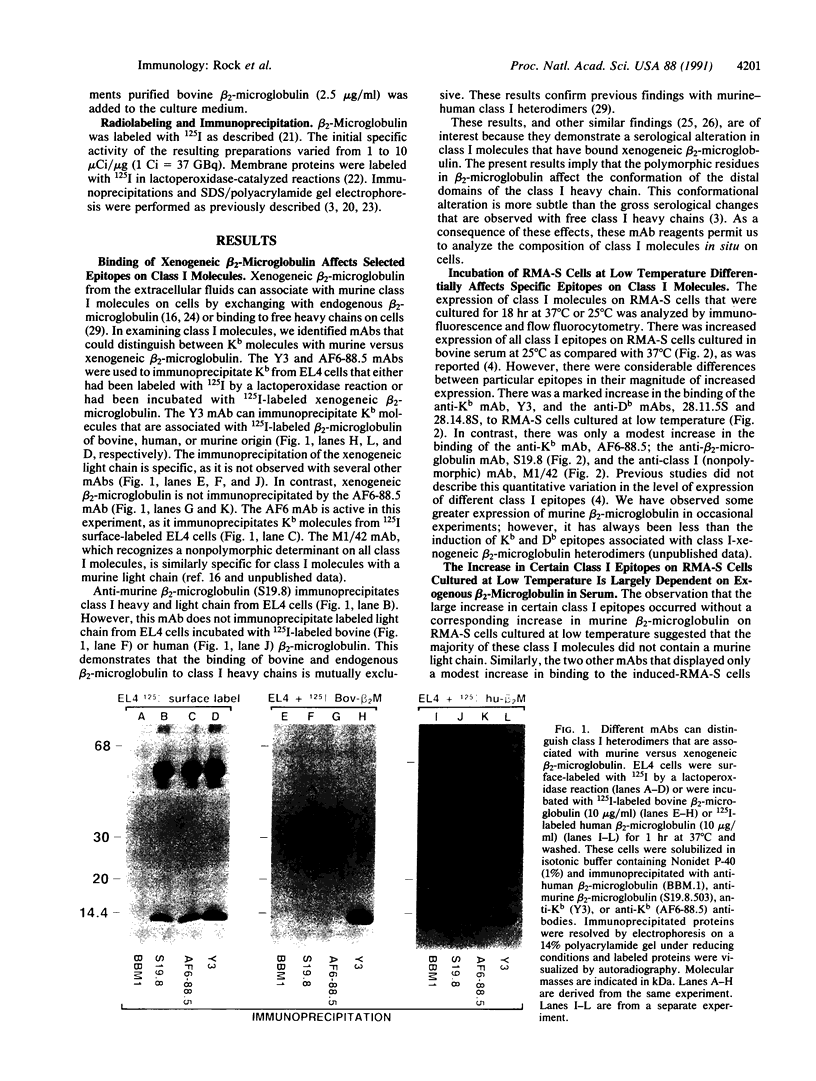
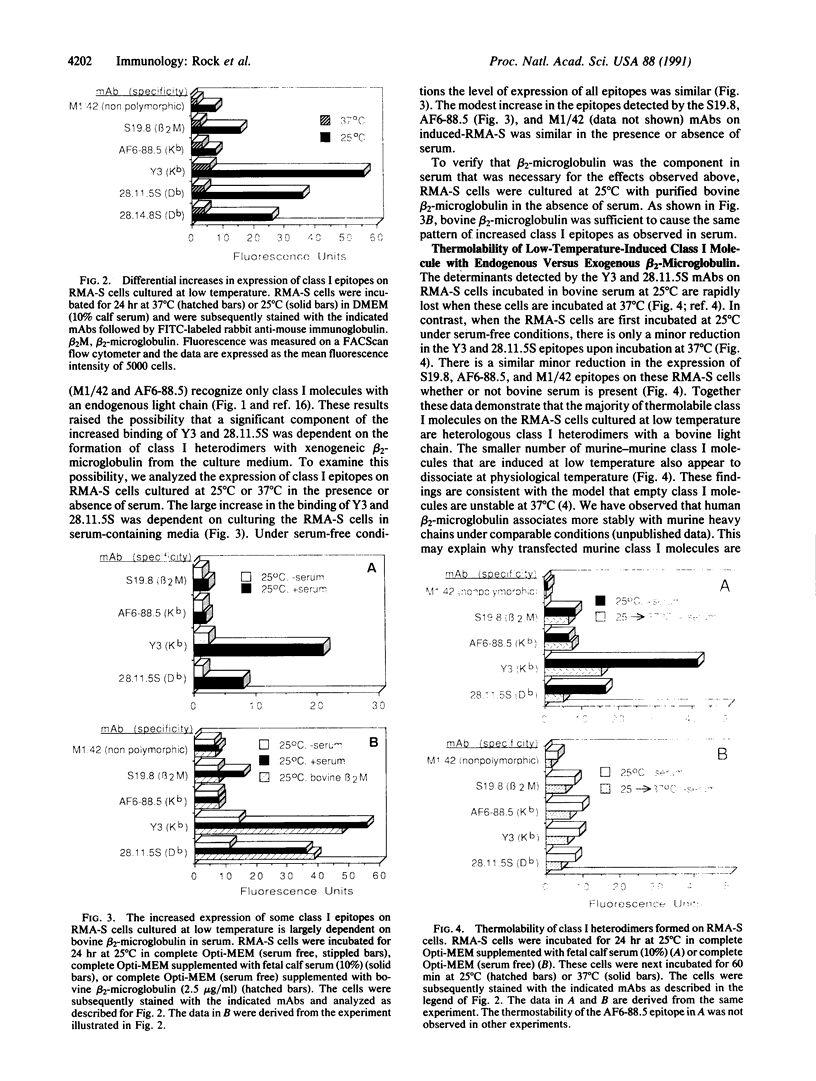
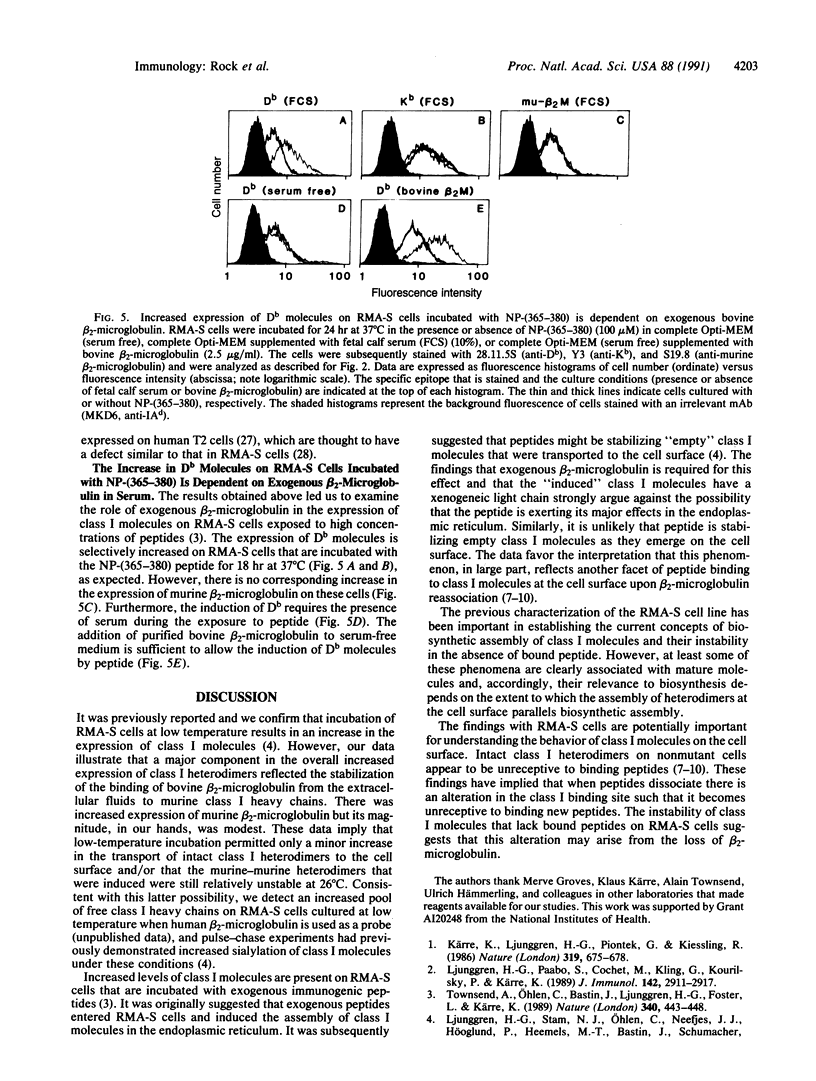
Abstract
RMA-S murine cells have a mutation that interferes with the assembly of class I major histocompatibility complex (MHC) heterodimers and are deficient in the expression of class I molecules on the cell surface. The mutant phenotype has been reported to be normalized upon incubation of RMA-S cells at 25 degrees C. We find that much of the increased expression of class I heterodimers is dependent on culturing RMA-S cells in bovine serum or with purified bovine beta 2-microglobulin. Furthermore, epitopes that are associated with class I MHC molecules that have bound xenogeneic beta 2-microglobulin are preferentially formed on RMA-S cells cultured at 25 degrees C. These heterologous class I molecules are thermolabile. Increased expression of class I molecules has also been observed on RMA-S cells incubated at 37 degrees C in the presence of class I-restricted peptides. We find that the increased expression of Db molecules induced by influenza virus nucleoprotein residues 365-380 is similarly dependent on culturing RMA-S cells in bovine serum or with purified bovine beta 2-microglobulin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Payne J. A., Murray R., Frelinger J. A., Cresswell P. Differential transport requirements of HLA and H-2 class I glycoproteins. Immunogenetics. 1989;29(6):380–388. doi: 10.1007/BF00375866. [DOI] [PubMed] [Google Scholar]
