Abstract
Persistent reduction of renal perfusion pressure induces renovascular hypertension by activating the renin-angiotensin-aldosterone system; however, the sensing mechanism remains elusive. Here we investigated the role of PGI2 in renovascular hypertension in vivo, employing mice lacking the PGI2 receptor (IP–/– mice). In WT mice with a two-kidney, one-clip model of renovascular hypertension, the BP was significantly elevated. The increase in BP in IP–/– mice, however, was significantly lower than that in WT mice. Similarly, the increases in plasma renin activity, renal renin mRNA, and plasma aldosterone in response to renal artery stenosis were all significantly lower in IP–/– mice than in WT mice. All these parameters were measured in mice lacking the four PGE2 receptor subtypes individually, and we found that these mice had similar responses to WT mice. PGI2 is produced by COX-2 and a selective inhibitor of this enzyme, SC-58125, also significantly reduced the increases in plasma renin activity and renin mRNA expression in WT mice with renal artery stenosis, but these effects were absent in IP–/– mice. When the renin-angiotensin-aldosterone system was activated by salt depletion, SC-58125 blunted the response in WT mice but not in IP–/– mice. These results indicate that PGI2 derived from COX-2 plays a critical role in regulating the release of renin and consequently renovascular hypertension in vivo.
Introduction
In renovascular hypertension, the reduction of renal blood flow due to renal artery stenosis originating from obstructive vascular diseases, such as atherosclerosis or fibromuscular dysplasia, induces excessive activation of the renin-angiotensin-aldosterone (RAA) system and leads to hypertension (1). In patients and animal models of renovascular hypertension, expression of COX-2, a rate-limiting enzyme for prostanoid synthesis, has been reported to be increased in the kidneys (2, 3). In addition, production of PGE2 and I2 in the kidney has been reported to be increased during renovascular hypertension (4, 5), suggesting that the prostanoids play an important role in the pathogenesis of renovascular hypertension. The roles of the prostanoids in renovascular hypertension, however, have not yet been fully defined.
The RAA system plays an important role in the maintenance of vascular tone, circulating blood volume, and electrolyte balance in the body. Renin is a rate-limiting enzyme involved in the activation of the RAA system and is secreted from the granular cells of juxtaglomerular apparatus (JGA) in the kidney. It converts plasma angiotensinogen to Ang I, which is successively changed to Ang II, a powerful vasoconstrictor, by angiotensin-converting enzyme present on the epithelial cells of pulmonary vasculatures. Ang II acts on the adrenal cortex and stimulates the secretion of aldosterone, which facilitates sodium reabsorption in the kidney and expands the circulating blood volume. Thus, Ang II and aldosterone are thought to be important players in the control of BP; therefore, renin secretion is precisely regulated through two major sensing mechanisms, along with regulation by the sympathetic nervous system. One mechanism is the baroreceptor mechanism, which senses the reduction in renal perfusion pressure and increases renin secretion (6). This mechanism is thought to reside in the renal vasculature itself and to be independent of renal tubular elements, although its exact location remains unknown. The other is the macula densa mechanism, which senses the decrease in the concentration of chloride ions in glomerular filtrate at the macula densa cells and increases renin secretion (6). The macula densa cell, a differentiated tubular epithelial cell, is one of those composing the JGA. These two sensing mechanisms transmit their information to the granular cells via the respective mediators (6). The role of the prostanoids as such mediators, however, especially in vivo, remains to be determined.
It is well established that cAMP works as a second messenger of renin secretagogues, such as norepinephrine, in the granular cells of JGA and that the increase in intracellular cAMP concentration induces renin secretion (7). PGE2 exerts its action through four subtype receptors, the EP1, EP2, EP3, and EP4, and PGI2 acts on its receptor IP. Stimulation of the EP2, EP4, and IP increases intracellular cAMP concentration, indicating that these receptors could mediate the stimulatory signal for renin secretion. In contrast, stimulation of the EP1 and EP3 leads to the increase in intracellular Ca2+ concentration and the decrease in intracellular cAMP concentration, respectively (8, 9). In addition, PGE2 and PGI2 have been reported to stimulate renin secretion in cultured juxtaglomerular (JG) cells (10). These results suggest that PGE2 and PGI2 work as mediators of renin secretion acting directly on the granular cells, while their in vivo actions in the regulation of renin secretion are not known.
In the present study, we attempted to clarify the roles of PGE2 and PGI2 in the pathogenesis of renovascular hypertension employing a two-kidney, one-clip (2K1C) hypertension model using mice lacking the EP1 (EP1–/– mice), EP2 (EP2–/– mice), EP3 (EP3–/– mice), EP4 (EP4–/– mice), or IP (IP–/– mice).
Results
Renovascular hypertension in 2K1C model.
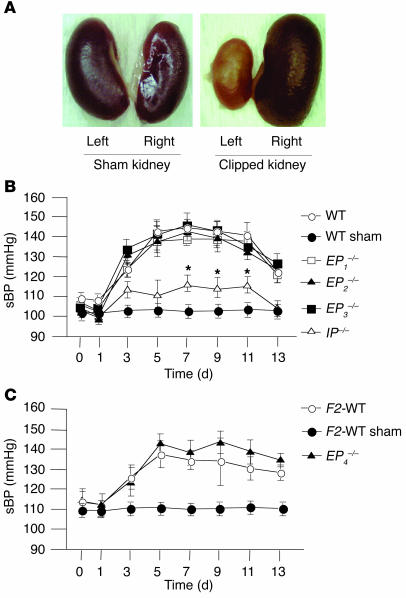
At day 14 of 2K1C, the left kidney became atrophic and the right kidney showed compensatory hypertrophy (Figure 1A). The degree of atrophic change estimated by weights of the left kidneys was similar among WT, EP1–/–, EP2–/–, EP3–/–, and IP–/– mice and between F2-WT and EP4–/– mice (data not shown), suggesting that the clipping of the renal artery induced a similar degree of ischemic stress to the left kidney in each mouse group.
