Abstract
We tested 240 patients with Plasmodium falciparum monoinfection for persistent parasite antigenemia after successful standardized antimalarial therapy by using the ICT Malaria Pf/Pv and OptiMAL-IT assays that detect the malaria antigens Plasmodium falciparum histidine-rich protein 2 (HRP2) and parasite lactate dehydrogenase (pLDH), respectively, as well as a panmalarial antigen (PMA). The patients were screened for antigenemia on days 0, 3, 7, and 14 of follow-up. On day 0, all 240 patients showed positive reactivity with both assays. Of the 229 cases with negative parasitemia on day 3, persistent antigenemia was observed in 207 (90.4%) of the cases: 188 (82.1%) for HRP2 antigen and 75 (32.8%) for PMA. There was a gradual decrease in antigenemia on follow-up to day 14; however, the drop in reactivity to PMA was less than that for HRP2 antigen. In contrast to HRP2 antigenemia, there was a significant decrease in pLDH antigenemia to 38.4% and to 14.8% (PMA) on day 3 (P < 0.03). The pLDH antigenemia level dropped further to 14.8% on day 7. There was no significant association of persistent antigenemia with gametocytemia. One case with gametocytemia was negative for both the antigens. In conclusion, the OptiMAL-IT assay is more sensitive than the ICT Malaria Pf/Pv test for monitoring therapeutic responses after antimalarial therapy since the LDH activity ceases when the malarial parasite dies.
Clinical diagnosis of malaria still relies upon identification of malaria parasites in Giemsa-stained blood smears of the peripheral blood. Recently, rapid diagnostic tests for the detection of Plasmodium falciparum infection has been introduced to overcome the problem of time constraints and low sensitivity in diagnosing malaria infections with a low level of parasitemia by microscopy. These rapid diagnostic tests are the immunochromatographic tests (ICT) based on the detection of antigen(s) released from the parasitized red blood cells. In the case of P. falciparum infection, these new rapid methods are based on detection of the histidine-rich protein 2 (HRP2; e.g., the ICT Malaria Pf, ParaSight-F, and ICT Malaria Pf/Pv tests) (1-6, 11, 20) or parasite lactate dehydrogenase (pLDH; e.g., OptiMAL-IT) (7-9, 11, 18-20). The sensitivities and specificities of each of these tests have been assessed in a range of clinical situations (1, 2, 5, 7, 11, 19), although the overall sensitivity and specificity of all of these assays to detect P. falciparum infection is high (>90%). However, the sensitivity of these assays decreases to <70% in parasitemia <50/μl. Further, these assays may remain positive due to persistence of P. falciparum HRP2 antigenemia after antimalarial therapy. This may result in a false-positive (FP) diagnosis of infection and thus may reduce the usefulness of the test in predicting treatment failure (4, 11, 12, 19). FP reactions have been reported in individuals with a history of recent fever and antimalarial treatment due to persistent circulation of HRP2 for up to 2 weeks after clearance of parasites or in patients who had circulating rheumatoid factors (9, 10, 13, 20).
The ICT Malaria Pf/Pv test is based on the use of HRP2 antigen to detect P. falciparum infection and a panmalarial antigen to detect Plasmodium vivax infection (7-9). In the present study, we evaluated the performance of the ICT Malaria Pf/Pv and OptiMAL-IT assays to detect persistent antigenemia after antimalarial therapy by using ICT assays in Punjab and Kuwait.
MATERIALS AND METHODS
Participants.
The present study was an extension of an earlier study conducted in Kuwait to evaluate the performance of the ICT Malaria Pf/Pv and OptiMAL-IT assays in detecting malaria infection in symptomatic patients (7). In the present study, we examined the persistence of parasite antigens in blood after the disappearance of asexual-stage parasitemia following antimalarial therapy. The study was conducted during the period September 1999 to August 2001. A total of 240 patients were admitted in the hospital with a microscopic diagnosis of P. falciparum monoinfection. The ICT was done on all enrolled patients to have baseline data on the rapid diagnostic test pattern.
The majority of malaria patients enrolled for the study were young adults from areas of malaria endemicity of Punjab reporting at the Health Centers and immigrants reporting at the District Health Centers in Kuwait (7). The mean age of patients was 21 years (range, 9 to 46 years) from both sexes, and the majority of them were from Southeast Asian countries. The majority of the patients had a history of malarial infections. The patients were treated either with a standardized supervised 3-day regimen of oral chloroquine (10 mg of base/kg of body weight on days 1 and 2 and 5 mg/kg on day 3) or quinine (500 mg every 8 h for 3 or 7 days) if they were infected in regions where the chloroquine-resistant strains were documented.
