Abstract
Hepatitis C virus (HCV) quasispeciation was studied in two children vertically coinfected with HCV and human immunodeficiency virus type 1 (HIV-1). HCV quasispecies diversification and liver injury were more significant in patient C1, who was immunocompetent with anti-HIV therapy, than in patient C2, who was immunosuppressed, in consistency with modulation of HCV quasispeciation and liver injury by immunocompetence in coinfected children.
To characterize the evolution of hepatitis C virus (HCV) disease and the influence of human immunodeficiency virus type 1 (HIV-1) and antiretroviral therapy (ART) (17), longitudinal sequence analysis of E2 envelope gene hypervariable region 1 (HVR1) was undertaken for two male Caucasian children (C1 and C2), who acquired HCV and HIV-1 infection by mother-to-child transmission.
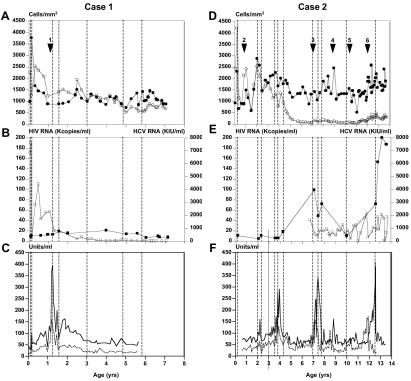
Patient C1, born in 1996, was treated from birth with zidovudine, which was complemented with lamivudine when HIV-1 cocultures became positive at 6 weeks of age. Early HIV-1 viremia correlated with a rise in CD8 cell counts, a sharp decline in CD4 counts, and an inversion of the CD4:CD8 ratio, consistent with acute HIV-1 infection (Fig. 1A and B) (3). HIV-1 viral load decreased and stabilized at the end of the first year of life, while CD4 counts remained within the normal range. At 1.16 years of age, combination ART (lamivudine-stavudine-ritonavir) was introduced, resulting in a further decline in HIV-1 viral load and a rise in CD4 counts (Fig. 1). Levels of alanine (ALT) and aspartate (AST) aminotransferases peaked 36 days later, followed by a decline over the following weeks without a change in treatment (Fig. 1C). Infection with HCV-1b (21) at 1.77 years of age was confirmed by PCR. Stored plasma samples were used retrospectively to measure HCV RNA levels, which were not influenced by ART (Fig. 1B). Between the ages of 3 and 6, HIV-1 levels declined but remained detectable, HCV viral load was stable, ALT and AST levels remained at twice normal or less, and CD4 counts declined but remained within the normal range (Fig. 1) (3). A liver biopsy performed at 5.83 years of age showed chronic hepatitis characterized by distorted lobular architecture together with mild, diffuse lobular inflammation with occasional nodular lymphoid infiltrate and prominent macro- and microvesicular steatosis. Portal tracts were expanded with mononuclear cell infiltrates, and interface hepatitis was present in the majority. Mild periportal and sinusoidal fibrosis was observed.
FIG. 1.
Clinical parameters measured in coinfected children. (A and D) CD4+ (open circles)- and CD8+ (filled circles)-T-lymphocyte counts were measured by flow cytometry. Introduction of and changes in antiretroviral therapy are indicated by arrowheads. Arrowhead 1, zidovudine-lamivudine-ritonavir; arrowhead 2, zidovudine monotherapy; arrowhead 3, zidovudine-lamivudine-saquinavir; arrowhead 4, amprenavir-didanosine-stavudine; arrowhead 5, lamivudine-stavudine-ritonavir-nelfinavir; arrowhead 6, didanosine-stavudine-lopinavir-ritonavir. (B and E) HIV-1 plasma RNA levels (open squares) were measured using the Quantiplex HIV RNA version 3.0 assay (Bayer, Pittsburgh, Pa.), with a sensitivity of 50 copies/ml; plasma HCV RNA levels (filled squares) were quantified using the COBAS Amplicor HCV Monitor assay version 2.0 (Roche Diagnostics, Montreal, Quebec, Canada), with a sensitivity of 100 IU/ml. (C and F) ALT aminotransferase (dashed lines) and AST aminotransferase (solid lines) levels were assayed on a Synchron LX20 system (Beckman Coulter, Palo Alto, Calif.). Sampling times are represented by vertical dashed lines.
