Abstract
Cerebral phaeohyphomycosis caused by Cladophialophora bantiana is a rare disease. We describe a heart and bilateral lung transplant recipient who was unsuccessfully treated for a C. bantiana brain abscess. This report compares the present case to those of other solid-organ transplant recipients with the same infection and to those of patients who did not receive transplants.
CASE REPORT
A 41-year-old woman who had heart and bilateral lung transplants in November 1997 secondary to sarcoidosis presented in August 2003 with a 3-day history of right facial numbness. She had a cough with green sputum for several weeks. She also had a 1-week history of right upper molar tooth pain, right earache, blurry vision, and right frontotemporal headache. She had no significant history of exposure to soil (gardening, landscaping, etc.). Her immunosuppressant regimen consisted of mycophenolate mofetil (750 mg per os [p.o.] every 12 h), tacrolimus (8 mg p.o. every 12 h), and prednisone (20 mg p.o. daily). Her immunosuppressant regimen was never increased because of rejection. Prior to presentation, she was stable over the 6-year posttransplant period. She recently completed a course of imipenem/cilastatin for Pseudomonas aeruginosa pneumonia. On admission her temperature was 99.7°F. Her lung exam was significant for diffuse rhonchi. On neurological exam, she had numbness to light touch on the right side of her face, maximum diplopia upon rightward gaze, decreased hearing on the right, and a positive (upgoing) Babinski on the right. Her chest radiograph showed right middle lobe consolidation, and her brain magnetic resonance image (MRI) showed a ring-enhancing right cerebellar lesion (Fig. 1). She was started on piperacillin/tazobactam, pyrimethamine, sulfadiazine, and dexamethasone before any culture results were available. On hospital day 3 she was taken to the operating room for a right craniotomy, evacuation, and partial decompression of a grossly purulent abscess. The Gram stain of the abscess showed few white blood cells and no organisms. The bacterial culture was sterile. Toxoplasma immunoglobulin G antibody was negative, and the pyrimethamine and sulfadiazine treatments were stopped. After the surgery the patient still had facial paresthesias, diplopia, and green sputum production but now also had dizziness. Eight days after the surgery a darkly pigmented mold was isolated from the brain abscess (Fig. 2). It was identified in our laboratory as Cladophialophora bantiana, because velvety gray colonies grew at 25, 37, and 42°C and a lactophenol stain of the slide culture showed oval conidia in long, sparsely branched, wavy chains (Fig. 3). This identification was confirmed by the Centers for Disease Control and Prevention (Atlanta, Ga.). Liposomal amphotericin B (5 mg/kg of body weight given intravenously daily) was begun on hospital day 11, and the next day voriconazole (300 mg p.o. every 12 h) and flucytosine (1.5 g p.o. every 6 h) were added. Her platelet count decreased from 187,000 to 44,000 cells/μl the following day, and the flucytosine treatment was stopped. Repeat brain MRI showed a progression of the ring-enhancing lesion into the pons and also the appearance of new bilateral cerebral lesions. She received a platelet transfusion and had a repeat craniotomy and evacuation of the abscess on hospital day 15. Again a purulent collection was removed, and C. bantiana grew from culture along with Mycobacterium chelonae, which was also isolated from sputum prior to this admission. A pathological examination of this sample showed septate branching hyphae and oval conidia on Gomori methenamine silver stain (Fig. 4). The liposomal amphotericin B was then increased to 10 mg/kg given intravenously daily. C. bantiana was also identified from sputum culture. On hospital day 20 the patient became critically ill, and she died on hospital day 45.
FIG. 1.
Coronal T1-weighted MRI of the brain, post-gadolinium administration, demonstrating a necrotic right cerebellar hemisphere lesion.
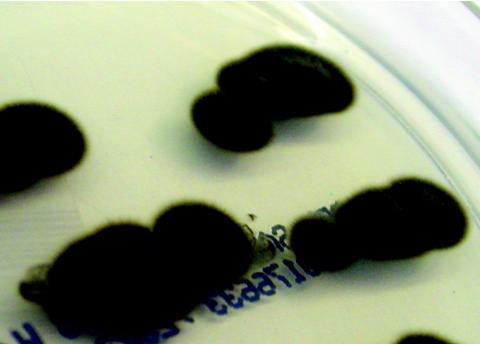
FIG. 2.
