Abstract
Clinical differences in histoplasmosis between North America and Brazil prompted investigation of experimental infection with representative strains. Mortality was higher with Latin American strains, and lung pathology showed large necrotizing granuloma with prominent neutrophilic infiltration. Chronic disease was unique to the North American strain.
Skin lesions were more common in cases of histoplasmosis from Brazil than in those from the United States, suggesting that genetic differences between isolates may affect clinical outcome (5). Latin American isolates were equally divided between Latin American classes 5 and 6. In contrast, most cases of histoplasmosis in the United States were caused by yps-3 nuclear gene class 2 strains (7). Lung involvement and mortality were more common in the Brazilian cases, as well. These observations suggested that North American and Latin American strains of Histoplasma capsulatum differ pathogenically (6). We now compare the course of infection using representative North American and Latin American isolates of H. capsulatum.
The North American class 2 strain was obtained from an immunosuppressed patient with disseminated histoplasmosis (2). Latin American strains were from patients with AIDS from Sao Paulo, Brazil (5). The intratracheal infection model with B6C3F1 mice used an inoculum of 104 yeast cells for survival, 103 yeast cells for fungal burden and pathology, and 50 yeast cells for chronic infection, as described previously (2, 4). Briefly, the inoculum, prepared freshly from frozen yeast and containing single cells or doublets, was introduced intratracheally. Fungal burden was determined on homogenized tissue by plating on brain heart infusion agar containing 10% sheep blood. Colony counts were determined after incubation for 10 days at 30°C. One-way analysis of variance was performed on the ranks of quantitative cultures. Pairwise comparisons were adjusted by using Dunnett's multiple comparison procedure, and survival times were compared using a Wilcoxon test.
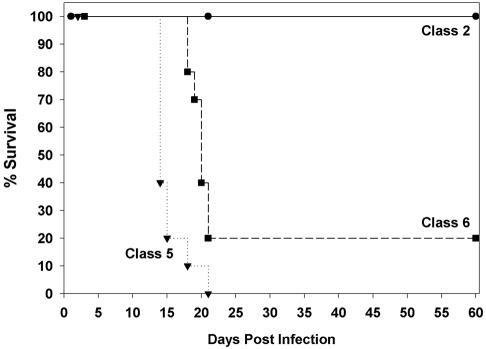
By day 21 of infection, all 10 mice inoculated with the Latin American class 5 isolate died, with no deaths occurring among the North American class 2 controls (Fig. 1). Mice infected with the Latin American class 6 strain also died more rapidly than those infected with the North American strain (P = 0.001).
FIG. 1.
Survival following infection with 104 yeast cells (n = 10 mice/group).
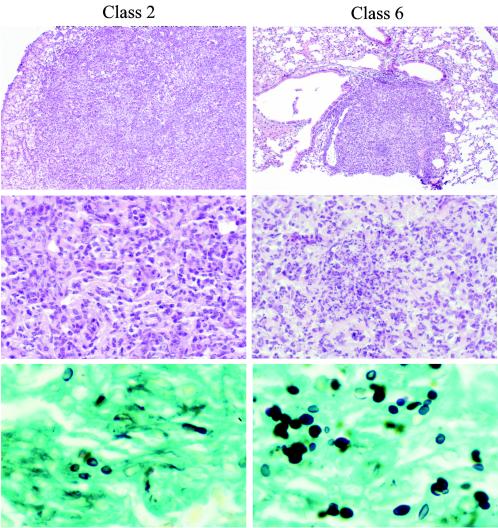
At day 14 of infection, nodules were seen on the surfaces of the lungs of mice infected with both Latin American strains but not with the North American isolate. Microscopically the nodules represented necrotizing granuloma (Fig. 2). The degrees of peribronchial and perivascular lymphoid infiltrates and fibroblast proliferation and the numbers of intra-alveolar macrophages and granulocytes were greater with the Latin American strains, and necrosis was prominent. Lung consolidation, however, was more prominent with the North American strain, but necrosis was not observed. Giant cells were not observed with either strain. Spleens in the mice infected with the North American and Latin American class 6 strains contained nonnecrotizing granuloma, which was less prominent with the Latin American class 5 strain. Staining characteristics of the yeast, including sizes between 2 and 4 μm, were similar between the strains.