Figure 1.
Morphological changes of the kidneys and renovascular hypertension in 2K1C model. (A) Representative photographs of the kidneys from sham-operated WT mice (left) and from mice subjected to 2K1C (right). At day 14 of 2K1C, the left kidney became atrophic and the right kidney showed compensatory hypertrophy. (B and C) Time course of sBP in WT, sham-operated WT, EP1–/–, EP2–/–, EP3–/–, and IP–/– mice (B), and in F2-WT, sham-operated F2-WT, and EP4–/– mice (C) in 2K1C model. Each point represents mean ± SEM of 8_10 mice per group. *P < 0.05 versus WT mice.
In WT and F2-WT mice, systolic BP (sBP) rose gradually and reached a plateau at day 5 of 2K1C, while there was no such increase in sBP in the sham-operated groups (Figure 1, B and C) measured by the tail-cuff method. In IP–/– mice, however, the increase in sBP was significantly lower than that observed in WT mice (Figure 1B), suggesting an important pathophysiological role of PGI2 in renovascular hypertension. In contrast, there were no significant differences in the degree and time-course of hypertension in EP1–/–, EP2–/–, EP3–/–, and EP4–/– mice compared with those in their respective WT mice (Figure 1, B and C), suggesting that there is a minor role, if any, for PGE2 in the present model of renovascular hypertension. When BP was measured directly through the cannula inserted into the carotid artery at day 7 of 2K1C, the increase in sBP was significantly lower in IP–/– mice compared with that in WT mice; these were 144 ± 12, 118 ± 6, and 104 ± 5 mmHg in WT, IP–/–, and sham-operated WT mice, respectively. This result confirmed the decreased susceptibility of IP–/– mice to renovascular hypertension as was observed by the tail-cuff method. The sBP in WT and F2-WT mice decreased gradually and returned to a preoperation level within 3 weeks after the operation, as the left kidney fell into severe atrophy. Although we performed the present study using female mice, the degree and time course of hypertension in male WT and IP–/– mice subjected to 2K1C were similar to those in female mice (data not shown), indicating that there was no gender difference in the present phenotype.
The basal levels of heart rate were not significantly different among WT, EP1–/–, EP2–/–, EP3–/–, and IP–/–, or between F2-WT and EP4–/– mice, and there were no significant changes in heart rate during the experiments in all groups of mice (data not shown). Plasma creatinine levels at day 14 of 2K1C were not significantly different among the mice groups (data not shown).
Activation of the RAA system in 2K1C model.
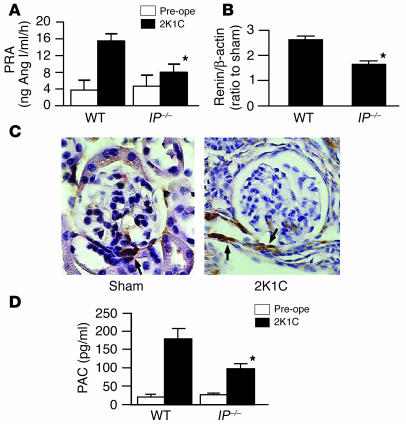
To explore the mechanism whereby PGI2 induces hypertension in 2K1C model, we examined the role of PGI2 in renin secretion by examining plasma renin activity (PRA). In WT mice, PRA increased significantly at day 7 of 2K1C, while there was no such increase in sham-operated WT mice (Figure 2A). In IP–/– mice, however, the increase in PRA was significantly lower than that in WT mice, suggesting a stimulatory effect of endogenous PGI2 on renin secretion. In contrast, there were no significant differences in the degree of increases in PRA between WT and EP2–/– or F2-WT and EP4–/– mice (data not shown). In sham-operated WT and IP–/– mice, there were no significant increases in PRA compared with preoperation values; these were 4.3 ± 1.2 and 5.0 ± 1.6 ng Ang I/ml/h, respectively.
Figure 2.
Activation of the RAA system in WT and IP–/– mice in 2K1C model. (A and B) Increases in PRA (A) and renin mRNA expression in the kidney (B) at day 7 of 2K1C. Each column represents mean ± SEM of 5_15 mice per group. In B values were expressed as a ratio of renin/β-actin mRNA of 2K1C mice to that of sham-operated mice. *P < 0.05 versus WT mice. (C) Immunohistochemical analysis of renin expression in the WT kidney in 2K1C model. The immunoreactivities of renin in the kidney from the sham-operated WT mouse were detected only in the cells of JGA (left). The immunoreactivities of renin in the kidney at day 7 of 2K1C were detected in the afferent arterioles adjacent to JGA in addition to in JG cells (right). Arrows indicate the renin immunoreactivities. Original magnification, ×400. (D) Increase in PAC at day 7 of 2K1C. Each column represents mean ± SEM of 5_15 mice per group. *P < 0.05 versus WT mice. Pre-ope, preoperation.