The velvety textured olivaceous-colored colonies of C. bantiana.
FIG. 3.
Lactophenochol stain of slide culture of brain abscess (original magnification, ×400).
FIG. 4.
Gomori methenamine silver stain of cerebellar tissue showing fungal elements (original magnification, ×630).
MIC, minimum lethal concentration (MLC), and synergy evaluations of various antifungal agents for C. bantiana isolated from this patient were obtained through the Fungus Testing Laboratory (San Antonio, Tex.). The MIC of amphotericin was 0.5 μg/ml at 48 h and 1.0 μg/ml at 72 h, which is considered moderately resistant in vitro; the flucytosine MIC was ≤0.125 μg/ml at 72 and 96 h; itraconazole, voriconazole, and posaconazole MICs were ≤0.015 μg/ml at 72 and 96 h; and the caspofungin MIC was 0.5 μg/ml at 48 and 72 h. The MLC of amphotericin B was >16 μg/ml at 72 and 96 h.
Synergy studies demonstrated MICs of ≤0.015 μg/ml for amphotericin B and ≤0.125 μg/ml for flucytosine at 48 and 72 h. Synergy levels were also observed at ≤0.03 μg/ml for caspofungin and ≤0.015 μg/ml for voriconazole at 48 and 72 h. Therefore, in vitro, these combinations appear indifferent. MLCs of amphotericin B and flucytosine were >2 μg/ml and >32 μg/ml, respectively, at 72 h. Therefore, no fungicidal activity was observed for these combinations at the highest concentrations tested. Specimens were evaluated for MICs and MLCs after the patient's death, and these results were not available at the time treatment decisions were made.
Phaeohyphomycosis (from the Greek word “phaeo,” meaning “dark”) is the infection caused by dematiaceous (dark-walled) molds. Dematiaceous fungi contain melanin pigments in their cell walls and spores. Although infections caused by dematiaceous fungi are rare, they are increasingly being recognized as causes of human disease (15). Their spectrum of disease includes soft-tissue infection (chromoblastomycosis), mycetoma, sinusitis, and brain abscesses. One particular species of dematiaceous fungus, C. bantiana, has a predilection for brain tissue. This species has had several name changes. It is now generally preferred that the genus and species name Cladophialophora bantiana be used to describe this organism, which is an important etiologic agent of cerebral phaeohyphomycosis. The older names Cladosporium trichoides, Cladosporium bantianum, Xylohypha bantiana, and Xylohypha emmonsii are synonyms or are related species.
In culture, C. bantiana grows slowly; colonies are mature within 15 days at 25 to 30°C. In gross terms, the colonies appear to have a velvety texture and are olive gray to black when viewed from the front and black when viewed from the back. Microscopically, the hyphae are brown and septate with smooth oval conidia that do not display dark scars of attachment, as has been described for other Cladosporium spp. The conidia form long, sparsely branched, wavy chains (17).
Transplant recipients.
There have been few reports of dematiaceous fungal infections in organ transplant recipients (19), and there are even fewer reports of cerebral phaeohyphomycosis in solid-organ transplant recipients. Brain abscesses in solid-organ transplant patients due to C. bantiana (or its synonyms) were identified in the literature by using a MEDLINE search for the years 1966 to the present. Keywords such as phaeohyphomycosis, dematiaceous fungi, Cladophialophora, Cladosporium, and Xylohypha were used. Seven such cases were identified (Table 1) (1, 2, 4, 9, 11, 16, 18) and, along with the present case, are compared with respect to epidemiology, immunosuppression, clinical features, therapy, and outcome.
TABLE 1.
Summary of data on cases of C. bantiana brain abscesses in solid-organ transplant recipients
| Reference | Age (sex)a | Transplanted organ | Abscess site | Treatment regimen | Outcome |
|---|---|---|---|---|---|
| 1 | 50 (M) | Liver | Temporoparietal lobe | Amphotericin B deoxycholateb | Died |
| 4 | 35 (M) | Kidney | Multiple, right parietal lobe | Amphotericin B deoxycholate | Unknown |
| 16 | 36 (F) | Kidney | Multiple, right hemisphere | Liposomal amphotericin B, flucytosine, itraconazole | Survived |
| 2 | 51 (M) | Kidney | Parietal lobe | Liposomal amphotericin B | Survived |
| 11 | 30 (F) | Heart | Cingulate gyrus | Amphotericin B lipid complex, changed to liposomal amphotericin B, changed to itraconazole | Died |
| 9 | 57 (M) | Heart | Cerebellum | Liposomal amphotericin B, flucytosine, changed to itraconazole | Died |
| 18 | 61 (M) | Kidney | Temporoparietal lobe | Fluconazole, then amphotericin B deoxycholate added after recurrence | Died |
| Present report | 41 (F) | Heart, bilateral lung | Cerebellum | Liposomal amphotericin B, voriconazole | Died |
M, male; F, female.
