Abstract
We describe the first case of distal renal tubular acidosis (dRTA) associated with primary sclerosing cholangitis. A 26-year-old Lao-Thai male patient presented with severe jaundice, metabolic acidosis and hypokalaemia. He was diagnosed of dRTA. Liver transplantation resulted in correction of electrolyte disturbances and hyperbilirubinaemia. A fludrocortisone-furosemide test revealed normal urinary acidification, demonstrating no residual dRTA. This observation suggests that dRTA may be an early manifestation of bilirubin-associated nephropathy or the consequence of an immune mechanism.
Keywords: distal renal tubular acidosis, liver transplantation, primary sclerosing cholangitis
Background
Renal tubular acidosis is considered when a relatively normal glomerular filtration rate is associated with metabolic acidosis, hyperchloremia and a normal plasma anion gap [1]. Distal renal tubular acidosis (dRTA) is characterized by an impairment of net acid secretion by the distal tubule and may be associated with hypokalaemia (type I) or hyperkalemia (type IV). Nephrocalcinosis, recurrent renal calculi and reduced bone mineral density are often present. It can be either inherited, idiopathic or acquired secondary to a variety of conditions [1, 2]. For example, autosomal dominant or recessive mutations in the anion exchanger 1 (AE1) gene encoding the erythroid and kidney anion exchanger 1 (chloride-bicarbonate) may result in dRTA with ovalocytosis or spherocytosis in patients from Southeast Asia [3]. The most common causes of secondary dRTA are autoimmune disorders such as systemic lupus erythematosus [4], Sjögren's syndrome [5] and primary biliary cirrhosis [6]. Of note, primary sclerosing cholangitis (PSC) is often compared to primary biliary cirrhosis, but no PSC-associated dRTA has been previously reported.
We report a case of type I dRTA occurring during the course of PSC that was corrected by a successful liver transplantation.
Case report
A 26-year-old Lao-Thai male patient with uneventful previous medical records was diagnosed as severe (Child score B7, Model for End-Stage Liver Disease (MELD) score 24) hepatic cirrhosis secondary to PSC in August 2012. Biliary magnetic resonance imaging revealed an irregular aspect of intrahepatic bile ducts with alternance of stenosis and dilatation (similar to a ‘stack of plates’). Liver biopsy showed mild inflammation in the portal spaces and major cholestasis in hepatocytes and bile ducts, with no sign of suppurated cholangitis, obliteration, or cholangiocarcinoma. Anti-mitochondrial, anti-smooth muscle, anti-liver cytosol, anti-endoplasmic reticulum, anti-neutrophil cytoplasmic and anti-nuclear antibodies were negative. No inflammatory bowel disease was detected.
During the following months he developed a marked hypokalaemia resistant to potassium replacement therapy, followed by metabolic acidosis. At referral to the renal department he had severe jaundice, generalized muscle weakness and bone pain. Body mass index was 22.3 kg/m2 and blood pressure was 110/70 mmHg with no sign of volume depletion. He was treated with esomeprazole, cholestyramine and ursodeoxycholic acid.