To determine if PGI2 has a stimulatory effect on renin production as well as secretion, we examined the expression of renin mRNA in the kidney. In WT mice, renal renin mRNA expression increased significantly at day 7 of 2K1C (Figure 2B). In IP–/– mice, however, the increase in the expression level was significantly lower than that in WT mice, suggesting that there is a stimulatory role of endogenous PGI2 in renin production. In contrast, there were no significant differences in the expression levels of renin mRNA among WT, EP1–/–, EP2–/–, EP3–/– kidneys or between F2-WT and EP4–/– kidneys (data not shown). The immunoreactivities of renin were detected only in JG cells in WT kidney at day 7 of sham operation (Figure 2C). In the clipped WT kidney, however, the immunoreactivities were found in the vascular cells of afferent arterioles near the glomerulus in addition to JG cells, indicating that transitional cells began to produce renin after 2K1C.
In accordance with the increase in PRA, plasma aldosterone concentration (PAC) increased significantly in WT mice at day 7 of 2K1C (Figure 2D). In IP–/– mice, however, the increase in PAC was significantly lower than that in WT mice, suggesting the activation of the RAA system by endogenous PGI2. In sham-operated WT and IP–/– mice, there were no significant increases in PAC compared with preoperation values; these were 19 ± 7 and 23 ± 4 pg/ml, respectively.
Expressions of COX mRNAs in the kidney and production of PGI2 in 2K1C model.
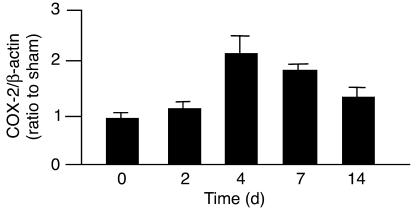
We further examined the expression of COX, the key enzymes for prostanoid synthesis. In WT kidney, the expression level of mRNA for COX-2, an inducible isoform of COX, increased significantly after the operation of 2K1C (Figure 3), indicating that the expression level of COX-2 mRNA increased, along with the increased expression of renin mRNA and the elevation of BP. There was no significant difference in the expression levels of COX-2 mRNA between WT and IP–/– kidneys (data not shown), suggesting that a similar degree of prostanoid synthesis took place in WT and IP–/– mice after 2K1C. In contrast, the expression levels of COX-1 mRNA in the kidney did not change significantly after the operation in either WT or IP–/– mice (data not shown).
Figure 3.
Expression of COX-2 mRNA in the WT kidney in 2K1C model. The expression of COX-2 mRNA increased significantly after the operation and reached a peak level at day 4 of 2K1C. Each column represents mean ± SEM of 5_12 mice per group. Values were expressed as a ratio of COX-2/β-actin mRNA of 2K1C mice to that of sham-operated mice.
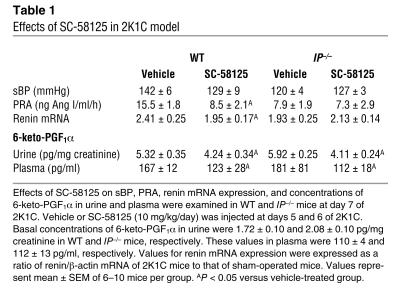
There were no significant differences in basal concentrations of 6-keto-PGF1α, a stable metabolite of PGI2, in either urine or plasma between WT and IP–/– mice (Table 1). In both WT and IP–/– mice, concentrations of 6-keto-PGF1α in urine and plasma increased significantly at day 7 of 2K1C compared with the basal values. There were no significant differences in these increases between WT and IP–/– mice, indicating that the COX-PGI2 system was stimulated to a similar extent in WT and IP–/– mice subjected to 2K1C.
Table 1.
Effects of SC-58125 in 2K1C model
Effects of SC-58125 in the 2K1C model.
To estimate the degree of participation of COX-2–derived PGI2 in the pathogenesis of renovascular hypertension, the effects of SC-58125 were examined. In WT mice, SC-58125 failed to affect BP in either WT or IP–/– mice, but SC-58125 significantly reduced the increases in PRA and renin mRNA expression in WT mice to levels similar to those in vehicle-treated IP–/– mice. In addition, SC-58125 had no effect on PRA or renin mRNA expression in IP–/– mice, suggesting that COX-2–derived PGI2 stimulated renin secretion and production. The concentrations of 6-keto-PGF1α in urine and plasma decreased significantly in both WT and IP–/– mice to a similar extent under SC-58125 treatment. SC-58125 had no effect on BP or PRA in sham-operated WT and IP–/– mice (data not shown).
Activation of the RAA system under low-salt diet with furosemide.
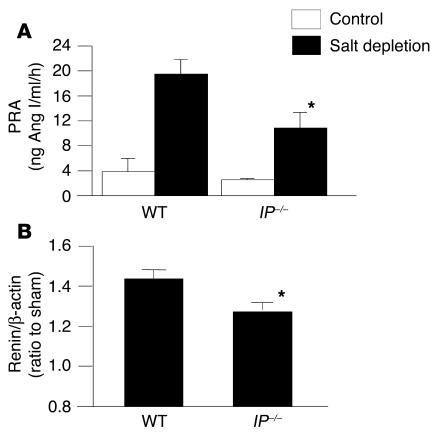
In WT mice subjected to low-salt diet with furosemide, PRA increased significantly compared with that in WT control mice (Figure 4A). In IP–/– mice under salt deficiency, however, the increase in PRA was significantly lower than that in WT mice. In EP2–/– and EP4–/– mice, the increases in PRA were similar to those in WT and F2-WT mice, respectively (data not shown). In addition, salt deficiency significantly increased the renin mRNA expression in the WT kidney compared with that in the control kidney. In IP–/– kidney, however, the degree of the increase was significantly lower than that in WT kidney (Figure 4B). These results suggest that PGI2 participates as an important mediator in the macula densa mechanism of renin release and production, as well as in the baroreceptor mechanism observed in 2K1C model.
Figure 4.