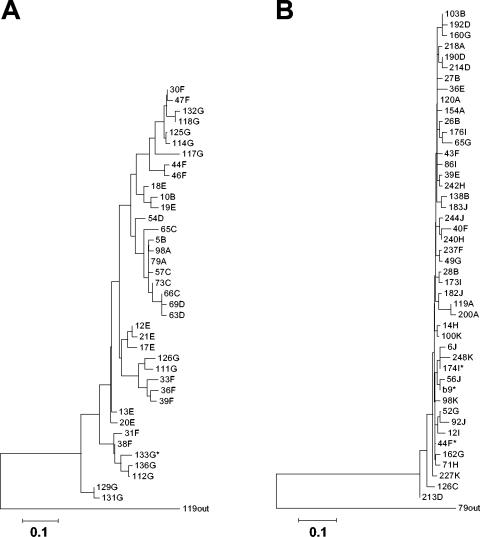
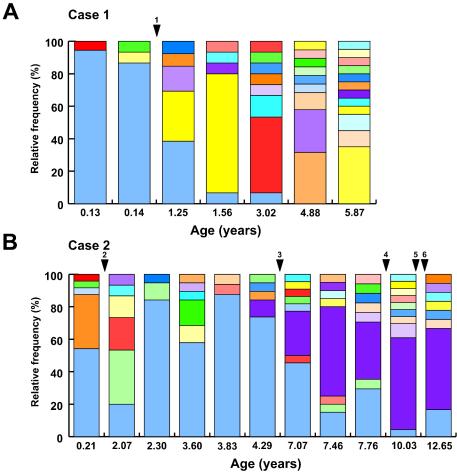
At 4.5 months later, changes in ART brought the HIV-1 viral load to <50 copies/ml and raised CD4 counts. Common clinical complications related to pediatric HIV infection were not seen. HCV RNA was extracted from plasma and was amplified using previously described primers and conditions (8, 9). Strict PCR precautions were adopted. A total of 115 independent subclones were sequenced. Multiple alignments were performed using Clustal X version 1.81 (30). Overall, accumulation of nucleotide substitutions was observed within HVR1 (nucleotides 1482 to 1562), while flanking E1 and E2 segments (1407 to 1481 and 1563 to 1670) remained stable. The mean frequencies of synonymous substitutions per synonymous site (dS) and of nonsynonymous substitutions per nonsynonymous site (dN) and the dN/dS ratio for HVR1 and flanking regions (22) were calculated using MEGA version 2.1 software (15). A dN/dS ratio of >1 reflects the occurrence of selective pressure. Within flanking regions, the mean dS was 0.013 ± 0.005 substitutions per site and the mean dN was 0.014 ± 0.005 (dN/dS ratio of 1.08). In contrast, the mean dS was 0.007 ± 0.003 within HVR1, with a mean dN of 0.183 ± 0.038 (dN/dS ratio of 26.1), consistent with strong selective pressure applied to HVR1. Phylogenetic reconstructions built using the neighbor-joining method (22) revealed a rapid increase in the complexity (number of variants) and diversity (genetic distance) of HCV quasispecies starting at 1.56 years of age, directly following control of HIV-1 replication by ART and a rise in CD4 counts (Fig. 2A). Except for variant 79A, predominant at birth, none of the initial variants was detected after initial sampling, indicative of the rapid evolution of the viral quasispecies (Fig. 3A).
FIG. 2.
