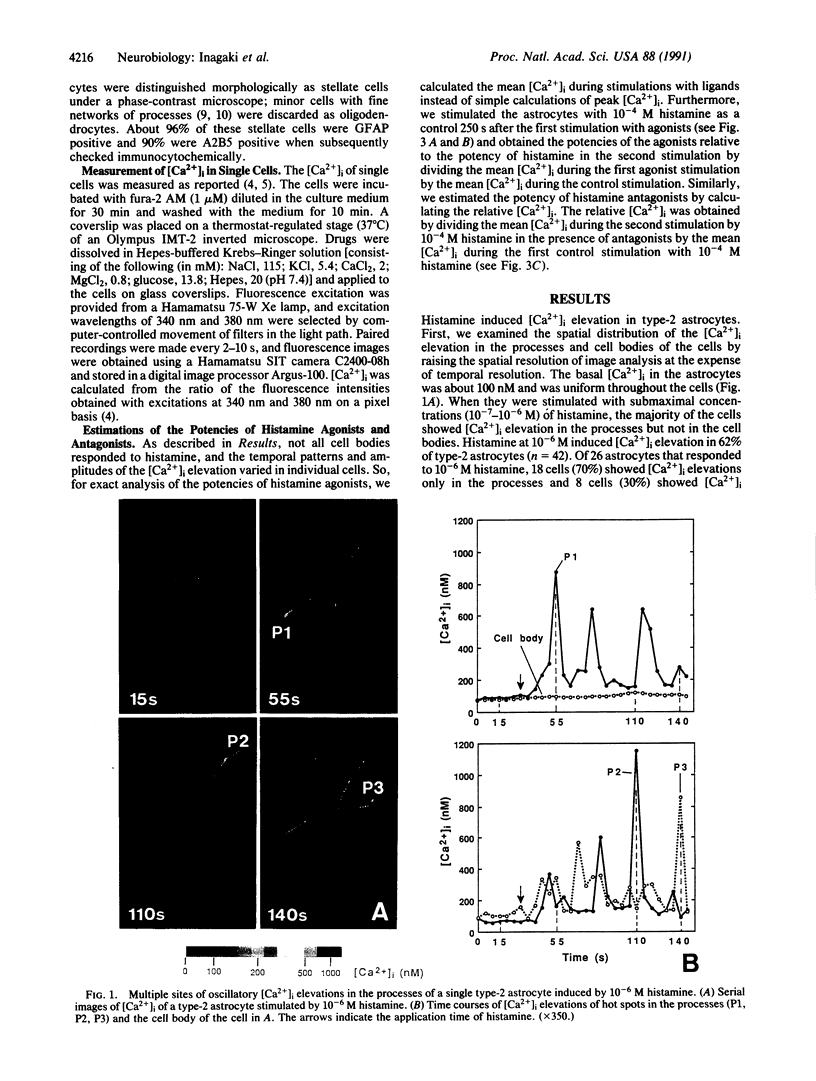
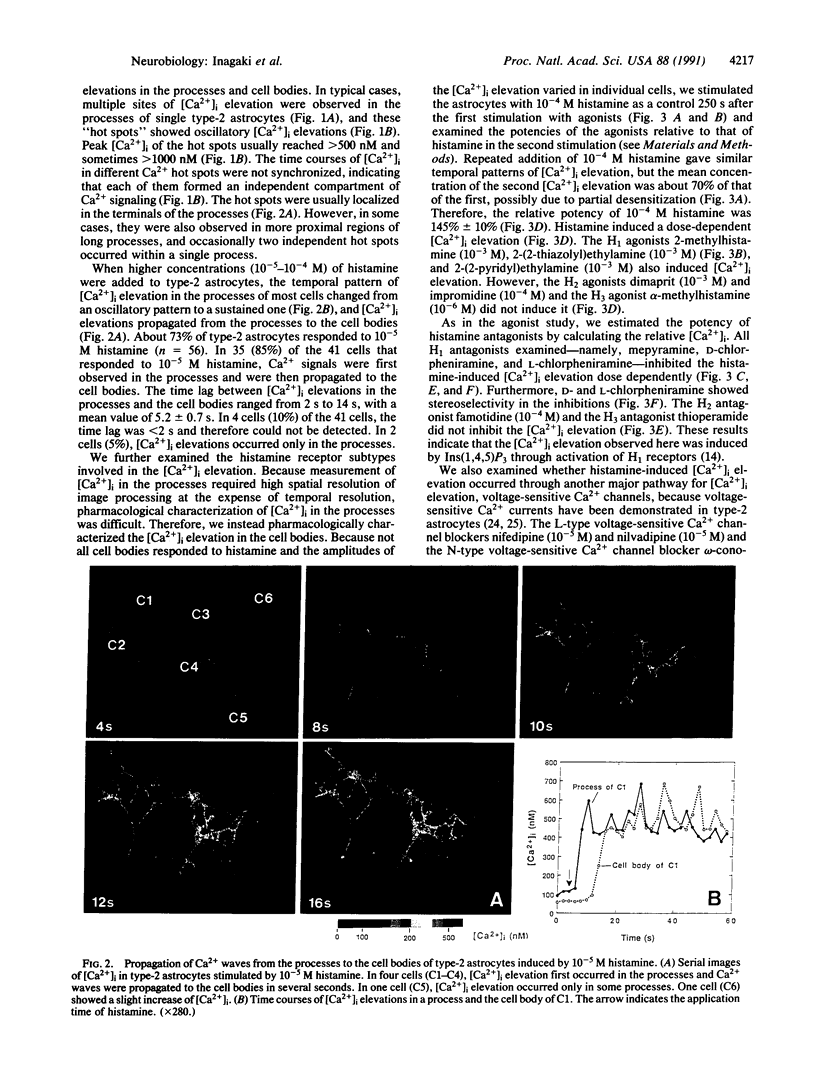
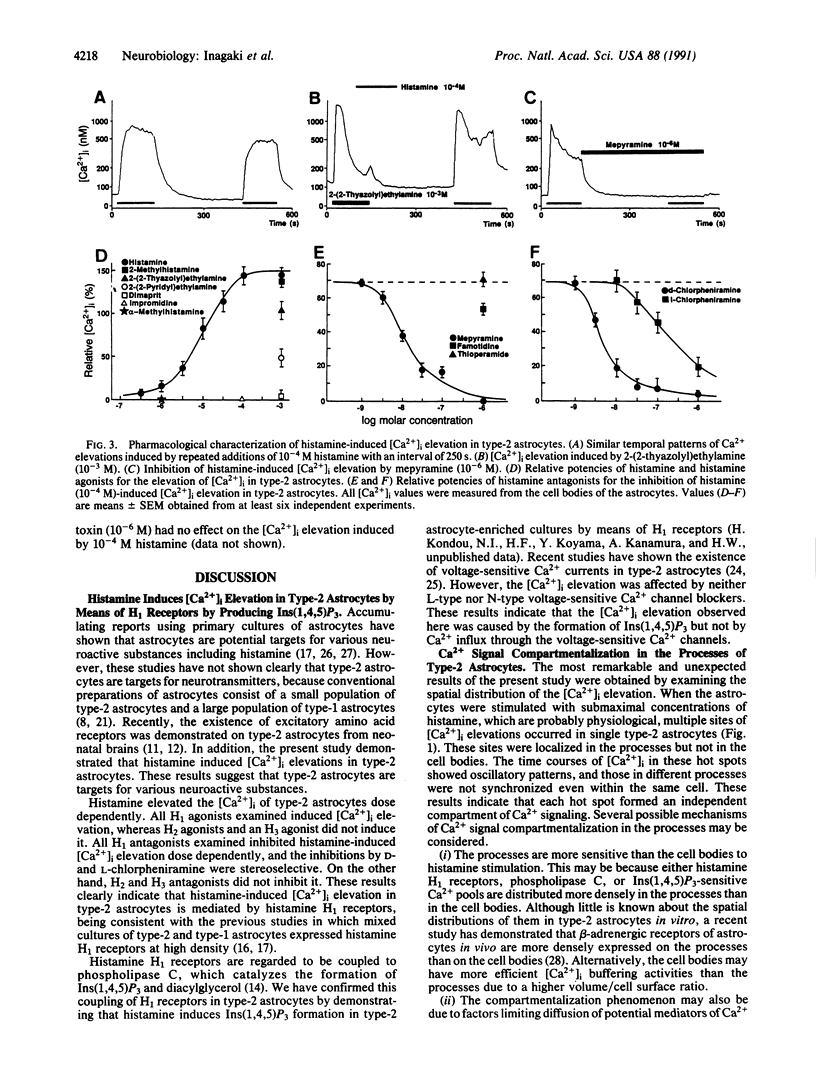
Abstract
Intracellular Ca2+ plays an important role in signal transduction as a second messenger. In various types of cells, inositol 1,4,5-trisphosphate-induced elevations of intracellular free Ca2+ concentration ([Ca2+]i) have been reported to be uniform in single cells or originate at discrete sites from which they then propagate throughout the cells. These observations so far imply that a single cell functions as a minimal unit for inositol 1,4,5-trisphosphate-induced Ca2+ signaling. In this study, we examined the effects of histamine on [Ca2+]i of type-2 astrocytes using fura-2-based digital imaging fluorescence microscopy and found an unusual type of Ca2+ signaling in these cells. Histamine induced [Ca2+]i elevation in type-2 astrocytes by means of histamine H1 receptors. Submaximal concentrations of histamine (10(-7)-10(-6) M) evoked multiple sites of oscillatory [Ca2+]i elevation in single type-2 astrocytes. These Ca2+ "hot spots" were localized in the processes of the astrocytes but not in the cell bodies. The time courses of [Ca2+]i oscillations in different hot spots were not synchronized, indicating that each of them formed an independent compartment of Ca2+ signaling. When higher concentrations (10(-5)-10(-4) M) of histamine were added, [Ca2+]i in the processes remained elevated at high levels and [Ca2+]i elevations propagated from the processes to the cell bodies. These results suggest that individual processes of type-2 astrocytes can form minimal units for Ca2+ signaling in response to submaximal concentrations of histamine and that single type-2 astrocytes may function as multiple units for Ca2+ signaling.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloisi F., Agresti C., Levi G. Establishment, characterization, and evolution of cultures enriched in type-2 astrocytes. J Neurosci Res. 1988 Oct-Dec;21(2-4):188–198. doi: 10.1002/jnr.490210211. [DOI] [PubMed] [Google Scholar]