PRA and renin mRNA expression in the kidney in a salt-deficiency model. Mice were subjected to a low-salt diet (0.12% NaCl) for 7 days and injected with furosemide (25 mg/kg, intraperitoneally) every day. PRA (A) and renin mRNA expression (B) increased significantly more than in control mice at day 7 of salt depletion. These increases were blunted significantly in IP–/– mice compared with those in WT mice. Each column represents mean ± SEM of 5_12 mice per group. Values in B were expressed as a ratio of renin/β-actin mRNA of salt-depleted mice to that of control mice. *P < 0.05 versus WT mice.
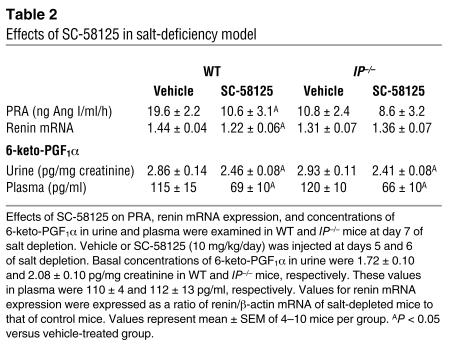
Expression levels of COX-2 mRNA increased significantly under salt deficiency in both WT and IP–/– kidneys to a similar degree; these were 21% ± 5% and 19% ± 3% increase over respective control levels. Expression levels of COX-1 mRNA, however, were unchanged in both WT and IP–/– kidneys (data not shown). Urinary contents of 6-keto-PGF1α increased significantly upon salt depletion in both WT and IP–/– mice compared with their respective control mice, but the plasma levels of 6-keto-PGF1α did not change significantly in either WT or IP–/– mice (Table 2), suggesting that PGI2 production increased mainly in renal tubular system under salt-deficient conditions. SC-58125 significantly reduced 6-keto-PGF1α contents in urine and plasma in both WT and IP–/– mice (Table 2). In addition, SC-58125 significantly reduced the increases in PRA and renin mRNA expression in WT mice subjected to salt depletion compared with those in vehicle-treated WT mice (Table 2). In IP–/– mice, however, SC-58125 had no significant effects on PRA or renin mRNA expression, supporting the theory that COX-2–derived PGI2 also participates in the regulation of renin release and production under salt-deficient conditions.
Table 2.
Effects of SC-58125 in salt-deficiency model
Effects of cicaprost and PGE2 on renin mRNA expression in the cultured cells rich in JG cells.
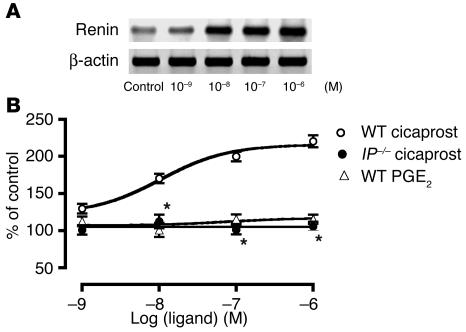
Cicaprost, an IP agonist, stimulated renin mRNA expression in the cultured JG cells prepared from WT mice in a concentration-dependent manner with a median effective concentration (EC50) value of 1.05 × 10–8 M (Figure 5). In the JG cells from IP–/– mice, however, the stimulatory effect of cicaprost on renin mRNA expression disappeared completely, indicating that the effect of cicaprost was mediated by the IP. In contrast, PGE2 did not stimulate the expression of renin mRNA at all (Figure 5), ruling out PGE2 as a direct stimulator of JG cells. In accordance with this result, all of the selective agonists for each EP subtypes failed to increase renin mRNA expression in the cultured JG cells prepared from WT mice (data not shown).
Figure 5.
Effects of cicaprost, an IP agonist, and PGE2 on renin mRNA expression in cultured cells rich in JG cells. (A) A representative photograph showing stimulatory effects of cicaprost on renin mRNA expression in cultured cells prepared from WT mice. (B) Cicaprost significantly increased the expression level of renin mRNA in a concentration-dependent manner in the cells prepared from WT mice, although the effects disappeared completely from the cells prepared from IP–/– mice. In contrast, PGE2 had no effect on renin mRNA expression in the cells prepared from WT mice. Each point represents mean ± SEM of 4 cell groups. *P < 0.05 versus WT mice.
Effects of cicaprost on renin activity and cAMP contents in the cultured cells rich in JG cells.
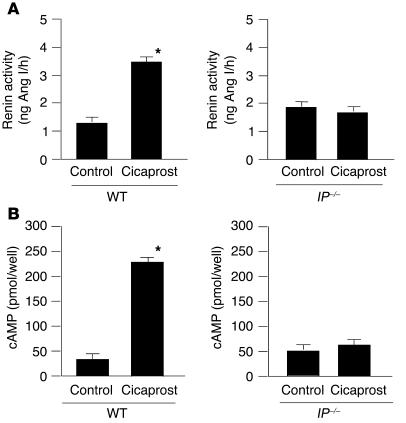
Cicaprost significantly increased renin activity in the cultured JG cells prepared from WT mice, but the effect disappeared in the cultured JG cells prepared from IP–/– mice (Figure 6A), showing a potent stimulatory effect of PGI2 through the IP on renin activity. In contrast, all of the selective EP agonists failed to increase renin activity in the cultured JG cells prepared from WT mice (data not shown).
Figure 6.
Effects of cicaprost on renin activity and cAMP contents in cultured cells rich in JG cells. Cicaprost (1 μM) significantly increased the renin activity (A) and cAMP contents (B) in the cells prepared from WT mice, while the effects disappeared completely in the cells prepared from IP–/– mice. Each point represents mean ± SEM of 4 cell groups. *P < 0.05 versus control.
