Abstract
The incidence of adverse drug reactions may be decreased by computerized physician order entry (CPOE) with decision support. The authors describe the development of a drug database model for computer-supported dose adjustment within a CPOE system. The following two core elements were included: (1) To allow electronic dose and volume calculation, the relation between strength (e.g., 5 mg/1 mL) and prescribed unit (e.g., 1 ampoule containing 2 mL) must be available in coded form. (2) The site of action along with the parent active ingredient, i.e., the pure drug without salt or ester, is necessary for linkage to knowledge bases. All complex examples of drugs that were examined could be described by the data model. With the ultimate goal of increasing prescribing effectiveness and quality the authors developed a drug database model for inclusion in a CPOE system, which allows dose calculations and may be coupled to decision support systems.
About one in seven hospitalized patients suffers from an adverse drug reaction,1 which increases the average length of hospital stay by two days and causes estimated costs of $2,400 U.S.2,3 One third of adverse drug reactions are judged to be preventable.4 Reasons preventable adverse drug reactions are not prevented include missing dissemination of knowledge about drugs5 and/or incorrect dosing.5,6 Especially in children, miscalculation of dosage may have fatal consequences.7 In one study, around half the surveyed physicians were unable to convert drug doses correctly from a percentage concentration or dilution to the more conventional units of concentration.8 Computerized physician order entry (CPOE) with decision support has been identified as a key quality improvement strategy in a health care system.9 Indeed, it has been shown that computerized alerts to physicians can decrease the incidence of adverse drug reactions.10,11 While some pioneering CPOE systems with decision support have been developed,12 they are not widely implemented in hospitals.13 Barriers for implementation might include lack of standards along with financial and cultural barriers.14
In the context of a project to individualize drug dose, we aimed to implement a CPOE with decision support on a model ward. For its development, we needed a complete and fully structured drug database as a central element of CPOE, which allowed: (1) the description of all drugs marketed in Germany, (2) calculation of prescribed drug dose, and (3) linkage to knowledge databases. Because we could not find a database fulfilling these requirements we developed a suitable drug database model. Development of a relational database model is time consuming, complex examples are needed to define the model, and the result is frequently not published. Publication, however, stimulates scientific discussion and facilitates interoperability between systems. In the current case report, we describe a drug database model for marketed drugs to be included in a CPOE system, which allows dose calculation and may be coupled to decision support systems.
Requirements of Data Model
In a first step, we defined the following model requirements: (1) calculation of prescribed dose for all dosage forms; (2) volume calculation of prescribed unit for liquid dosage forms; (3) possibility to link evidence-based information to the database to increase drug safety and effectiveness and to maximize the specificity of electronic alerts; (4) provision of meaningful default values and drop down lists to facilitate prescribing; and (5) inclusion of all marketed drugs in one database.
We then developed the model in an iterative process starting from a database of all drugs marketed in Germany,15 an expert system giving dose recommendations in patients with renal insufficiency,16 and a prototype for documentation of prescriptions in inpatients.
Results
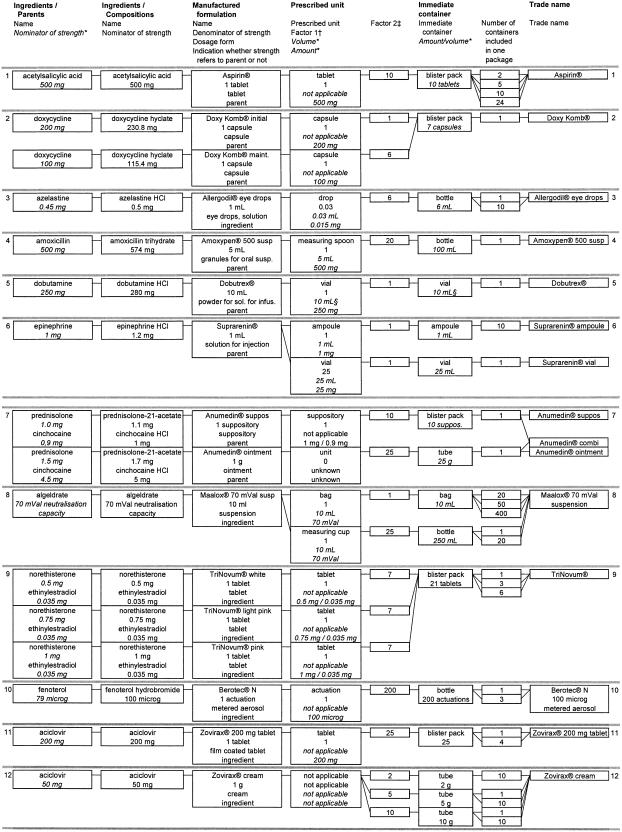
Core elements of a drug order are trade name, dosage form, strength, dosage regimen (amount of prescribed units and timing of drug administration), and route of administration. While drug databases of marketed drugs contain trade name, dosage form, and strength, they usually do not allocate a unit of prescription or route of administration to the trade name. For a prescription-oriented drug database, both are necessary. To allow electronic dose calculation, the relation between the denominator of strength and prescribed unit must be available in coded form. While these are usually identical for most solid, single-dose dosage forms (e.g., tablet), this does not hold true for liquid dosage forms (e.g., strength, 50 mg/1 mL; prescribed unit, measuring spoon). Therefore, a factor relating volume or amount included in one prescribed unit to the denominator of strength must be defined, which similarly can be used for calculation of the volume of the prescribed unit and calculation of the actually applied dosage after early discontinuation of an infusion (model requirements 1 and 2). Without this factor the prescribed dose and volume cannot be calculated. For topical dosage forms like creams, the prescribed unit usually cannot be quantified. Accordingly, a factor relating strength and prescribed unit cannot be defined, which means that it is not possible to calculate the dosage unless the prescribed unit and the included amount of active ingredient can be indicated quantitatively (e.g., apply 10 cm, 10 mg/1 cm). A schematic representation of the drug database model is shown in ▶; its application to 12 commercially available preparations is shown in ▶.
