Abstract
Voltage-gated Ca2+ (Cav) channels regulate a variety of biological processes, such as muscle contraction, gene expression, and neurotransmitter release. Cav channels are subject to diverse forms of regulation, including those involving the Ca2+ ions that permeate the pore. High voltage-activated Cav channels undergo Ca2+-dependent inactivation (CDI) and facilitation (CDF), which can regulate processes such as cardiac rhythm and synaptic plasticity. CDI and CDF slightly differ between Cav1 (L-type) and Cav2 (P/Q-, N-, and R-type) channels. Human embryonic kidney cells transformed with SV40 large T-antigen (HEK-293T) are advantageous for studying CDI and CDF of a particular type of Cav channel. HEK-293T cells do not express endogenous Cav channels, and Cav channels can be exogenously expressed at high levels in these cells through transient transfection. This protocol explains how to characterize and analyze Ca2+-dependent modulation of recombinant Cav channels in HEK-293T cells.
Overview
Voltage-gated Cav channels mediate Ca2+ signals that regulate cellular excitability, muscle contraction, gene expression, and hormone/neurotransmitter release. Cav channels are multi-subunit complexes that consist of a pore-forming α1 subunit and auxiliary subunits, Cavβ and Cavα2δ (Simms and Zamponi, 2014). Ten genes encode distinct α1 subunits that display distinct pharmacological and biophysical properties (Cav1.x-Cav3.x). Mutations in these genes have been associated with disorders such as epilepsy, migraine, deafness, and congenital stationary night blindness (Pietrobon, 2010, Striessnig et al., 2010).
The α1 subunit mediates Ca2+ entry, and the auxiliary subunits regulate trafficking and other properties of the channel. In addition, the Cav1 and Cav2 α1 subunits are constitutively associated with calmodulin (CaM), which is essential for Ca2+-dependent inactivation (CDI) and facilitation (CDF). The N- and C-terminal lobes of CaM each contain 2 EF-hand Ca2+ binding domains. Increases in global and local Ca2+ entry through Cav channels are sensed by the N- and C-terminal lobes of CaM, respectively, which play distinct roles in CDI of Cav1 and Cav2 channels. CDI of Cav1 channels is mediated by the C-lobe of CaM and is insensitive to strong intracellular Ca2+ buffering. In contrast, CDI of Cav2 channels is mediated by the N-lobe of CaM and is inhibited by strong intracellular Ca2+ buffering. Interestingly, CDF of Cav2.1 channels is mediated by the C-lobe of CaM, and is spared by high concentrations of intracellular EGTA. The complex regulation of Cav channels by CaM has been described in recent reviews (Christel and Lee, 2012, Ben-Johny and Yue, 2014).
It should be noted that factors other than Ca2+ can influence inactivation of Cav1 and Cav2 channels. For example, inactivation of Cav channels can be driven by a purely voltage-dependent mechanism (voltage-dependent inactivation, VDI), which is seen for IBa. “Fast” VDI occurs from the resting (closed) state (Patil et al., 1998), while “slow” VDI occurs not only from the fast-inactivated state but also the open state (Sokolov et al., 2000). Both forms of VDI are influenced by Cavβ subunits. Some Cavβ subunits, like Cavβ1b promote VDI, while the membrane-associated Cavβ, Cavβ2a, diminishes VDI (Buraei and Yang, 2010). Since CDI can be masked by strong VDI, the use of Cav channels containing Cavβ2a is a common approach in studies of CDI (Lee et al., 2000, DeMaria et al., 2001).
The α1 subunit of Cav channels is subject to alternative splicing, which increases the functional diversity of Cav channels (Lipscombe et al., 2013). Such splicing events can either heighten or dampen Ca2+-dependent regulation of Cav channels. For example, inclusion of the alternatively spliced exon 42A of Cav1.3 channels results in enhanced CDI due to a truncation of a distal C-terminal autoregulatory domain (Singh et al., 2008). In addition, alternative splicing of exon 37 in Cav2.1 alters CDF (Soong et al., 2002, Chaudhuri et al., 2005). These examples illustrate the capability of splicing events to serve as molecular switches for CDI and CDF of Cav channels.