Cicaprost significantly increased cAMP contents in the cultured cells prepared from WT mice, while the effect disappeared in the cultured cells prepared from IP–/– mice (Figure 6B). This result suggests that the stimulatory effects of cicaprost by way of the IP on the renin mRNA expression and renin activity in the JG cells would be mediated by the increase in intracellular cAMP concentration.
Discussion
The prostanoids have long been suggested to be involved in the regulation of the RAA system. Their exact contribution to the pathophysiology of the activated RAA system in vivo and the types of the responsible prostanoids remain elusive, however. Here we showed that IP deficiency significantly suppressed the development of renovascular hypertension in vivo, demonstrating for the first time that endogenous PGI2 plays an important role in the pathogenesis of renovascular hypertension by way of the IP. In contrast, the deficiency of EP1–/–, EP2–/–, EP3–/–, or EP4–/– did not attenuate at all the development of renovascular hypertension in the 2K1C model. This suggests that endogenous PGE2 does not participate in the pathogenesis of renovascular hypertension, at least in the present model of renovascular hypertension. There may be, however, some fixation of particular genes in EP4–/– mice due to a selective bleeding as described. Therefore, it would be necessary to give care to the difference in genetic background when considering in vivo phenotype of EP4–/– mice, because the factors responsible for BP response would be different according to a genetic background.
The pivotal role of the RAA system in the development of renovascular hypertension is well-established. In IP–/– mice, the increases in PRA and PAC were significantly lower compared with those in WT mice, indicating the stimulatory actions of endogenous PGI2 on renin and succeeding aldosterone secretions. In addition, the expression level of renin mRNA was significantly elevated in the WT kidney after the operation, although the elevation was significantly blunted in IP–/– kidney, indicating that endogenous PGI2 also stimulates renin production. These results suggest that the role of PGI2 in the development of renovascular hypertension was mediated by activation of the RAA system.
Immunohistochemical analysis detected the renin immunoreactivities only in JG cells in the kidney from sham-operated mice. In the kidney of 2K1C, however, the renin immunoreactivities were additionally found in the afferent arterioles adjacent to the glomeruli. This expansion of renin immunoreactivities toward afferent arterioles corresponds to the fact that intermediated cells in the media of the afferent arteriole can produce renin granules upon a decrease in perfusion pressure of the kidney (6). We previously showed that the IP was expressed in smooth muscle cells of the afferent arterioles in the murine kidney (11). Taken together, these findings suggest that PGI2 produced in the arterioles could act at the IP on intermediate cells transformed to contain renin granules as well as at the IP on JG cells.
We determined which isoform of COXs is responsible for production of PGI2 participating in the pathogenesis of renovascular hypertension. Expression of COX-2 mRNA, but not COX-1 mRNA, increased significantly after the operation in both WT and IP–/– kidneys to a similar degree. In addition, PRA and the expression level of renal renin mRNA in SC-58125–treated WT mice were significantly lower than those in vehicle-treated WT mice and were similar to those in vehicle-treated IP–/– mice. Moreover, SC-58125 failed to affect these parameters in IP–/– mice. These results suggest that COX-2–derived PGI2 is responsible for the activation of the RAA system in renovascular hypertension. Nevertheless, SC-58125 failed to significantly suppress sBP. Several investigators have reported the effects of COX inhibitors in renovascular hypertension, although the results are controversial. Some reports suggested significant suppressive effects of COX-2 inhibitors on BP in renovascular hypertension (12, 13). In contrast, the absence of effects of these inhibitors on BP and renin production in the 2K1C model have been shown (14, 15). This discrepancy may be derived from the effects of COX-2 inhibitors on a variety of systemic actions of the prostanoids in the body irrespective of their potent inhibitory effect on renin secretion and production, as shown in this study. Otherwise, the failure of SC-58125 to suppress BP elevation in the present study may originate from its relatively moderate effect on PGI2 production due to a mild dose protocol.
We examined whether PGI2 also plays a role in the regulation of the RAA system through the macula densa mechanism under salt-deficient conditions. In WT mice, a low-salt diet with furosemide significantly increased PRA and renin mRNA expression, indicating an activation of the RAA system. In IP–/– mice, however, these increases were significantly blunted, indicating that endogenous PGI2 also participates in macula densa mechanism of renin release and production. In addition, SC-58125 significantly reduced salt deficiency–induced increases in PRA and renin mRNA expression in WT mice to a level similar to those in vehicle-treated IP–/– mice. These inhibitory effects of SC-58125 on the RAA system, however, disappeared in IP–/– mice, suggesting that COX-2–derived PGI2 participates in the macula densa mechanism.
In both the 2K1C and salt restriction model, the activation of the RAA system in IP–/– mice was significantly blunted compared with that in WT mice. The activity of the RAA system was still significantly higher in IP–/– mice compared with that in control mice, however. This result suggests that the IP-independent mechanism through factors other than PGI2, such as sympathetic nervous system and nitric oxide, would be involved in the activation of the RAA system in the pathogenesis of renovascular hypertension and under the condition of salt deficiency. In addition, possible contribution of other prostanoids in the regulation of the RAA system, such as PGD2, PGF2α, and TXA2, could not be excluded in the present study.