Treatment for left subphrenic abscess culture which grew Candida albicans.
Eight cases of Cladophialophora brain abscesses occurred in solid-organ transplant recipients: two heart recipients, four kidney recipients, one liver recipient, and, in the present case, a heart and bilateral lung recipient. Five were men and three were women. The mean age was 41, and the time to onset of fungal infection from the time of original transplant ranged from 9 to 120 months, with the majority occurring before 20 months. In only two patients was soil exposure history elicited; one worked as a florist.
Six of the seven patients whose immunosuppression regimen was reported had prednisone or prednisolone as a part of the regimen. The glucocorticoid was combined with azathioprine and cyclosporine for three patients, with azathioprine and tacrolimus for one patient, with tacrolimus and mycophenolate mofetil for one patient, and with azathioprine alone for one patient. The seventh patient was taking methotrexate, tacrolimus, and mycophenolate mofetil but no glucocorticoid.
Presenting symptoms in four of the eight patients included headaches. Two of these four patients also had focal neurological deficits, one with left arm and leg weakness and one with facial paresthesias. The presenting symptoms of the other four patients were hemiparesis, seizures, ataxia, and arm pain and numbness.
Seven of the eight patients had the diagnosis made during life, and all had the organism cultured from intracranial contents. In five cases, neurosurgery was part of the therapeutic plan (four craniotomies and one excisional biopsy). In the other two cases, the only procedure was a diagnostic stereotactic brain biopsy. All but one patient were initially treated with some formulation of amphotericin. Two patients received amphotericin B deoxycholate, and one patient received liposomal amphotericin as his or her sole antifungal therapy. Three patients who received liposomal amphotericin and one who received amphotericin B lipid complex (and subsequently liposomal amphotericin) also received itraconazole or voriconazole with or without flucytosine. Mortality was 71% in those patients whose outcome was reported (five of seven patients).
Phaeohyphomycosis in patients who did not receive a transplant.
There have been case reports and reviews of cerebral phaeohyphomycosis in patients not receiving transplants, with or without any apparent underlying medical conditions (5, 6, 14, 20).
A comprehensive review of central nervous system (CNS) phaeohyphomycosis was recently published (14). Forty-eight of the 101 cases were caused by C. bantiana. The average age of the patients who had not received transplants was 35, and the mortality of the same population was 71%. Several of the patients who had not received transplants had risk factors for infection, such as glucocorticoid treatment (4), diabetes mellitus, lymphoma with neutropenia, eye trauma, and intravenous drug use.
In 1989, Dixon et al. (3) reviewed culture-documented human C. bantiana infections, 26 of which involved the CNS. The mean age of subjects with CNS disease was 30 years. Of the 12 subjects who had occupational histories recorded, 9 were associated with traumatic or inhalation exposure to soil. The majority of the subjects did not have any physiological factor predisposing them to infection. However, some subjects were taking medications which could possibly predispose them to infections. These included antibiotics, corticosteroids, and azathioprine. Additional associated conditions causing possible predisposition to infection included chronic purulent otitis, interstitial pulmonary fibrosis, alcohol abuse, traumatic scalp lacerations, and penetrating ocular injury. Four subjects had other infections suggestive of defective cell-mediated immunity or neutrophil dysfunction: Nocardia spp. (3) and prior facial phaeohyphomycosis with Alternaria spp. Presenting symptoms included headache, usually for longer than 1 week's duration, hemiparesis, or focal deficits, including seizures, cranial nerve deficits, or corticospinal and cerebellar deficits. Patients were treated with surgical resection and/or antifungal therapy consisting of amphotericin B deoxycholate and flucytosine, either alone or in combination. The survival of those patients whose diagnosis was made before autopsy was 45% (3).