Phylogenetic analysis of HCV HVR1 sequences derived from peripheral blood or liver samples obtained from coinfected children. (A) Case 1. A total of 115 independently derived HVR1 sequences (mean of 16.4 clones per time point; n = 13 to 20) were analyzed using the neighbor-joining method as described in the text. A transition/transversion ratio of 0.5 was used, and 500 bootstrap resamplings were performed. Letters correspond to the specific time point after birth at which sequences were isolated (A, 0.13 years; B, 0.14 years; C, 1.25 years; D, 1.56 years; E, 3.02 years; F, 4.88 years; G, 5.87 years). The asterisk indicates a sequence identical to that of a liver-derived variant. (B) Case 2. A total of 212 independently derived HVR1 sequences (mean of 21.2 clones per time point; n = 16 to 24) were analyzed as described above. Letters correspond to the specific time points after birth at which sequences were isolated (A, 0.21 years; B, 2.07 years; C, 2.30 years; D, 3.60 years; E, 3.83 years; F, 4.29 years; G, 7.07 years; H, 7.46 years; I, 7.76 years; J, 10.03 years; K, 12.65 years). Asterisks indicate the sequence of a liver-derived variant (b9) or sequences identical thereto. Predominant variants from the first time points were used as reciprocal outgroups in both analyses. The scale bars represent 0.1 nucleotide substitutions per site.
FIG. 3.
Longitudinal analysis of the distribution of HVR1 variants in two coinfected children. Each color corresponds to identical variants in within-group analysis but not in between-group analysis. (A) Case 1. A total of 115 independently derived clones were analyzed (mean of 16.4 clones per time point; n = 13 to 20). (B) Case 2. A total of 212 independently derived clones were analyzed (mean of 21.2 clones per time point; n = 16 to 24). Introduction of and changes in antiretroviral therapy are indicated by arrowheads. Arrowhead 1, zidovudine-lamivudine-ritonavir; arrowhead 2, zidovudine monotherapy; arrowhead 3, zidovudine-lamivudine-saquinavir; arrowhead 4, amprenavir-didanosine-stavudine; arrowhead 5, lamivudine-stavudine-ritonavir-nelfinavir; arrowhead 6, didanosine-stavudine-lopinavir-ritonavir.
HIV-1 infection was confirmed by viral coculture in patient C2 (born in 1990). CD4 counts dropped between 2 weeks and 7 months of age, indicative of moderate immunosuppression (3) (Fig. 1D). Zidovudine monotherapy was introduced, resulting in a rebound of CD4 counts which then declined to 88/mm3 by 6 years of age (Fig. 1D). Oral candidiasis was a recurrent finding, while additional drug combinations failed to control HIV-1 replication (Fig. 1E). Elevations of ALT and AST levels were observed in three instances following changes in ART (Fig. 1F). Infection with HCV-1a excluded, in retrospect erroneously, by negative serology at ages 4, 8.5, 10, and 12 years was diagnosed by PCR at age 12.5. It was determined retrospectively that C2 was HCV RNA positive from birth, consistent with mother-to-child transmission (Fig. 1E). A liver biopsy (age 12.5) showed no lobular inflammation and rare steatotic hepatocytes. Most portal tracts were normal, with the rare presence of portal inflammation, interface hepatitis, and fibrosis. One focus of micronodular formation was observed. A total of 212 independent recombinants were sequenced, and no significant accumulation of nucleotide substitutions was observed within HVR1 over 12.5 years of follow-up (mean dS of 0.042 ± 0.024; mean dN of 0.011 ± 0.003; dN/dS ratio of 0.262). A similar situation was found within flanking regions (mean dS of 0.043 ± 0.018; mean dN of 0.003 ± 0.001; dN/dS ratio of 0.0698). HVR1 sequences were tightly clustered, and no reliable phylogenetic connectedness could be inferred (Fig. 2B). In addition, one of the main variants present in patient C2 soon after birth (variant 120A) was clearly detectable in blood 12.5 years later (Fig. 3B). These results suggest an absence of immune selective pressure applied to HVR1 sequences in patient C2. Indeed, despite a high circulating viral load, C2 was repeatedly found to be HCV seronegative, likely an outcome of HIV-induced immunosuppression (5, 12).