FIG. 2.
Hematoxylin and eosin staining of lung tissue. Chronic inflammation was characteristic of class 2 infection (top panel), consisting of macrophages, lymphocytes, fibroblasts, and rare granulocytes but no necrosis (middle panel). H. capsulatum yeast forms were scattered diffusely in the Gomori methenamine silver stain (bottom panel). Lungs of mice infected with class 6 showed immature granuloma (top panel) with prominent early caseation necrosis and numerous macrophages and neutrophils (middle panel), and GMS stain shows yeast forms confined focally within granuloma (bottom panel). Magnifications of top, middle, and bottom panels, ×21.75, × 108.75, and ×435, respectively (original magnifications, ×25, ×125, and ×500, respectively).
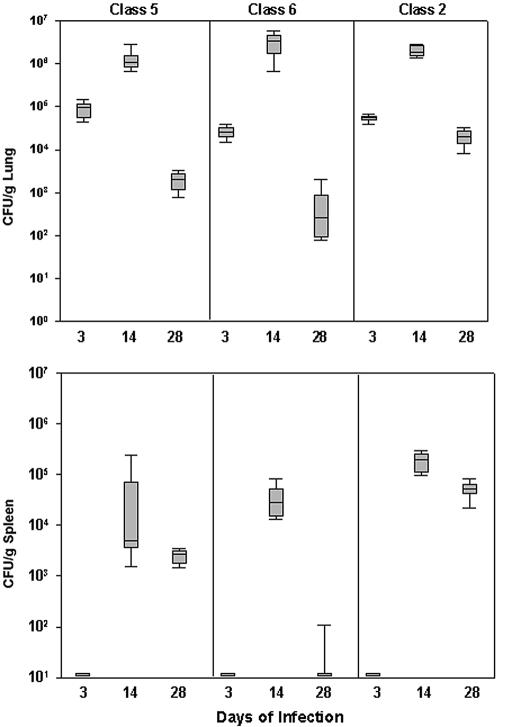
The highest fungal burden occurred at day 14 of infection and was similar among the groups (Fig. 3). Fungal clearance was slowest with the North American strain, and at day 28, the fungal burden in lung and spleen was higher in that group (P < 0.001).
FIG. 3.
Fungal burden following infection with 103 yeast cells. Each box represents the 25th and 75th percentiles, and the bars represent the 10th and 90th percentiles. Blocks at the bottom of the graph without bars represent groups where there were no positive cultures.
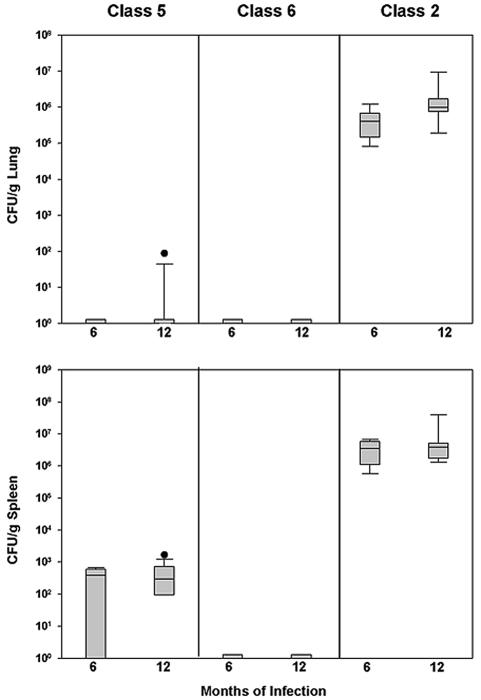
Chronic disease following infection with 50 yeast cells (4) was unique to the North American strain. Four of ten mice infected with the North American strain died between the 6th and 12th months, and the fungal burden remained high in the six survivors (Fig. 4). With the Latin American class 5 strain, cultures of lungs at 6 months were negative in all eight animals, while cultures of spleen were positive in five of eight. At 12 months, cultures of lung were negative in 9 of 10 mice, but organisms were present in spleen at a low colony count in eight (median 2.99 × 102 CFU/gm). Cultures of the lungs and spleen were uniformly negative at months 6 and 12 in all mice infected with the Latin American class 6 strain.