Other regulators of CDI and CDF of Cav channels include a family of Ca2+ binding proteins (CaBPs) that are related to CaM (Haeseleer et al., 2000). Unlike CaM, CaBPs contain at least one nonfunctional EF-hand, and are almost exclusively expressed in neurons. CaBPs may compete with and/or allosterically modulate CaM interactions with Cav1 channels, which inhibits CDI. For Cav2.1 channels, one CaBP family member, CaBP1 strongly inhibits CDI and VDI, and voltage-dependent activation. The regulation of Cav channels by CaBPs has been recently reviewed (Christel and Lee, 2012, Lee et al., 2014).
The methods described below present the basic framework for characterizing CDI and CDF of recombinant Cav channels in HEK-293T cells. These cells have proven ideal for studying the molecular determinants and biophysical mechanisms underlying CDI and CDF of Cav channels. Because of the presence of CDI/CDF regulatory factors such as CaBPs and alternative splicing in neurons, additional insights into the neurophysiological significance of CDI and CDF may be gained by studying these processes in various neuronal cell-types.
MATERIALS
Detailed recipes (<R>) are listed at the end of this protocol.
Reagents
-
Cav channel cDNAs (examples shown below)
Cav2.1 (GenBank : NM023035.1) or Cav2.2 (GenBank: AF055477)
Cavβ2a (GenBank: NM053581)
α2δ1 (GenBank: NM21948.1)
pEGFP (Life Technologies)
Culture medium: DMEM (GIBCO, 11965) + 10% FBS (Atlanta Biologicals, S11150)
DMEM (GIBCO, 11965)
Extracellular recording solution <R>
FuGene 6 Transfection Reagent (Promega, E2691)
HEK-293T cells (ATCC, CRL-11268)
Internal recording solution<R>
Versene (GIBCO, 15010-066)
Equipment
Micropipettor (P1000)
Borosilicate glass capillaries (WPI, TW 150-4)
Cell Culture Dish (Corning, 35 mm × 10 mm)
Data acquisition and analysis software
Micropipette puller
Microforge
Patch-clamp electrophysiology setup including inverted fluorescence microscope, micromanipulators, amplifier, and perfusion setup
Tissue culture hood Tissue culture incubator (37°C and humidified atmosphere with 5% CO2)
METHOD
Transient transfection
-
1
Plate HEK-293T cells in 35 mm culture dishes. Cells should be maintained in DMEM+ 10% FBS (2 mL) in a tissue culture incubator at 37°C with a humidified atmosphere and 5% CO2.
-
2
When cells are ~ 70% confluent, begin transient transfection with the cDNAs corresponding to the Cav channel of interest.
-
3
About 30 minutes prior to transfection, aspirate culture medium and replace with fresh medium.
-
4
In a sterile microfuge tube (1.5 mL), combine FuGene 6 transfection reagent (6 μL) with DMEM medium (100 μL). Then gently mix.
Notes:
For every 3 μg of DNA, use 2 μL of FuGene.
Use DMEM without FBS for transfection mix.
-
5
To the same tube, add cDNAs corresponding to the α1 subunit (1.5 μg), Cavβ and α2δ (0.5 μg each), and pEGFP (50 ng). Then gently mix.
Note: pEGFP is used to help identify transfected cells via GFP fluorescence.
-
6
Let mix sit at room temperature for 15 minutes.
-
7
Next, add transfection mix to the cells in a drop wise manner covering the entire dish.
-
8
Cells should be ready for recordings after incubation at 37°C for 24–72 hours. If waiting 48 hours, replace with fresh medium after 24 hours.