Metabolic acidosis [arterial pH 7.34, partial pressure of carbon dioxide (pCO2) 27 mmHg, bicarbonatemia 15 mmol/L] was associated with hyperchloremia (117 mmol/L), a normal plasma anion gap [17.6 mmol/L (normal 16 ± 4 mmol/L); 18.1 mmol/L after correction for albumin, albuminemia 38 g/L), a positive urinary anion gap (7.6 mmol/L) and an inappropriate urinary pH of 6. Hypokalaemia (2.7 mmol/L) was associated with a normal electrocardiogram, inadapted renal potassium loss (urinary potassium excretion 88 mmol/day), normal blood magnesium (1.0 mmol/L), increased plasma renin activity (188 pg/mL) and normal aldosterone level (13 ng/dL). Serum creatinine was 80 µmol/L (normal range 70–110 μmol/L). Urinalysis showed urinary sodium at 39 mmol/L (fractional excretion of sodium 1.3%), moderate proteinuria (protein:creatinine ratio 650 mg/g, urinary albumin:creatinine ratio 50 mg/g), calcium:creatinine ratio of 10.7 mmol/g and the absence of glycosuria, aminoaciduria, inappropriate phosphaturia, leukocyturia and haematuria. Urinary NH4 and citrate were not measured. Liver investigations showed severe cholestasis with a total bilirubin value of 831 µmol/L (normal <21 µmol/L), mainly conjugated [762 µmol/L (normal <3.4 µmol/L)]. Blood cell count was indicative of regenerative normocytic anaemia (haemoglobin 9.5 g/dL, mean cell volume 82 fL, reticulocytes 119.000/mm3), thrombocytosis (456.000/mm3) and leukocytosis (11.670/mm3). Heterozygous beta thalassemia [haemoglobin (Hb) A 93.0%, Hb A2 6.0%, Hb F <1%] was demonstrated by electrophoresis. Testing for SCL4A1 gene mutation, commonly associated with dRTA in the Southeast Asian population [3], was negative. Simple renal cortical cysts but no calcinosis or lithiasis were noticed on computed tomography. Osteodensitometry was normal.
During the alkali infusion test, the urine to blood pCO2 gradient did not rise normally (i.e. >30 mmHg when bicarbonaturia approaches 80 mEq/L), indicative of a low rate of H+ secretion [7, 8]. In the present case, the maximal urine to blood pCO2 gradient at bicarbonaturia of 80 mEq/L was 1.2 mmHg while urinary pH and pCO2 were 7.1 and 33 mmHg, respectively.
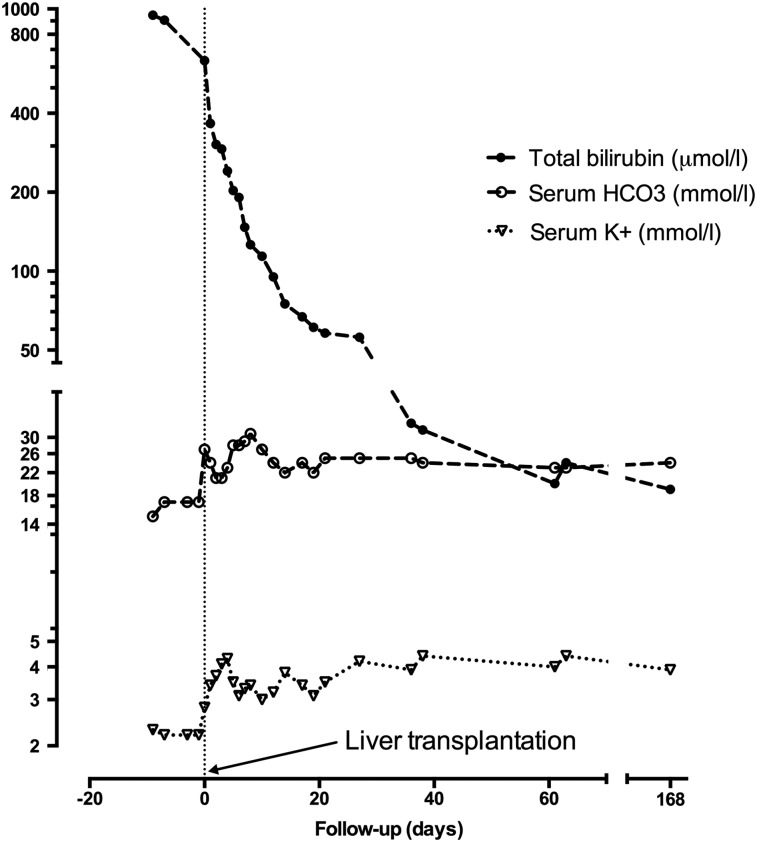
The opportunity of liver transplantation was taken and the patient was transplanted in July 2013, before a furosemide-fludrocortisone test and a kidney biopsy were performed. Native liver biopsy confirmed the diagnosis of PSC. Induction immunosuppressive therapy consisted of basiliximab, methylprednisolone, tacrolimus and mycophenolate mofetil. Maintenance immunosuppression included prednisolone, tacrolimus and mycophenolate mofetil. As shown in Figure 1, hypokalaemia and acidosis disappeared upon correction of bilirubinemia. Renal function was normal during the initial postoperative period. A mild and stable increase in serum creatinine (130 µmol/L) without proteinuria (proteinuria:creatininuria ratio 50 mg/g) occurred about 1 month later and was attributed to calcineurin inhibitor toxicity. A fludrocortisone-furosemide test was normal 6 months after liver transplantation with normal urinary acidification and urinary pH of 4.77.