- Bernabeu C., van de Rijn M., Lerch P. G., Terhorst C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984 Apr 12;308(5960):642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Ferrier P., Layet C., Caillol D. H., Jordan B. R., Lemonnier F. A. The association between murine beta 2-microglobulin and HLA class I heavy chains results in serologically detectable conformational changes of both chains. J Immunol. 1985 Aug;135(2):1281–1287. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Harris A. W. Immunoglobulin of T lymphoma cells. Biosynthesis, surface representation, and partial characterization. Biochemistry. 1975 May 6;14(9):1826–1834. doi: 10.1021/bi00680a004. [DOI] [PubMed] [Google Scholar]
- Hosken N. A., Bevan M. J. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science. 1990 Apr 20;248(4953):367–370. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Strominger J. L. Dissociation and exchange of the beta 2-microglobulin subunit of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5834–5838. doi: 10.1073/pnas.76.11.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski S., Takeshita T., Boehncke W. H., Takahashi H., Boyd L. F., Germain R. N., Berzofsky J. A., Margulies D. H. Excess beta 2 microglobulin promoting functional peptide association with purified soluble class I MHC molecules. Nature. 1991 Jan 3;349(6304):74–77. doi: 10.1038/349074a0. [DOI] [PubMed] [Google Scholar]
- Kubota K. Association of serum beta 2-microglobulin with H-2 class I heavy chains on the surface of mouse cells in culture. J Immunol. 1984 Dec;133(6):3203–3210. [PubMed] [Google Scholar]
- Kvist S., Hamann U. A nucleoprotein peptide of influenza A virus stimulates assembly of HLA-B27 class I heavy chains and beta 2-microglobulin translated in vitro. Nature. 1990 Nov 29;348(6300):446–448. doi: 10.1038/348446a0. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Päbo S., Cochet M., Kling G., Kourilsky P., Kärre K. Molecular analysis of H-2-deficient lymphoma lines. Distinct defects in biosynthesis and association of MHC class I heavy chains and beta 2-microglobulin observed in cells with increased sensitivity to NK cell lysis. J Immunol. 1989 Apr 15;142(8):2911–2917. [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Db class I major histocompatibility complex binding of an exogenous influenza peptide. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):301–304. doi: 10.1073/pnas.88.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L. E., Gamble S. R., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Kb class I major histocompatibility complex binding of exogenous peptides. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7517–7521. doi: 10.1073/pnas.87.19.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Yeh E. T., Gramm C. F., Haber S. I., Reiser H., Benacerraf B. TAP, a novel T cell-activating protein involved in the stimulation of MHC-restricted T lymphocytes. J Exp Med. 1986 Feb 1;163(2):315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Springer T. A., Mescher M. F. Characterization of an anti-H-2 monoclonal antibody and its use in large-scale antigen purification. J Immunol. 1981 Sep;127(3):923–930. [PubMed] [Google Scholar]
- Tada N., Kimura S., Hatzfeld A., Hämmerling U. Ly-m11: the H-3 region of mouse chromosome 2 controls a new surface alloantigen. Immunogenetics. 1980;11(5):441–449. doi: 10.1007/BF01567813. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Potter T. A., Sherman L. A. The role of beta 2-microglobulin in peptide binding by class I molecules. Science. 1990 Dec 7;250(4986):1423–1426. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]