Figure 1.
Schematic representation of the drug database model. Primary keys are shown in bold, look-up tables are not shown.
Figure 2.
Exemplary application of the data fields to comprehensively describe 12 commercially available preparations. *Information not included directly in database but derived from other fields included in database. For calculation see box “calculations” in ▶.
†Factor relating volume or amount included in one unit of prescription to denominator of strength.
‡Factor relating volume or amount included in immediate container to denominator of strength.
The combination of active ingredient and route of administration governs the site of action. While, for example, vancomycin given orally acts only locally in the gut lumen, it exerts systemic effects after intravenous administration. In case of electronic detection of possible adverse drug reactions, it may be useful to add the category potentially systemic (examples are timolol eye drops17 or topical application of corticosteroids,18 which are intended to act locally but may occasionally induce systemic effects). The site of action along with the parent active ingredient, i.e., the pure drug without salt, ester, or another chemical combination in which the drug may exist,19 is necessary for unequivocal linkage to knowledge bases (model requirement 3).
To facilitate prescribing, default values like default route of administration or default prescribed unit have to be defined (model requirement 4). In addition, the user interface should differ for prescription of single-dose dosage forms like tablets compared with complex infusions, in which it is indispensable to furnish the system with extensive information.
It can be decided with certainty only whether the objective that all drugs marketed in Germany can be described adequately by the model after all drugs have been entered without difficulties into the database (model requirement 5). This is a very laborious task considering that roughly 59,000 preparations are available in Germany.15 We thus have chosen the approach of searching for appropriate and complex test drugs, an approach that has, by definition, a random aspect. However, so far, no additional constellation could be found or thought of that could not be entered into the database without ambiguity or loss of precision.
Several (active) ingredients may be combined in a given manufactured formulation (e.g., antibiotics or antihypertensive drugs). Moreover, different manufactured formulations may be included in one package. Pertinent examples are sequential preparations of oral contraceptives, doxycycline preparations containing a starting dose of 200 mg, and maintenance doses of 100 mg. On the other hand, a given preparation may be dispensed in different immediate containers, which are used as prescription units for inpatients (for example, a solution for injection supplied in either an ampoule or a vial). All these constellations can be described adequately by the data model.
While most liquid dosage forms are ready for use, it is common practice, especially for drugs with moderate stability, to be supplied by the manufacturer as solid (e.g., lyophilized) dosage forms, which have to be dissolved immediately before application. In this case the following two situations may apply:
The concentration must be constant, because the prescribed unit is assumed to always contain the same amount of drug. An example is a powder for oral suspension, in which the application unit is a measuring spoon. The dosage form is nearly ready for use and is always diluted with the same volume.
For some intravenously applied solutions, the volume used to dissolve the drug may change from patient to patient. In case the powder is dissolved with the solution prepared for the infusion of another drug (e.g., cefuroxime for injection admixed in intravenous infusion with heparin20), the prescribed element contributes no additional volume. The relation between amount of active ingredient and volume may change, which means that the concentration has to be defined for each patient. The indicated volume in the database corresponds to the maximal volume.
To deal with this issue, a pertinent field has been introduced in the table prescribed unit of the data model (▶).