Fig. 1.
Improvement in distal renal tubular acidosis-associated disorders (metabolic acidosis and hypokalaemia) was concomitant with the decrease in total bilirubin following liver transplantation.
DISCUSSION
To our knowledge, this is the first described case of type I dRTA associated with PSC. Interestingly, renal tubular dysfunction was corrected by liver transplantation.
Since the patient originated from Laos, an inherited mechanism—namely SLC4A1 gene mutation—was suspected and eliminated. This gene encodes for anion exchanger 1 (AE1), a bicarbonate/chloride transporter localized in both red cell membrane and basolateral membrane of the collecting tubule alpha-intercalated cell. Its mutations are common in Southeast Asia and result in dRTA and ovalocytosis, which can occur in adulthood [3]. Two other mutations in genes encoding for the α4 (ATP6V0A4) and β1 (ATP6V1B1) subunits of the V-ATPase of the alpha-intercalated cells of the collecting duct can also cause hereditary dRTA. We did not perform gene sequencing for these genes because the onset of the disease occurs in childhood (ATP6V0A4) and is associated with sensorineural deafness (ATP6V1B1). Moreover, altered urinary acidification could not be attributed to impaired distal sodium delivery [9], as fractional excretion of sodium was not low.
Immune disorders are frequent causes of acquired dRTA. Of note, the ion disturbances of dRTA were corrected in this patient a few weeks after liver transplantation with immunosuppressive therapy. Sjögren's syndrome is associated with dRTA, and several reports suggest that autoantibodies against carbonic anhydrase II enzyme [10] or vacuolar H+-ATPase pump in the collecting duct [11] are involved in the pathogenesis of dRTA. In a recent unusual case of multiorgan autoimmunity, circulating autoantibodies directed against intercalated cells were apparently involved in the pathogenesis of dRTA [12]. Primary biliary cirrhosis is an autoimmune liver disease associated with dRTA and tubulointerstitial nephritis that is alleviated by steroid therapy [6]. However, PSC is not considered an autoimmune disease, but rather an inflammatory condition. Male predominance, lack of a defined pathogenic autoantigen and the potential role of the innate immune system suggest that it may be due to dysregulation of immunity [13].
Although frequently overlooked, bilirubin is a recognized renal toxin, and in the present case bilirubin levels were normalized concomitant to the correction of dRTA. Rafat et al. [14] recently reported the case of an adult kidney recipient who developed a malignant cholangiocarcinoma 4 years after transplantation and progressively lost transplant function in parallel with a continuous increase in bilirubinemia. Kidney biopsy showed bile thrombi in dilated tubules and bile granules in the cytoplasm of tubular epithelial cells, but no interstitial inflammation. Because the patient had no jaundice-associated confounding factor such as sepsis, heart failure or hepatorenal syndrome that could explain his kidney failure, the authors suggested that bilirubin per se should be seen as a cause of acute tubular necrosis. van Slambrouck et al. [15] studied 41 autopsies and 3 renal biopsies in 44 jaundiced patients with acute kidney injury. Bile casts were observed 24 times, with involvement of distal nephron segments in 18 mild cases and extension to proximal tubules in 6 more severe cases and they proposed the term bile cast nephropathy. Griffin et al. [16] reported a 49-year-old man with progressive obstructive jaundice secondary to PSC who developed acute anuric renal failure requiring prolonged haemodialysis. Renal biopsy showed evidence of tubular epithelial toxicity, pigmented casts, interstitial fibrosis and ischaemic glomerular changes. After orthotopic liver transplantation, there was an immediate urine output recovery, followed by gradual improvement in renal function to a serum creatinine nadir of 210 µmol/L. In the present case, liver transplantation also permitted correction of dRTA biological abnormalities. Since kidney biopsy was not performed, whether interstitial inflammation or bile granules in the cytoplasm of distal tubular epithelial cells participated in dRTA cannot be confirmed.