- Arbonés L., Picatoste F., García A. Histamine H1-receptors mediate phosphoinositide hydrolysis in astrocyte-enriched primary cultures. Brain Res. 1988 May 31;450(1-2):144–152. doi: 10.1016/0006-8993(88)91554-5. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Brew H., Gray P. T., Mobbs P., Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986 Dec 4;324(6096):466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Giovannini C., Suergiu R., Levi G. Expression of excitatory amino acid receptors by cerebellar cells of the type-2 astrocyte cell lineage. J Neurochem. 1989 Jan;52(1):1–9. doi: 10.1111/j.1471-4159.1989.tb10890.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansson E. Astroglia from defined brain regions as studied with primary cultures. Prog Neurobiol. 1988;30(5):369–397. doi: 10.1016/0301-0082(88)90008-1. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Takagi H., Takeda N., Kubota Y., Tohyama M., Watanabe T., Wada H. Fine structure of histaminergic neurons in the caudal magnocellular nucleus of the rat as demonstrated by immunocytochemistry using histidine decarboxylase as a marker. J Comp Neurol. 1984 Oct 20;229(2):233–241. doi: 10.1002/cne.902290208. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Hamberger A. Glial cell function: uptake of transmitter substances. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Fukui H., Taguchi Y., Wang N. P., Yamatodani A., Wada H. Characterization of histamine H1-receptors on astrocytes in primary culture: [3H]mepyramine binding studies. Eur J Pharmacol. 1989 Nov 28;173(1):43–51. doi: 10.1016/0014-2999(89)90007-1. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Yamatodani A., Ando-Yamamoto M., Tohyama M., Watanabe T., Wada H. Organization of histaminergic fibers in the rat brain. J Comp Neurol. 1988 Jul 15;273(3):283–300. doi: 10.1002/cne.902730302. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K. Occurrence and functional significance of serotonin and catecholamine uptake by astrocytes. Biochem Pharmacol. 1986 Jul 15;35(14):2273–2281. doi: 10.1016/0006-2952(86)90451-x. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Ffrench-Constant C., Raff M. C. The macroglial cells of the rat optic nerve. Annu Rev Neurosci. 1989;12:517–534. doi: 10.1146/annurev.ne.12.030189.002505. [DOI] [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prell G. D., Green J. P. Histamine as a neuroregulator. Annu Rev Neurosci. 1986;9:209–254. doi: 10.1146/annurev.ne.09.030186.001233. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Takemura H., Hughes A. R., Horstman D. A., Thastrup O. How do inositol phosphates regulate calcium signaling? FASEB J. 1989 Jun;3(8):1899–1905. doi: 10.1096/fasebj.3.8.2542110. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Williams B. P., Miller R. H. The in vitro differentiation of a bipotential glial progenitor cell. EMBO J. 1984 Aug;3(8):1857–1864. doi: 10.1002/j.1460-2075.1984.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Jacob R. Calcium oscillations in non-excitable cells. Trends Neurosci. 1989 Feb;12(2):43–46. doi: 10.1016/0166-2236(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol. 1986 Dec;103(6 Pt 1):2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M., Evêquoz-Mercier V., Perrottet P., Buchner E. Honeybee retinal glial cells transform glucose and supply the neurons with metabolic substrate. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8727–8731. doi: 10.1073/pnas.85.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Walz W., Hertz L. Functional interactions between neurons and astrocytes. II. Potassium homeostasis at the cellular level. Prog Neurobiol. 1983;20(1-2):133–183. doi: 10.1016/0301-0082(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Taguchi Y., Shiosaka S., Tanaka J., Kubota H., Terano Y., Tohyama M., Wada H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984 Mar 12;295(1):13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Levi G., Johnstone S. R., Riddle P. N. Cerebellar astroglial cells in primary culture: expression of different morphological appearances and different ability to take up [3H]D-aspartate and [3H]GABA. Brain Res. 1983 Nov;312(2):265–277. doi: 10.1016/0165-3806(83)90143-8. [DOI] [PubMed] [Google Scholar]