-
9
To isolate cells for electrophysiological recordings, aspirate culture medium from transfected cells and replace with Versene (1 mL) for 1 minute. After 1 minute, aspirate the Versene and gently triturate the cell by pipetting up and down 10 times in culture medium (1 mL) with a P1000 micropipettor. Finally, for low-density plating, add cell suspension (1 to 2 drops) to culture dishes containing culture medium (2 mL).
Notes:
Allow cells to rest for at least 2 hours prior to recording at 37°C.
Do not use trypsin-EDTA to isolate cells, as it may proteolyze channels
Whole-cell voltage-clamp recording
-
10
Prior to recording, use a micropipette puller to produce electrodes with a resistance of 4–6 Megaohms (MΩ) in the extracellular recording solution. Polish the tips with a microforge. Electrodes can be coated with Sylgard to reduce pipette capacitance, although this is generally unnecessary.
-
11
Remove culture medium and fill culture dish with extracellular recording solution (1 mL).
-
12
After identifying a GFP-positive cell that has a spherical/boxlike shape, fill electrode with internal recording solution and begin electrophysiological recordings.
Notes:
Avoid oval and triangular shaped cells, which may yield distorted currents due to an inability to properly clamp the membrane voltage.
The internal recording contains adenosine triphosphate (ATP) which may undergo auto-hydrolysis under some conditions. Therefore, while working solutions may be maintained at room temperature, for prolonged experiments, maintain the internal recording solution on ice.
-
13
Obtain a gigaohm (GΩ) seal and change the holding voltage (Vh) to -80 mV.
-
14
Apply negative pressure to rupture the membrane to gain whole-cell access. Before running voltage protocols, reduce capacitance and series resistance electronically (60–70%).
Characterization of CDI
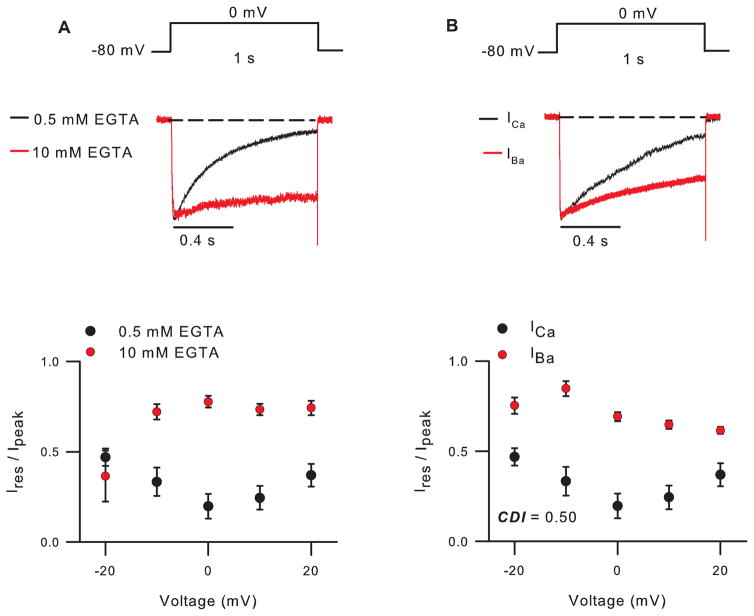
For Cav2 channels, the internal recording solution should contain a relatively low concentration of EGTA (0.5 mM), as higher concentrations inhibit CDI (Fig. 1A). By contrast, CDI of Cav1 channels can be recorded with internal recording solutions containing a higher concentration of EGTA (5 – 10 mM).
Figure 1. CDI of Cav2.2 channels.