Discussion of findings.
In comparing the epidemiology, clinical features, therapy, and outcome of patients with C. bantiana brain abscesses in the general population to those of patients with prior solid-organ transplantation, the only apparent difference was the much higher rate of administration of immunosuppressant medications in the transplant group. Most cases seem to occur in the first year following transplant, the same time frame as that seen with other transplant-related infections (cytomegalovirus, Pneumocystis jiroveci pneumonia) associated with reduced CD4+ cell numbers or function. This infection is uniformly difficult to treat, with a high mortality. It is hard to establish an incidence for this disease because it is not reportable and seems to occur sporadically and rarely. It is possible, though unlikely, that mild cases of cerebral phaeohyphomycosis are missed or cured with empirical antifungal therapy.
The site of fungal entry is not well understood for solid-organ transplant recipients with cerebral phaeohyphomycosis. Hematogenous dissemination from a pulmonary source is one possibility. In the present series, two patients (including the case patient) had C. bantiana isolated from lung specimens, and an additional patient had a skin lesion from which C. bantiana was identified (which might represent a site of entry or another metastatic lesion). In three of the seven patients, multiple brain abscesses were seen, adding more evidence to support a hematogenous source of the abscesses. Local spread from the sinuses or ears could also account for brain abscesses. However, the location of abscesses in the five patients who initially presented with single brain abscesses, namely, parietal, temporoparietal, cingulate gyrus, and cerebellum (2), makes this mechanism of contiguous spread unlikely.
Treatment of established infection has been problematic, and the role of surgical evacuation versus antifungal therapy alone is unknown. Although the optimal antifungal therapy is not known, some authors recommend combining amphotericin B with flucytosine and either itraconazole or voriconazole (12). One of the two patients who survived in the present series received a combination of liposomal amphotericin B, flucytosine, and itraconazole (16). In vitro data suggest that itraconazole, voriconazole, and amphotericin B have activity against C. bantiana, with the azoles having lower MICs than amphotericin (7, 8, 13). The azole and flucytosine MICs for our isolate also were lower than those of amphotericin. However, it is difficult to apply this in vitro data to decisions regarding the treatment of human infection. The National Committee for Clinical Laboratory Standards (NCCLS) published standards for antifungal susceptibility testing of filamentous fungi (10). Although most data regarding amphotericin relates to Aspergillus spp., treatment failures have occurred with MICs above 2 μg/ml. Little data exists regarding flucytosine, azole, and echinocandin MICs for dematiaceous fungi. The C. bantiana isolate described in this case report appeared to be moderately resistant to amphotericin and sensitive to flucytosine and the azoles tested, and they were not synergistic in vitro. It is possible that one contributing reason for our patient's poor outcome is that part of the antifungal regimen (amphotericin) was ineffective for this strain of C. bantiana. The role of corticosteroids and the short delay of therapy in the outcome of these infections is also unknown. Once an effective treatment strategy can be established, the next step should be early laboratory detection of this fungus. Empirical therapy specific for dematiaceous fungi is not feasible, because such infections are uncommon even in medical centers with a great deal of experience with immune-suppressed patients. Presently, treatment is individualized after the diagnosis is made by culture, but susceptibility testing has not been completely evaluated and correlated with clinical outcome in animals or humans. If a consistent in vitro assay of susceptibility can be developed and correlated with outcome, it would be helpful to evaluate all new antifungals (and possibly combinations of available agents) for potential activity against this rare but very serious fungus.
Acknowledgments
We thank Mary Brandt from the Centers for Disease Control and Prevention for confirming the species, and we also thank the mycology staff at Temple University Hospital.