In conclusion, to the best of our knowledge, this case report is the first description of type I dRTA associated with PSC. It illustrates the putative immunological as well as toxic bilirubin-associated mechanisms that may link both entities and reminds us that distal tubular renal function should be carefully assessed in PSC patients with acid–base or electrolyte disorders.
Conflict of interest statement
None declared.
References
- 1.Soriano JR. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol 2002; 13: 2160–2170 [DOI] [PubMed] [Google Scholar]
- 2.Haque SK, Ariceta G, Batlle D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant 2012; 27: 4273–4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrong O, Bruce LJ, Unwin RJ et al. . Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int 2002; 62: 10–19 [DOI] [PubMed] [Google Scholar]
- 4.Li SL, Liou LB, Fang JT et al. . Symptomatic renal tubular acidosis (RTA) in patients with systemic lupus erythematosus: an analysis of six cases with new association of type 4 RTA. Rheumatology 2005; 44: 1176–1180 [DOI] [PubMed] [Google Scholar]
- 5.Shioji R, Furuyama T, Onodera S et al. . Sjögren's syndrome and renal tubular acidosis. Am J Med 1970; 48: 456–463 [DOI] [PubMed] [Google Scholar]
- 6.Komatsuda A, Wakui H, Ohtani H et al. . Tubulointerstitial nephritis and renal tubular acidosis of different types are rare but important complications of primary biliary cirrhosis. Nephrol Dial Transplant 2010; 25: 3575–3579 [DOI] [PubMed] [Google Scholar]
- 7.Halperin ML, Goldstein MB, Haig A et al. . Studies on the pathogenesis of type I (distal) renal tubular acidosis as revealed by the urinary PCO2 tensions. J Clin Invest 1974; 53: 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batlle D, Grupp M, Gaviria M et al. . Distal renal tubular acidosis with intact capacity to lower urinary pH. Am J Med 1982; 72: 751–758 [DOI] [PubMed] [Google Scholar]
- 9.Batlle DC, von Riotte A, Schlueter W. Urinary sodium in the evaluation of hyperchloremic metabolic acidosis. N Engl J Med 1987; 316: 140–144 [DOI] [PubMed] [Google Scholar]
- 10.Takemoto F, Hoshino J, Sawa N et al. . Autoantibodies against carbonic anhydrase II are increased in renal tubular acidosis associated with Sjögren syndrome. Am J Med 2005; 118: 181–184 [DOI] [PubMed] [Google Scholar]
- 11.DeFranco PE, Haragsim L, Schmitz PG et al. . Absence of vacuolar H(+)-ATPase pump in the collecting duct of a patient with hypokalemic distal renal tubular acidosis and Sjögren's syndrome. J Am Soc Nephrol 1995; 6: 295–301 [DOI] [PubMed] [Google Scholar]
- 12.Van den Wildenberg MJ, Hoorn EJ, Mohebbi N et al. . Distal renal tubular acidosis with multiorgan autoimmunity: a case report. Am J Kidney Dis 2015; 65: 607–610 [DOI] [PubMed] [Google Scholar]
- 13.Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol 2009; 31: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafat C, Burbach M, Brochériou I et al. . Bilirubin-associated acute tubular necrosis in a kidney transplant recipient. Am J Kidney Dis 2013; 61: 782–785 [DOI] [PubMed] [Google Scholar]
- 15.Van Slambrouck CM, Salem F, Meehan SM et al. . Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int 2013; 84: 192–197 [DOI] [PubMed] [Google Scholar]
- 16.Griffin M, Grande J, Wiesner R et al. . Prolonged anuria complicating primary sclerosing cholangitis: successful outcome following orthotopic liver transplantation. Am J Kidney Dis 1998; 31: 360–363 [DOI] [PubMed] [Google Scholar]