Finally, we examined whether PGI2 acts directly at JG cells. In the cultured cells from WT mice, cicaprost exhibited a potent stimulatory effect on renin mRNA expression and renin activity, effects that disappeared completely in cultured cells from IP–/– mice. In contrast, PGE2 and selective EP agonists had no effect on renin mRNA expression and renin activity in the cultured cells from WT mice. In addition, cicaprost potently increased cAMP contents in the cultured cells in an IP-dependent manner. In accordance with this result, a stimulatory effect of PGI2 on renin production and cAMP contents in cultured JG cells has been reported (10). These results indicate that PGI2 is a potent stimulant of renin mRNA expression acting directly on JG cells and that the effect of PGI2 is mediated by increased cAMP contents. In contrast to the present result, however, several reports have suggested a stimulatory effect of PGE2 on renin mRNA expression in cultured JG cells (10, 16). Although the reason for this discrepancy is not clear, it may be derived from a species difference. In fact, a reported EC50 value of PGE2 in renin mRNA expression in cultured murine JG cells is more than two rank orders higher compared with that of PGI2 (10), indicating a low potency of PGE2 in mouse JG cells. Otherwise, this discrepancy may be attributed to a difference in the experimental condition.
In conclusion, COX-2–derived PGI2 plays a critical role through the IP in regulating the release of renin and consequently renovascular hypertension in vivo. The mediatory role of PGI2 also occurred in the macula densa mechanism of renin secretion, indicating that PGI2 is a key molecule in the regulation of renin release.
Methods
Mice.
Generation and maintenance of EP1–/–, EP2–/–, EP3–/–, EP4–/–, and IP–/– mice have been reported (17–20). The EP1–/–, EP2–/–, EP3–/–, and WT control mice have a genetic background similar to C57BL/6 mice. Most EP4–/– mice die postnatally as a result of patent ductus arteriosus and do not survive at all in the C57BL/6 background. Therefore, F2 progenies of surviving EP4–/– mice and their WT littermates were maintained independently in the mixed genetic background of 129/Ola and C57BL/6. For the experiments using EP4–/– mice, F2-WT mice having this genetic background were used as a control. All experiments, which were approved by the Asahikawa Medical College Committee on Animal Research, were performed using 10- to 20-week-old female mice.
The 2K1C procedure as a model of renovascular hypertension.
The 2K1C procedure was performed according to the reported method (21) with some modification. Mice were anesthetized with ketamine (100 mg/kg, intraperitoneally) and xylazine (5 mg/kg, intraperitoneally), and were placed in a prone position under a control of body temperature. The left kidney was exposed through the posteroabdominal incision, and the renal artery was individualized from the renal vein and nerves over a short segment, along which a fluorocarbon thread (Siglon; Sunline Co. Ltd.) 5 mm long, with a diameter of 0.11 mm, was placed. A clip was then made by twisting 10 mm of copper wire around the renal artery and the thread, which was removed after the clipping. In a pilot experiment, the diameter of the stenosis was estimated by measuring the diameter of the clips, which were removed from mice 24 hours after the operation. Digitalized photographs of the clips captured by CCD camera were analyzed by the software program NIH image, and the diameter of the clips thus measured was 0.11 ± 0.01 mm (n = 9). Renal blood flow estimated by laser-Doppler method (ALF21; Advantec MFS Inc.) fell to a level of about 30% ± 1.6% of the preclipping value (n = 5). The procedure gave a fairly constant degree of elevation in BP, and 91% of WT mice subjected to 2K1C (n = 109) showed the increase in sBP greater than 50% of the mean increase of all WT mice. A sham procedure, which included the entire surgery with the exception of arterial clipping, was applied in control mice.
To examine the effect of SC-58125 (Cayman Chemical Co.), a selective COX-2 inhibitor (22, 23), in the 2K1C model we administered it at days 5 and 6 of 2K1C. SC-58125 dissolved in DMSO at a concentration of 20 mg/ml was injected intraperitoneally at a dose of 0.5 μl/g body weight (10 mg/kg body weight) using a microsyringe (no. 710; Hamilton Co.). The dose of DMSO had no effect on BP, PRA, and renin mRNA expression. We chose a dose protocol of SC-58125, 10 mg/kg for 2 days, because SC-58125 administrated at 20 mg/kg for 2 days had an inhibitory effect on COX-1, which was estimated by the reduction of plasma level of TXB2 (22), a COX-1–related metabolite. In addition, we found a sign of gastric damage by high doses of SC-58125, which was represented by reduction of gastric weight (23).
The incidence of minute renal infarction after the operation was estimated by examining the degree of sclerotic change in glomeruli at day 7 of 2K1C because no gross infarction was detected histologically. There was no significant difference in the percentage of sclerotic or collapsed glomeruli between WT and IP–/– kidneys; these were 4.7% ± 1.8% (n = 10) and 5.0% ± 3.2% (n = 12), respectively. This result indicates that the tendency to thrombosis found in IP–/– mice was not apparent in the present 2K1C model.
BP was measured by a method using tail plethysmography (Softron Co. Ltd.) as reported (24). We also measured BP directly from the carotid artery in some experiments in which the left carotid artery was cannulated with a polyurethane catheter (Instech Laboratories Inc.) at day 7 of 2K1C, and the BP was measured with a polygraph (San-Ei Instrument Co. Ltd.) after recovery from anesthesia.
Low-salt diet with furosemide.
Mice were fed with a normal diet containing 0.33% NaCl or a low-salt diet containing 0.12% NaCl (Oriental Yeast Co. Ltd.) for 7 days. Mice on a low-salt diet also received an intraperitoneal injection of furosemide (25 mg/kg) everyday. At days 5 and 6 of salt depletion, 10 mg/kg of SC-58125 was injected intraperitoneally.
Measurements of PRA and PAC.
