review of full economic evaluations. Nephrology (Carlton) 2014; 19: 459–470 [DOI] [PubMed] [Google Scholar]
- 10.ERA-EDTA Registry. ERA-EDTA Registry 2003 Annual Report. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands, May 2005 [Google Scholar]
- 11.ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2013. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands, 2015 [Google Scholar]
- 12.Jain AK, Blake P, Cordy P et al. . Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23: 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goovaerts T, Jadoul M, Goffin E. Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 2005; 20: 1842–1847 [DOI] [PubMed] [Google Scholar]
- 14.Lacson E, Wang W, DeVries C et al. . Effects of a nationwide predialysis educational program on modality choice, vascular access and patient outcomes. Am J Kidney Dis 2011; 58: 235–242 [DOI] [PubMed] [Google Scholar]
- 15.Golper T. Patient education: can it maximize the success of therapy? Nephrol Dial Transplant 2001; 16: 20–24 [DOI] [PubMed] [Google Scholar]
- 16.Johansson L. Shared decision making and patient involvement in choosing home therapies. J Ren Care 2013; 39: 9–15 [DOI] [PubMed] [Google Scholar]
- 17.Morton RL, Tong A, Howard K et al. . The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ 2010; 340: c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castledine CI, Gilg JA, Rogers C et al. . Renal centre characteristics and physician practice patterns associated with home dialysis use. Nephrol Dial Transplant 2013; 28: 2169–2180 [DOI] [PubMed] [Google Scholar]
- 19.Medworq. (Title translated from Dutch) Healthy Kidneys, network of experts. http://www.gezondenieren.nl/over-ons/voor-professionals/#main-nav (17 June 2015, date last accessed)
- 20.Renine. (Title translated from Dutch) EDTA code list primary diagnoses (version 2008). https://www.renine.nl/page?id=forms (19 November 2014, date last accessed)
- 21.Charlson ME, Pompei P, Ales KL et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J et al. . Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 23.ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2012. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands, 2014 [Google Scholar]
- 24.de Mutsert R, Snijder MB, van der Sman-de Beer F et al. . Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol 2007; 18: 967–974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.