A,B) Top, Voltage protocols and representative current traces. Currents were leak-subtracted using the P/4 method. Bottom, Ires/Ipeak represents residual current amplitude at the end of the pulse normalized to the peak current amplitude, and is plotted against test voltage. In A, internal recording solution contained 0.5 mM or 10 mM EGTA. In B, internal recording solution contained 0.5 mM and extracellular solution contained 10 mM Ca2+ (ICa ) or Ba2+ (IBa ). CDI = Ires/Ipeak for IBa - Ires/Ipeak for ICa, where Ires/Ipeak for IBa = 0.69 ± 0.02 and ICa = 0.19 ± 0.07 for test pulse to 0 mV. Currents and averaged data for ICa (n=10) and IBa (n=8) are from different cells.
-
15
Ca2+ currents (ICa) can be evoked by a long (1 second) depolarizing test pulse from Vh (-80 mV) to voltages ranging from -20 mV to +20 mV (Fig. 1A). Inactivation can be measured as the residual current amplitude at the end of the pulse normalized to the peak current amplitude (Ires/Ipeak). A U-shaped curve is observed when Ires/Ipeak is plotted against test voltages (Fig. 1), with maximal inactivation at the test voltage evoking maximal inward ICa.
-
16
With Ba2+ as the permeant ion, Cav channels undergo inactivation that is voltage-rather than Ca2+ -dependent. To measure voltage-dependent inactivation (VDI), repeat step 16 with extracellular solution containing Ba2+ instead of Ca2+ (Fig. 1B). Ba2+-containing solution can be exchanged for the Ca2+-containing solution via a gravity-driven or pressurized perfusion system. Alternatively, population averages of cells recorded in Ca2+- or Ba2+-containing solution are often used, since the differences in CDI and VDI are robust.
Note: Unlike Ca2+, Ba2+ binds poorly to calmodulin (CaM), an essential mediator of CDI.
-
17
A convenient metric for CDI is the difference between Ires/Ipeak for ICa and IBa (Fig. 1B).
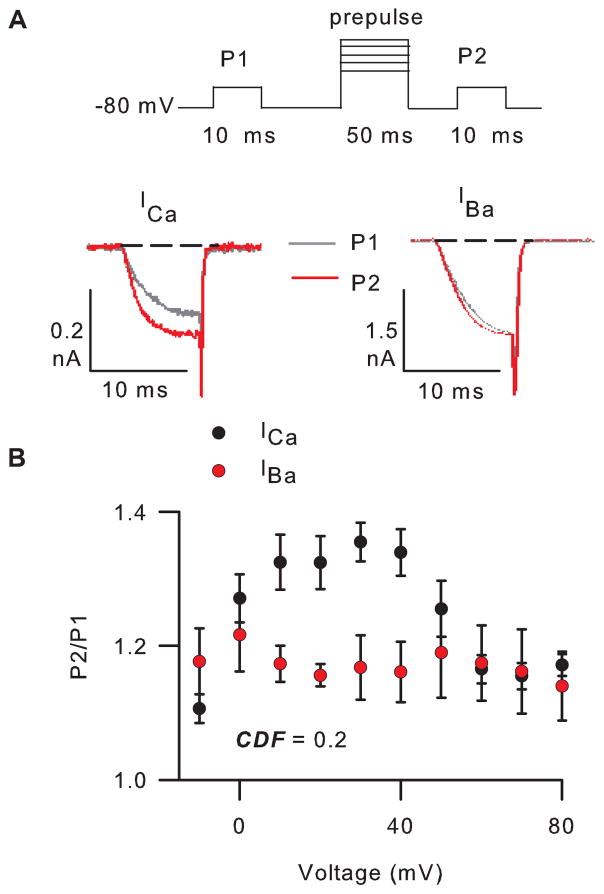
Characterization of CDF for Cav2.1 channels
-
18
To characterize CDF of Cav2.1 channels, the internal recording solution should contain a relatively high concentration of EGTA (10 mM), which will minimize CDI.
-
19
To measure CDF, a test pulse from Vh (-80 mV) to 0 mV (P1) is given 1 s before, and 5 ms after (P2), a 50-ms prepulse to various voltages (Fig. 2A).