PRA was measured according to the reported method (25) with some modifications. In short, blood (400 μl) was collected from the heart, and 8 μl of 0.5 M EDTA (pH 8.0) was added. After centrifugation at 1,500 g for 10 minutes, plasma was collected and stored at –80°C until use. To determine PRA, plasma (10 μl) was incubated for 1 hour at 37°C with 10 μl of plasma prepared from nephrectomized mice 36 hours after the operation plus 10 μl of phosphate buffer (50 mM, pH 6.6). The generated Ang I was measured by an enzyme immunoassay (EIA) kit (Peninsula Laboratory Inc.). Residual renin activity in plasma from nephrectomized mice was subtracted from the PRA of each sample. The PAC was measured by an EIA kit (Cayman Chemical Co.).
Examination of renin and COX mRNA expressions in the kidney.
After the kidney was excised, the renal cortex was separated from the medulla along the inner stripe of outer medulla, frozen in liquid nitrogen, and stored at –80°C until use. Total RNA (2 μg), which was isolated from the renal cortex using Isogen (Nippon Gene Co. Ltd.), was reverse-transcribed as reported (26). The resulting cDNA was amplified by PCR using primer sets corresponding to the respective mRNA for renin, COX-1, COX-2, and β- actin (27, 28). The quantity of PCR product was determined by real-time PCR analysis using a Lightcycler apparatus (Idaho Technology Inc.) and DNA Master SYBR Green I (Roche Molecular Biochemicals).
Immunohistochemical analysis of renin expression in the WT kidney.
After fixation by perfusion and immersion with 4% paraformaldehyde, the kidney was embedded in paraffin. Tissue sections (5 μm thick) prepared from paraffin-embedded kidneys were washed in a buffer containing 0.05% polyoxyethylene sorbitan monolaurate (Tween 20; ICN Biomedicals Inc.) and incubated with normal rabbit serum for 10 minutes at room temperature. These tissue sections were then incubated with the first Ab for renin (Swant) for 20 minutes at room temperature. After the sections were incubated with secondary Ab (Histofine; Nichirei Corp.) for 20 minutes at room temperature, the immunocomplexes were visualized using the strepto-avidin-biotin complex method (Nichirei Corp.). Each section was counterstained with hematoxylin (Wako Pure Chemical Industries Ltd.). As a negative control, we used preimmune mouse IgG (DAKO Corp.).
Measurements of 6-keto-PGF1 α >and creatinine contents in plasma and urine.
Plasma and urinary contents of 6-keto-PGF1α, a stable metabolite of PGI2, were measured by an EIA kit (Cayman Chemical Co.). Contents of creatinine in plasma and urine were measured by the method of Folin-Wu (described in ref. 29).
Isolation and culture of JG cells.
JG cells were separated and cultured according to the reported method (30). The kidneys were removed, decapsulated, and minced with a surgical blade. After the minced tissue was incubated with 0.1% collagenase for 15 minutes at 37°C, it was filtered through a 40-μm nylon mesh. The filtered cells were put on 15 ml of 30% isosmotic Percoll solution (Amersham Biosciences) and centrifuged at 27,000 g for 20 minutes at 4°C. The cell layer enriched with JG cells near the surface was collected and washed (31). The cells (105) were then plated onto a collagen-coated dish (60 mm) in 5 ml of culture medium: RPMI-1640 supplemented with 2% FBS, transferrin (10 μg/ml), insulin (10 μg/ml), sodium selenite (0.67 ng/ml), penicillin (50 U/ml), and streptomycin (50 μg/ml) (Invitrogen Corp.). The cells were cultured for 20 hours in a humidified atmosphere containing 5% CO2 at 37°C. After the culture medium was changed to a fresh one containing indomethacin (10 μM), various concentrations of cicaprost (Schering AG), an IP agonist, or PGE2 (Cayman Chemical Co.) were added, and the cells were incubated further for 20 hours. In some experiments, the cells were incubated with 1 μM of DI-004, AE1-259, AE-248, and AE1-329 (Ono Pharmaceutical Co.); these are the selective agonists for EP1, EP2, EP3, and EP4, respectively (32, 33). After the cell culture, total RNA was prepared, and RT-PCR analyses for renin mRNA expression were performed. For the examination of renin activity, the cells were disrupted by sonication and were centrifuged at 2,000 g for 5 minutes. Then the supernatant was used for the measurement of renin activity.
The JG cells were identified as the positive cells for renin immunoreactivity. We applied the prepared cells onto a glass slide optimized for tissue culture (Falcon; BD) and incubated them with an Ab for renin (Swant). After staining of the cells with hematoxylin, we counted a number of cells positive for renin immunoreactivity and determined the purity of the cells. The average content of the renin-positive cells was 26% of the total cells. The other 74% cells were mainly spindle-shaped and might represent fibroblastic or mesangial cells (31), although we could not identify the types of these cells.
Measurements of cAMP contents in the cultured cells.
The isolated cells rich in JG cells (2 × 104) were plated onto a 24-well culture plate in 0.5 ml of the culture medium. After 24 hours of culture, the cells were washed twice with a serum-free medium, and 0.5 ml of RPMI-1640 containing 1 mM 3-isobutyl-1-methylxanthine and 0.1% BSA was added. After preincubation for 10 minutes at 37°C, the cells were stimulated with 1 μM of cicaprost for 30 minutes. The incubations were terminated by adding 0.5 ml of 6% perchloric acid, and the cAMP contents were measured as reported (26).
Data analysis.
All data were expressed as mean plus or minus SEM. Statistical comparison of data were made with repeated two-way ANOVA followed by a Bonferroni/Dunn test for multiple comparison. Differences were considered significant if P values were less than 0.05.
Acknowledgments
We thank K. Nakaya and T. Yokoyama for help in breeding and maintenance of mice, K. Nakanishi for experimental assistance, and Y. Takashima for secretarial assistance. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and by the Research Grant for Cardiovascular Disease (14A-1) from the Ministry of Health and Welfare. This work was also supported by grants from Ono Pharmaceutical Co., the Smoking Research Foundation, and Hokkaido Heart Association.