REFERENCES
- 1.Aldape, K. D., H. S. Fox, J. P. Roberts, N. L. Ascher, J. R. Lake, and H. A. Rowley. 1991. Cladosporium trichoides cerebral phaeohyphomycosis in a liver transplant recipient. Report of a case. Am. J. Clin. Pathol. 95:499-502. [DOI] [PubMed] [Google Scholar]
- 2.Arunkumar, M. J., V. Rajshekhar, M. J. Chandy, P. P. Thomas, and C. K. Jacob. 2000. Management and outcome of brain abscess in renal transplant recipients. Postgrad. Med. J. 76:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon, D. M., T. J. Walsh, W. G. Merz, and M. R. McGinnis. 1989. Infections due to Xylohypha bantiana (Cladosporium trichoides). Rev. Infect. Dis. 11:515-525. [DOI] [PubMed] [Google Scholar]
- 4.Gupta, S. K., K. S. Manjunath-Prasad, B. S. Sharma, V. K. Khosla, V. K. Kak, M. Minz, and V. K. Sakhuja. 1997. Brain abscess in renal transplant recipients: report of three cases. Surg. Neurol. 48:284-287. [DOI] [PubMed] [Google Scholar]
- 5.Henry, C., E. Song, A. Kellen, F. Raal, S. Miller, and V. Davis. 1989. Cerebral phaeohyphomycosis caused by Xylohypha bantiana. Eur. J. Clin. Microbiol. Infect. Dis. 8:984-988. [DOI] [PubMed] [Google Scholar]
- 6.Horre, R., and G. de Hoog. 1999. Primary cerebral infections by melanized fungi: a review. Studies Mycol. 43:176-193. [Google Scholar]
- 7.Johnson, E. M., A. Szekely, and D. W. Warnock. 1999. In vitro activity of Syn-2869, a novel triazole agent, against emerging and less common mold pathogens. Antimicrob. Agents Chemother. 43:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 9.Keyser, A., F. X. Schmid, H. J. Linde, J. Merk, and D. Birnbaum. 2002. Disseminated Cladophialophora bantiana infection in a heart transplant recipient. J. Heart Lung Transplant. 21:503-505. [DOI] [PubMed] [Google Scholar]
- 10.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. NCCLS, Wayne, Pa.
- 11.Olayemi, O. O., L. M. Dowdry, S. M. Mallon, and T. Cleary. 2001. Cerebral phaeohyphomycosis due to a novel species: report of a case and review of the literature. Transplantation 71:1343-1346. [DOI] [PubMed] [Google Scholar]
- 12.Perfect, J. R. 2002. Treatment of fungal infections. Infect. Dis. Special Ed. 5:55-60. [Google Scholar]
- 13.Radford, S. A., E. M. Johnson, and D. W. Warnock. 1997. In vitro studies of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob. Agents Chemother. 41:841-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revankar, S. G., D. A. Sutton, and M. G. Rinaldi. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:208-216. [DOI] [PubMed] [Google Scholar]
- 15.Rossman, S. N., P. L. Cernoch, and J. R. Davis. 1996. Dematiaceous fungi are an increasing cause of human disease. Clin. Infect. Dis. 22:73-80. [DOI] [PubMed] [Google Scholar]
- 16.Salama, A. D., T. Rogers, G. M. Lord, R. I. Lechler, and P. D. Mason. 1997. Multiple Cladosporium brain abscesses in a renal transplant patient: aggressive management improves outcome. Transplantation 63:160-162. [DOI] [PubMed] [Google Scholar]
- 17.Schell, W. A., I. F. Salkin, and M. R. McGinnis. 2003. Bipolaris, Exophiala, Schedosporium, Sporothrix, and other dematiaceous fungi, p. 1820-1847. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. R. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 18.Silveira, E. R., M. A. Resende, V. S. Mariano, W. A. Coura, L. D. Alkmim, L. B. Vianna, C. E. Starling, G. G. Cruz, L. H. A. Benicio, A. M. Paula, J. A. Gomes, G. D. Santos, M. A. M. Macedo, R. E. Salum, M. Gontijo, A. L. Rabello, and R. B. Caligiorne. 2003. Brain abscess caused by Cladophialophora (Xylohypha) bantiana in a renal transplant patient. Transplant. Infect. Dis. 5:104-107. [DOI] [PubMed] [Google Scholar]
- 19.Singh, N., F. Y. Chang, T. Gayowski, and I. R. Marino. 1997. Infections due to dematiaceous fungi in organ transplant recipients: case report and review. Clin. Infect. Dis. 24:369-374. [DOI] [PubMed] [Google Scholar]
- 20.Walz, R., M. Bianchin, M. L. Chaves, M. R. Cerski, L. C. Severo, and A. T. Londero. 1997. Cerebral phaeohyphomycosis caused by Cladophialophora bantiana in a Brazilian drug abuser. J. Med. Vet. Mycol. 35:427-431. [PubMed] [Google Scholar]