-
20
Repeat step 21 with Ba2+ as the charge carrier (Fig. 2B).
Note: The test pulse for P1 and P2 should be set about -10 mV for IBa as compared to that used for ICa. Due to surface charge screening effects, Ba2+ causes a negative shift in the voltage-dependence of activation, which should be compensated for in channel modulation protocols.
-
21
Facilitation (F) is measured as the ratio of P2 to P1 and plotted against prepulse voltage. If P2/P1 is greater than 1, then the current is facili0ated.
-
22
A convenient metric for CDF is the difference between facilitation of ICa and IBa at the prepulse voltage eliciting maximal facilitation of ICa (Fig. 2B).
Figure 2. CDF of Cav2.1 channels.
A) Top, voltage protocol and representative ICa and IBa evoked by 10-ms test pulses to 0 mV and -10 mV, respectively, before (grey) and after (red) a 50-ms prepulse to +30 mV. Currents were leak-subtracted using the P/4 method. B) The ratio of P2/P1 current amplitudes is plotted against prepulse voltage. CDF = the difference in P2/P1 for ICa and IBa using a +30-mV prepulse, where P2/P1 for ICa =1.4 ± 0.03 (n=4) and for IBa =1.2 ± 0.05 (n=4) for a +30-mV prepulse. Currents were recorded in 10 mM extracellular Ca2+ or Ba2+ and 10 mM intracellular EGTA. Currents and averaged data for ICa and IBa are from different cells.
RECIPES
Extracellular recording solution
| Reagent | Final concentration (in mM) |
|---|---|
| CaCl2 or BaCl2 | 10 |
| Tris | 150 |
| MgCl2 | 1 |
pH to 7.3 with methanesulfonic acid. Osmolarity should be around 290–310. Filter and store at at 4 °C.
Intracellular recording solution
| Reagent | Final concentration (in mM) |
|---|---|
| EGTA | 0.5 or 10 |
| N-methyl-D-glucamine (NMDG) | 140 |
| HEPES | 10 |
| MgCl2 | 2 |
| Mg-ATP | 2 |
pH to 7.3 with methanesulfonic acid. Osmolarity should be around 290–310. Filter and store at Sterile filter and store in aliquots at -20°C.
Acknowledgments
This work was supported by grants from the NIH (DC009433, NS084190) and a Carver Research Program of Excellence Award.
References
- Ben-Johny M, Yue DT. Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J Gen Physiol. 2014;143:679–692. doi: 10.1085/jgp.201311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D, Alseikhan BA, Chang SY, Soong TW, Yue DT. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J Neurosci. 2005;25:8282–8294. doi: 10.1523/JNEUROSCI.2253-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christel C, Lee A. Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim Biophys Acta. 2012;1820:1243–1252. doi: 10.1016/j.bbagen.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria CD, Soong T, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CL, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Fakler B, Kaczmarek LK, Isom LL. More than a pore: ion channel signaling complexes. J Neurosci. 2014;34:15159–15169. doi: 10.1523/JNEUROSCI.3275-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Allen SE, Toro CP. Control of neuronal voltage-gated calcium ion channels from RNA to protein. Trends Neurosci. 2013;36:598–609. doi: 10.1016/j.tins.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. CaV2.1 channelopathies. Pflugers Arch. 2010;460:375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82:24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov S, Weiss RG, Timin EN, Hering S. Modulation of slow inactivation in class A Ca2+ channels by beta-subunits. J Physiol. 2000;527:445–454. doi: 10.1111/j.1469-7793.2000.t01-1-00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong TW, DeMaria CD, Alvania RS, Zweifel LS, Liang MC, Mittman S, Agnew WS, Yue DT. Systematic identification of splice variants in human P/Q-type channel a12.1 subunits: implications for current density and Ca2+-dependent inactivation. J Neurosci. 2002;22:10142–10152. doi: 10.1523/JNEUROSCI.22-23-10142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]