Discussion
We have described a model of a drug database to be included in a CPOE system, which allows dose and volume calculation and may be coupled to decision support systems. To the best of our knowledge, the literature describing the structure of a drug database to be included in a CPOE in sufficient detail is scarce. A prescription is frequently coded by trade name, dosage form, strength, dosage regimen (amount of prescribed units and timing of drug administration), and possibly route of administration.21,22,23 The Italian VIDEOFAR drug database,24 the European OPADE project,25 and the National Drug Code (NDC26) published by the U.S. Food and Drug Administration present a relational model of a drug database. In VIDEOFAR a base code is introduced, which allows identification of the active principle regardless of the different possible salt forms of the active ingredient. The NDC, which has the advantage of being in the public domain, allocates the route(s) of administration to each product. However, neither VIDEOFAR nor NDC are designed for inclusion in a CPOE system with dose adjustment, because calculation of the prescribed dose is not possible for all dosage forms. The model of the OPADE project25 includes a prescribed unit, which is the unit used to order the manufactured preparation (e.g., ampoule). However, because the relationship between the prescribed unit and the denominator of strength is not defined (e.g., strength, 5 mg/1 mL and 1 ampoule, 2 mL) neither the prescribed dose nor the volume of it can be calculated. To allow dose calculation, we have introduced the prescribed unit and the factor describing the relation between the denominator of strength and the prescribed unit.
Sperzel et al19 point out that multiple levels of description can be applied to drugs. They present five levels of drug description from ingredient, to which information about drug interaction can be linked in general, over clinical drug to the packaged drug product. The concept of clinical drug is useful for prescription and groups together all manufactured formulations having the same set of therapeutically active ingredients and associated strengths within the same dosage form (e.g., any ampicillin, 250-mg capsule). Because the pertinent information is available in coded form in our database, the different views on drugs as described by Sperzel et al19 may be easily extracted. The concept of clinical drug is used in the RxNorm project included in the Unified Medical Language System (UMLS), an ambitious project of the U.S. National Library of Medicine.27 More than two thirds of a broadly used electronic drug file could successfully be parsed into the semantic normal form of the clinical drug.28 Compared with our model, RxNorm may not present all necessary information on package size, e.g., volume included in one vial. In addition, only up to three ingredients per drug are currently coded.29 This may not, however, be sufficient if, for instance, a knowledge database on drug interactions were to be linked to RxNorm because numerous drugs contain more than three active ingredients and because also pharmacologically inactive ingredients may cause drug interactions.30
To benefit from the information included in the database and to ease interoperability with other systems, thesauri must be used when populating the database.31 Thesauri of the dosage forms and the routes of administration are available from both the European (EMEA) and the U.S. Regulatory Authorities (FDA).32,33 The U.S. National Committee on Vital and Health Statistics recently defined essential and desirable features of terminologies and analyzed several drug terminologies.34 The question arises of who will maintain the whole drug database, which is mostly country specific. One possibility is the Regulatory Authority, which should elaborate strict editorial rules for allocating the information to the fields and may demand that the pharmaceutical companies supply the necessary information when applying for the registration of a new drug or changing a registration. Ideally, this database will be available to all interested parties.
If electronic prescription and other parts of the electronic patient record are used regularly in daily practice, the data may be used further to evaluate safety issues, treatment processes, and other outcomes, i.e., for epidemiologic research. Potential effects of individual functional capabilities on prescribing quality and, ultimately, patient safety have recently been reviewed.35
With the ultimate goal of increasing prescribing effectiveness and quality we have described a model of a drug database suitable for inclusion in a CPOE, which allows dose calculations and may be coupled to decision support systems.
Supported by BMBF grant #01EC9902 from the German Federal Ministry for Education and Research.
Part of this information has been presented in abstract and poster form: Martin-Facklam M, Haefeli WE, Martin P. Essential data fields for a drug database enabling electronic dose calculation of complex applications. Eur J Clin Pharmacol. 2002;58:S82.
References
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalised patients. JAMA. 1998;279:1200–5. [DOI] [PubMed] [Google Scholar]
- 2.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalised patients. JAMA. 1997;277:307–11. [PubMed] [Google Scholar]
- 3.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalised patients. JAMA. 1997;277:301–6. [PubMed] [Google Scholar]
- 4.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 5.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274:35–43. [PubMed] [Google Scholar]
- 6.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in paediatric inpatients. JAMA. 2001;285:2114–20. [DOI] [PubMed] [Google Scholar]
- 7.Koren G, Barzilay Z, Greenwald M. Tenfold errors in administration of drug doses: a neglected iatrogenic disease in pediatrics. Pediatrics. 1986;77:848–9. [PubMed] [Google Scholar]
- 8.Rolfe S, Harper NJN. Ability of hospital doctors to calculate drug doses. BMJ. 1995;310:1173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiff GD, Rucker D. Computerised prescribing. JAMA. 1998;279:1024–9. [DOI] [PubMed] [Google Scholar]
- 10.Rind DM, Safran C, Phillips RS, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalised patients. Arch Intern Med. 1994;154:1511–7. [PubMed] [Google Scholar]
- 11.Pestotnik SL, Classen DC, Evans RS, Burke JP. Implementing antibiotic practice guidelines through computer-assisted decision support: clinical and financial outcomes. Ann Intern Med. 1996;124:884–90. [DOI] [PubMed] [Google Scholar]
- 12.Doolan DF, Bates DW, James BC. The use of computers for clinical care: a case series of advanced U.S. sites. J Am Med Inform Assoc. 2003;10:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke K. NHS misses target for introducing electronic records. BMJ. 2002;324:870. [Google Scholar]
- 14.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348:2526–34. [DOI] [PubMed] [Google Scholar]
- 15.Gelbe Liste Pharmindex. MediMedia, Medizinische Medien Informations GmbH. Available at: <http://www.gelbe-liste.de/>. Accessed January 22, 2004.