Coinfection with HIV-1 worsens the prognosis of HCV disease (23, 27), while HCV-associated liver disease is a common cause of HIV-associated morbidity and mortality (19, 28). HCV exists in its host as a collection of variants termed quasispecies (18), whose evolution correlates with viral persistence (6, 7, 16, 34), long-term outcome of HCV disease (13), and response to interferon treatment (9, 24). Studies of HCV-infected agammaglobulinemic patients (11) and adults coinfected with HIV-1 (17, 25, 26, 31) have suggested that relative immunocompetence modulates HCV quasispecies diversity. Results obtained with patient C1, in whom constant remodeling of HCV quasispecies and accumulation of NS mutations in HVR1 were observed, support this interpretation. They also explain the stable quasispeciation profile seen in patient C2, who exhibited variant persistence, low CD4 counts, and functional immunodeficiency. While levels of quasispecies complexity at the last time point were comparable in both patients, the small genetic distances observed for patient C2 were consistent with random nondirectional drift. The higher number of infecting variants may also have contributed to overall quasispecies complexity in patient C2.
HCV can be transmitted from mother to child (29, 33, 35), but coinfection with HCV and HIV-1 in children is rare by all accounts. To our knowledge, our work provides the first description of long-term coinfection in children following mother-to-child transmission and highlights what may represent important characteristics of long-term pediatric HIV-1-HCV coinfection. (i) Diagnosis of HCV infection in coinfected children may in some cases be delayed, as HCV-specific humoral immunity is often impaired in HIV-infected subjects (5, 12). (ii) Early development of liver disease is present, typified by transient elevations of transaminases, moderate hepatic inflammation, and early fibrosis. This contrasts with the relatively mild clinical and histological pictures observed in pediatric HCV disease, with which less liver fibrosis and inflammation are observed than in adults after a similar disease duration (2, 10, 32). Differences in route of infection (prenatal versus perinatal), pathogenic properties, and variant composition of infecting HCV isolates may also have contributed to the patterns of liver disease progression (2, 32). (iii) Exacerbation of hepatic abnormalities correlates with changes in ART. As in adults (1, 14), initiation of combination ART was associated with increased CD4 counts, rapid remodeling of HCV quasispecies, and transaminase elevations for patient C1. This is consistent with the notion that immunocompetence leads to more aggressive development of HCV-associated hepatic inflammation. However, elevations of transaminase levels were also seen in patient C2 in the absence of HCV-specific humoral immunity, suggesting the possible involvement of medication toxicity (20) and/or cell-mediated immunity in HCV-associated liver disease (4).
Nucleotide sequence accession numbers
All nucleotide sequence information obtained in this study was submitted to GenBank (accession numbers AY385797 to AY386123).
Acknowledgments
This research protocol was approved by the Ethics Review Board of Sainte-Justine Hospital, where the research was conducted. We thank Silvie Valois, Martine Caty, and Ampha Khammy for expert technical assistance and Laurent Knafo for automated DNA sequencing.
This work was supported in part by the Canadian Institutes for Health Research (CIHR)/Health Canada Research Initiative on Hepatitis C (grant EOP-41537), by CANFAR, the Canadian Foundation for AIDS Research (grant 013515), and by the Réseau SIDA-maladies infectieuses of the Fonds de la recherche en santé du Québec (FRSQ). H.S. is a chercheur-boursier of the FRSQ. M.T. is the recipient of a Graduate Scholarship from the Fondation de l'Hôpital Sainte-Justine.