Footnotes
See the related Commentary beginning on page 757.
Nonstandard abbreviations used: EIA, enzyme immunoassay; JG, juxtaglomerular; JGA, juxtaglomerular apparatus; 2K1C, two-kidney, one-clip; PAC, plasma aldosterone concentration; PRA, plasma renin activity; RAA, renin-angiotensin-aldosterone; sBP, systolic BP.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Pickering, T.G., and Laragh, J.H. 1991. Renovascular hypertension. In The kidney. B.M. Brenner and F.C. Rector, Jr., editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 1940–1967.
- 2.Hartner A, Goppelt-Struebe M, Hilgers KF. Coordinate expression of cyclooxygenase-2 and renin in the rat kidney in renovascular hypertension. Hypertension. 1998;31:201–205. doi: 10.1161/01.hyp.31.1.201. [DOI] [PubMed] [Google Scholar]
- 3.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am. J. Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 4.Imanishi M, Tsuji T, Nakamura S, Takamiya M. Prostaglandin I2/E2 ratios in unilateral renovascular hypertension of different severities. Hypertension. 2001;38:23–29. doi: 10.1161/01.hyp.38.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Jackson EK, Gerkens JF, Brash AR, Branch RA. Acute renal artery constriction increases renal prostaglandin I2 biosynthesis and renin release in the conscious dog. J. Pharmacol. Exp. Ther. 1982;222:410–413. [PubMed] [Google Scholar]
- 6.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol. Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz A, Wagner C. Cellular control of renin secretion. J. Exp. Biol. 1999;202:219–225. doi: 10.1242/jeb.202.3.219. [DOI] [PubMed] [Google Scholar]
- 8.Ushikubi F, Hirata M, Narumiya S. Molecular biology of prostanoid receptors; an overview. J. Lipid Mediat. Cell Signal. 1995;12:343–359. doi: 10.1016/0929-7855(95)00022-i. [DOI] [PubMed] [Google Scholar]
- 9.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 10.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am. J. Physiol. 1996;271:F659–F669. doi: 10.1152/ajprenal.1996.271.3.F659. [DOI] [PubMed] [Google Scholar]
- 11.Oida H, et al. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br. J. Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imanishi M, et al. Aspirin lowers BP in patients with renovascular hypertension. Hypertension. 1989;14:461–468. doi: 10.1161/01.hyp.14.5.461. [DOI] [PubMed] [Google Scholar]
- 13.Wang JL, Cheng HF, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers BP in a model of renovascular hypertension. Hypertension. 1999;34:96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 14.Hartner A, Cordasic N, Goppelt-Struebe M, Veelken R, Hilgers KF. Role of macula densa cyclooxygenase-2 in renovascular hypertension. Am. J. Physiol. 2003;284:F498–F502. doi: 10.1152/ajprenal.00136.2002. [DOI] [PubMed] [Google Scholar]
- 15.Mann B, et al. Acute upregulation of COX-2 by renal artery stenosis. Am. J. Physiol. 2001;280:F119–F125. doi: 10.1152/ajprenal.2001.280.1.F119. [DOI] [PubMed] [Google Scholar]
- 16.Yang T, et al. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J. Biol. Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 17.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 18.Hizaki H, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP2. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segi E, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem. Biophys. Res. Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 20.Ushikubi F, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 21.Wiesel P, Mazzolai L, Nussberger J, Pedrazzini T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension. 1997;29:1025–1030. doi: 10.1161/01.hyp.29.4.1025. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J. Clin. Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seibert K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension. 1996;28:1064–1069. doi: 10.1161/01.hyp.28.6.1064. [DOI] [PubMed] [Google Scholar]
- 25.Menard J, Catt KJ. Measurement of renin activity, concentration and substrate in rat plasma by radioimmunoassay of angiotensin I. Endocrinology. 1972;90:422–430. doi: 10.1210/endo-90-2-422. [DOI] [PubMed] [Google Scholar]
- 26.Fujino T, et al. Effects of the prostanoids on the proliferation or hypertrophy of cultured murine aortic smooth muscle cells. Br. J. Pharmacol. 2002;136:530–539. doi: 10.1038/sj.bjp.0704749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruna RD, Kurtz A, Corvol P, Pinet F. Renin mRNA quantification using polymerase chain reaction in cultured juxtaglomerular cells. Short-term effects of cAMP on renin mRNA and secretion. Circ. Res. 1993;73:639–648. doi: 10.1161/01.res.73.4.639. [DOI] [PubMed] [Google Scholar]
- 28.Yang T, et al. Renin expression in COX-2-KO mice on normal or low-salt diets. Am. J. Physiol. 2000;279:F819–F825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 29.Bonsnes RW, Taussky HH. On the colorimetric determination of creatinine by the Jaffe reaction. J. Biol. Chem. 1945;158:581–591. [Google Scholar]
- 30.Gambaryan S, et al. Endogenous or overexpressed cGMP-dependent protein kinases inhibit cAMP-dependent renin release from rat isolated perfused kidney, microdissected glomeruli, and isolated juxtaglomerular cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9003–9008. doi: 10.1073/pnas.95.15.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Della Bruna R, Pinet F, Corvol P, Kurtz A. Regulation of renin synthesis by second messengers in isolated mouse juxtaglomerular cells. Cell Physiol. Biochem. 1991;1:98–110. [Google Scholar]
- 32.Kabashima K, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest. 2002;109:883–893. doi:10.1172/JCI200214459. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzawa T, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]