FIG. 4.
Fungal burden following infection with 50 yeast cells. Each box represents the 25th and 75th percentiles, and the bars represent the 10th and 90th percentiles. Blocks at the bottom of the graph without bars represent groups where there were no positive cultures. Circles denote outliers outside the 90th percentile. Differences in both tissues were statistically significant (P < 0.001).
These findings suggest that the course of infection differed between the Latin American and North American strains of H. capsulatum. Mortality was higher with the Latin American strains, while fungal burden was similar to that with the North American strain. Grossly visible pulmonary nodules were unique to infection with the Latin American strains, and a neutrophilic infiltrate was prominent microscopically. Increased mortality at comparable fungal burden and prominent neutrophilic infiltration suggest that the inflammatory response may have contributed to the poor outcome.
Members of our group have previously reported that mice infected with our North American strain of H. capsulatum developed chronic disseminated histoplasmosis (4). Here fungal clearance was greatest and chronic disease was not observed with the Latin American strains. Low-level persistence in the spleen was noted with class 5 infection.
Knowledge of strain-related differences in histoplasmosis remains incomplete. Variation in mortality was reported with different North American strains, but the comparison was not made in the same experiment and the inoculum was not administered intratracheally (1, 3). Whether our observations represent species- or strain-specific characteristics requires investigation, however. If our findings are confirmed, studies of additional strains, including the conidium form of the organism, should be considered.
Acknowledgments
This work was supported by the Department of Veterans' Affairs. E. Keath is a Burroughs Wellcome Scholar in Pathogenic Mycology, and Katia Alves is a Fogarty Fellow (grant no. D43-TW00003).
We also thank Takao Kasuga for critical review of the manuscript and Blair Wheat for editorial assistance. We acknowledge L. Joseph Wheat's guidance in the conduct of the project and assistance in preparation of the manuscript.
Experimental protocols approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee were followed in the conduct of animal studies presented in this report.
REFERENCES
- 1.Anderson, K. L., and S. Marcus. 1969. Variability in virulence of strains of Histoplasma capsulatum. Am. Rev. Respir. Dis. 99:608-609. [DOI] [PubMed] [Google Scholar]
- 2.Connolly, P., J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 1999. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 43:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drouhet, E., and J. Schwarz. 1956. Comparative studies with 18 strains of Histoplasma. J. Lab. Clin. Med. 47:129-139. [PubMed] [Google Scholar]
- 4.Durkin, M., S. Kohler, C. Schnizlein-Bick, A. LeMonte, P. Connolly, J. S. Goldberg, T. Garringer, and L. J. Wheat. 2001. Chronic infection and reactivation in a pulmonary challenge model of histoplasmosis. J. Infect. Dis. 183:1822-1824. [DOI] [PubMed] [Google Scholar]
- 5.Karimi, K., L. J. Wheat, P. Connolly, G. Cloud, R. Hajjeh, E. Wheat, K. Alves, C. D. Lacaz, and E. Keath. 2002. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. J. Infect. Dis. 186:1655-1660. [DOI] [PubMed] [Google Scholar]
- 6.Kasuga, T., T. J. White, G. Koenig, J. McEwen, A. Restrepo, E. Castaneda, L. C. Da Silva, E. M. Heins-Vaccari, R. S. De Freitas, R. M. Zancope-Oliveira, Z. Qin, R. Negroni, D. A. Carter, Y. Mikami, M. Tamura, M. L. Taylor, G. F. Miller, N. Poonwan, and J. W. Taylor. 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol. Ecol. 12:3383-3401. [DOI] [PubMed] [Google Scholar]
- 7.Keath, E. J., G. S. Kobayashi, and G. Medoff. 1992. Typing of Histoplasma capsulatum by restriction fragment length polymorphisms in a nuclear gene. J. Clin. Microbiol. 30:2104-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]