REFERENCES
- 1.Babik, J. M., and M. Holodniy. 2003. Impact of highly active antiretroviral therapy and immunologic status on hepatitis C virus quasispecies diversity in human immunodeficiency virus/hepatitis C virus-coinfected patients. J. Virol. 77:1940-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badizadegan, K., M. M. Jonas, M. J. Ott, S. P. Nelson, and A. R. Perez-Atayde. 1998. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology 28:1416-1423. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1994. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb. Mortal. Wkly. Rep. 43(RR-12):1-11. [Google Scholar]
- 4.Cerny, A., and F. V. Chisari. 1999. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 30:595-601. [DOI] [PubMed] [Google Scholar]
- 5.Cribier, B., D. Rey, C. Schmitt, J. M. Lang, A. Kirn, and F. Stoll-Keller. 1995. High hepatitis C viraemia and impaired antibody response in patients coinfected with HIV. AIDS 9:1131-1136. [DOI] [PubMed] [Google Scholar]
- 6.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 7.Farci, P., J. Bukh, and R. H. Purcell. 1997. The quasispecies of hepatitis C virus and the host immune response. Springer Semin. Immunopathol. 19:5-26. [DOI] [PubMed] [Google Scholar]
- 8.Farci, P., A. Shimoda, A. Coiana, G. Diaz, G. Peddis, J. C. Melpolder, A. Strazzera, D. Y. Chien, S. J. Munoz, A. Balestrieri, R. H. Purcell, and H. J. Alter. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339-344. [DOI] [PubMed] [Google Scholar]
- 9.Farci, P., R. Strazzera, H. J. Alter, S. Farci, D. Degioannis, A. Coiana, G. Peddis, F. Usai, G. Serra, L. Chessa, G. Diaz, A. Balestrieri, and R. H. Purcell. 2002. Early changes in hepatitis C viral quasispecies during interferon therapy predict the therapeutic outcome. Proc. Natl. Acad. Sci. USA 99:3081-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Monzon, C., P. Jara, M. Fernandez-Bermejo, L. Hierro, E. Frauca, C. Camarena, C. Diaz, A. De la Vega, J. Larrauri, C. Garcia-Iglesias, M. J. Borque, P. Sanz, L. Garcia-Buey, J. A. Moreno-Monteagudo, and R. Moreno-Otero. 1998. Chronic hepatitis C in children: a clinical and immunohistochemical comparative study with adult patients. Hepatology 28:1696-1701. [DOI] [PubMed] [Google Scholar]
- 11.Gaud, U., B. Langer, T. Petropoulou, H. C. Tomas, and P. Karayiannis. 2003. Changes in hypervariable region 1 of the envelope 2 glycoprotein of hepatitis C virus in children and adults with humoral immune defects. J. Med. Virol. 69:350-356. [DOI] [PubMed] [Google Scholar]
- 12.George, S. L., J. Gebhardt, D. Klinzman, M. B. Foster, K. D. Patrick, W. N. Schmidt, B. Alden, M. A. Pfaller, and J. T. Stapleton. 2002. Hepatitis C virus viremia in HIV-infected individuals with negative HCV antibody tests. J. Acquir. Immune Defic. Syndr. 31:154-162. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, J., N. Furusyo, I. Ariyama, Y. Sawayama, Y. Etoh, and S. Kashiwagi. 2000. A relationship between the evolution of hepatitis C virus variants, liver damage, and hepatocellular carcinoma in patients with hepatitis C virus viremia. J. Infect. Dis. 181:1523-1527. [DOI] [PubMed] [Google Scholar]
- 14.John, M., J. Flexman, and M. A. French. 1998. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS 12:2289-2293. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 16.Lechner, F., D. K. H. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao, Q., S. C. Ray, O. Laeyendecker, J. R. Ticehurst, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2001. Human immunodeficiency virus seroconversion and evolution of hepatitis C virus quasispecies. J. Virol. 75:3259-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martell, M., J. I. Esteban, J. Quer, J. Genesca, A. Weiner, R. Esteban, J. Guardia, and J. Gomez. 1992. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J. Virol. 66:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Carbonero, L., V. Soriano, E. Valencia, J. Garcia-Samaniego, M. Lopez, and J. Gonzalez-Lahoz. 2001. Increasing impact of chronic viral hepatitis on hospital admissions and mortality among HIV-infected patients. AIDS Res. Hum. Retrovir. 17:1467-1471. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Carbonero, L., M. Nunez, P. Rios, M. Perez-Olmeda, J. Gonzalez-Lahoz, and V. Soriano. 2002. Liver injury after beginning antiretroviral therapy in HIV/hepatitis C virus co-infected patients is not related to immune reconstitution. AIDS 16:1423-1425. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, D., B. Willems, and G. Delage. 1994. Use of the 5′ noncoding region for genotyping hepatitis C virus. J. Infect. Dis. 169:473-475. [DOI] [PubMed] [Google Scholar]