- 16.DOSING, Tools for Drug Information & Drug Safety. Available at: <http://www.dosing.de>. Accessed January 22, 2004.
- 17.Netland PA, Weiss HS, Stewart WC, Cohen JS, Nussbaum LL. Cardiovascular effects of topical carteolol hydrochloride and timolol maleate in patients with ocular hypertension and primary open-angle glaucoma. Night Study Group. Am J Ophthalmol. 1997;123:465–77. [DOI] [PubMed] [Google Scholar]
- 18.Aalto-Korte K, Turpeinen M. Quantifying systemic absorption of topical hydrocortisone in erythroderma. Br J Dermatol. 1995;133:403–8. [DOI] [PubMed] [Google Scholar]
- 19.Sperzel WD, Broverman CA, Kapusnik-Uner JE, Schlesinger JM. The need for a concept-based medication vocabulary as an enabling infrastructure in health informatics. Proc AMIA Symp. 1998:865–9. [PMC free article] [PubMed]
- 20.Zinacef® (cefuroxime for injection). US Product Information of October 2001.
- 21.Grönroos PE, Irjala KM, Huupponen RK, Scheinin H, Forsström J, Forsström JJ. A medication database—a tool for detecting drug interactions in hospital. Eur J Clin Pharmacol. 1997;53:13–7. [DOI] [PubMed] [Google Scholar]
- 22.McDonald CJ, Blevins L, Tierney WM, Martin DK. The Regenstrief medical records. MD Comput. 1988;5:34–47. [PubMed] [Google Scholar]
- 23.Pryor TA. The HELP medical record system. MD Comput. 1988;5:22–33. [PubMed] [Google Scholar]
- 24.Caffari B, Raschetti R. The logical structure of the VIDEOFAR drug data base. Ann Ist Super Sanita. 1991;27:195–200. [PubMed] [Google Scholar]
- 25.Séné B, Venot A, de Zegher I, et al. A general model of drug prescription. Meth Inf Med. 1995;34:310–7. [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration, Center for Drug Evaluation and Research. National Drug Code Directory. Available at: <http://www.fda.gov/cder/ndc/index.htm>. Accessed January 22, 2004.
- 27.UMLS Information, RxNorm Project. Available at: <http://umlsinfo.nlm.nih.gov/RxNorm.html>. Accessed January 22, 2004.
- 28.Nelson SJ, Brown SH, Erlbaum MS, et al. A semantic normal form for clinical drugs in the UMLS: early experiences with the VANDF. Proc AMIA Symp. 2002:557–61. [PMC free article] [PubMed]
- 29.A Guide to RxNorm. Available at: <http://www.nlm.nih.gov/research/umls/rxnorm_guide.pdf>. Accessed April 29, 2004.
- 30.Martin-Facklam M, Burhenne J, Ding R, et al. Dose-dependent increase of saquinavir bioavailability by the pharmaceutic aid cremophor EL. Br J Clin Pharmacol. 2002;53:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Board of Directors of the American Medical Informatics Association. Standards for medical identifiers, codes, and messages needed to create an efficient computer-stored medical record. J Am Med Inform Assoc. 1994;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDER Data Standards Manual. Available at: <http://www.fda.gov/cder/dsm/>. Accessed January 22, 2004.
- 33.Pharmeuropa. Standard terms, pharmaceutical dosage forms, routes of administration, containers. Strasbourg: Council of Europe, January 2000.
- 34.NCVHS. Patient Medical Record Information Terminology Analysis Reports. Prepared for the National Committee on Vital and Health Statistics Subcommittee on Standards and Security. Available at: <http://www.ncvhs.hhs.gov/>. Accessed April 29, 2004.
- 35.Bell DS, Cretin S, Marken RS, Landman AB. A conceptual framework for evaluating outpatient electronic prescribing systems based on their functional capabilities. J Am Med Inform Assoc. 2004;11:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]