- 22.Nei, M., and T. Gojobori. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3:418-426. [DOI] [PubMed] [Google Scholar]
- 23.Papaevangelou, V., H. Pollack, G. Rochford, R. Kokka, Z. Hou, D. Chernoff, B. Hanna, K. Krasinsky, and W. Borkowsky. 1998. Increased transmission of vertical hepatitis C virus (HCV) infection to human immunodeficiency virus (HIV)-infected infants of HIV- and HCV-coinfected women. J. Infect. Dis. 178:1047-1052. [DOI] [PubMed] [Google Scholar]
- 24.Polyak, S. J., S. McArdle, S. L. Liu, D. G. Sullivan, M. Chung, W. T. Hofgartner, R. L. Carithers, B. J. McMahon, J. I. Mullins, L. Corey, and D. R. Gretch. 1998. Evolution of hepatitis C virus quasispecies in hypervariable region 1 and the putative interferon sensitivity-determining region during interferon therapy and natural infection. J. Virol. 72:4288-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roque-Afonso, A. M., M. Robain, D. Simoneau, P. Rodriguez-Mathieu, M. Gigou, L. Meyer, and E. Dussaix. 2002. Influence of CD4 cell counts on the genetic heterogeneity of hepatitis C virus in patients coinfected with human immunodeficiency virus. J. Infect. Dis. 185:728-733. [DOI] [PubMed] [Google Scholar]
- 26.Sherman, K. E., C. Andreatta, J. O'Brien, A. Gutierrez, and R. Harris. 1996. Hepatitis C in human immunodeficiency virus-coinfected patients: increased variability in the hypervariable envelope coding domain. Hepatology 23:688-694. [DOI] [PubMed] [Google Scholar]
- 27.Spengler, U., and J. K. Rockstroh. 1998. Hepatitis C in the patient with human immunodeficiency virus infection. J. Hepatol. 29:1023-1030. [DOI] [PubMed] [Google Scholar]
- 28.Tedaldi, E. M., R. K. Baker, A. C. Moorman, C. F. Alzola, J. Furhrer, R. E. McCabe, K. C. Wood, S. D. Holmberg, and HIV Outpatient Study Investigators. 2003. Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 36:363-367. [DOI] [PubMed] [Google Scholar]
- 29.Thomas, S. L., M. L. Newell, C. S. Peckham, A. E. Ades, and A. J. Hall. 1998. A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. Int. J. Epidemiol. 27:108-117. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toyoda, H., Y. Fukuda, Y. Koyama, J. Takamatsu, H. Saito, and T. Hayakawa. 1997. Effect of immunosuppression on composition of quasispecies population of hepatitis C virus in patients with chronic hepatitis C coinfected with human immunodeficiency virus. J. Hepatol. 26:975-982. [DOI] [PubMed] [Google Scholar]
- 32.Vogt, M., T. Lang, G. Frosner, C. Klingler, A. F. Sendl, A. Zeller, B. Wiebecke, B. Langer, H. Meisner, and J. Hess. 1999. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N. Engl. J. Med. 341:866-870. [DOI] [PubMed] [Google Scholar]
- 33.Weiner, A. J., M. M. Thaler, K. Crawford, K. Ching, J. Kansopon, D. Y. Chien, J. E. Hall, F. Hu, and M. Houghton. 1993. A unique, predominant hepatitis C virus variant found in an infant born to a mother with multiple variants. J. Virol. 67:4365-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiner, A. J., H. M. Geysen, C. Christopherson, J. E. Hall, T. J. Mason, G. Saracco, F. Bonino, K. Crawford, C. D. Marion, K. A. Crawford, M. Brunetto, P. J. Barr, T. Miyamura, J. McHutchinson, and M. Houghton. 1992. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA 89:3468-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weintrub, P. S., G. Veereman-Wauters, M. J. Cowan, and M. M. Thaler. 1991. Hepatitis C virus infection in infants whose mothers took street drugs intravenously. J. Pediatr. 119:869-874. [DOI] [PubMed] [Google Scholar]