For each patient, a finger prick was made, and 50 μl of blood was collected in a preheparinized Eppendorf tube for ICT Malaria Pf/Pv test and OptiMAL-IT assay and thick/thin blood smear for Giemsa-microscopy. All specimens were coded, and the microscopists and the ICT readers were blinded to each other and to the outcome of chemotherapy.
Clinical evaluation, parasitemia, and ICT antigen testing were performed on days 0, 3, 7, and 14. Informed consent to participate in the study was obtained from participants and the Ethical Committee of the local Health Division approved the study.
Microscopy of Giemsa-stained blood films.
Thick and thin blood films were stained with 10% Giemsa stain for 10 min and examined by two experienced microscopists who had no knowledge of patient disease status or nationality to avoid any bias in blood film readings. Asexual- and sexual-stage parasite densities were counted per 200 leukocytes and were then expressed in microliters, assuming a leukocyte count of 8,000/μl (7, 14, 15). Thick films were considered negative if no parasites were seen in at least 100 consecutive oil immersion fields.
ICT Malaria Pf/Pv test.
This is a rapid, in vitro immunodiagnostic test for the detection of circulating P. falciparum HRP2 and an antigen common to all four species of malaria, panmalarial antigen (PMA), in whole blood. The test uses two specific monoclonal antibodies that have been immobilized across the test strip. The test was performed according to the manufacturer's instructions (Now ICT MALARIA; Binax, Inc., Portland, Maine). Briefly, 15 μl of whole blood was added to a sample pad impregnated with colloidal gold-labeled antibodies to the malarial antigens. The blood sample was allowed to run up the full length of the strip after the addition of buffer A. The result was read after 10 min. The test was valid only if the control line was observed. The results were interpreted as follows: P. falciparum infection was indicated by a line next to the P. falciparum HRP2 antigen mark; P. falciparum infection or a mixed infection was indicated by a line next to the HRP2 antigen and the PMA marks; a mixed infection of all three or P. vivax, P. ovale, or P. malariae was indicated by a line next to the PMA mark only. A limitation of this test is that it cannot speciate P. falciparum mixed infections.
OptiMAL-IT assay.
The OptiMAL-IT assay is based on the detection of intact and functional pLDH specific for P. falciparum and pan-pLDH (PMA), an antigen that is common to all four species of malaria, in whole blood. The test uses two monoclonal antibodies that have been immobilized across the test strip. The assay was performed according to the manufacturer's instructions (DiaMed AG, Cressier, Switzerland). Briefly, 1 drop of whole blood was mixed with 2 drops of lysis buffer A, which disrupts the red blood cells and releases pLDH, and the specimens were allowed to migrate to the top of the strip. After 8 min, the strips was placed in washing buffer B that cleared the hemoglobin from the strip. In the pLDH assay there are two diagnostic zones of reaction containing monoclonal anti-pLDH and PMA. The anti-pLDH antibody that recognizes only P. falciparum is present in the bottom reaction zone, and the PMA is present immediately above this zone. A third reaction zone containing a pan-specific monoclonal antibody is present at the top of the test strip and serves as a positive control for the assay.
Statistical analysis.
Data were collected and analyzed by using the SPSS statistical program. ICT antigen test results were considered FP if they were positive for HRP2, pLDH, or PMA but negative for asexual-stage parasites microscopically with or without gametocytes on days 0, 3, 7, and 14 after therapy. The percent ratio of persistent antigen reactivity was expressed as a proportion of the total FP cases, i.e., antigen positive but microscopy negative, and the total patients, i.e., 229 admitted with P. falciparum monoinfection, for each antigen tested on follow-up.
RESULTS
A total of 240 patients with a microscopic diagnosis of P. falciparum monoinfection were admitted during the study period. The patients were treated with a standardized supervised 3-day regimen of oral chloroquine (10 mg of base/kg [body weight] on days 1 and 2 and 5 mg/kg on day 3) or quinine (500 mg every 8 h for 3 or 7 days). The patients were monitored by clinical review, microscopy, and ICT assays on days 0, 3, 7, and 14.
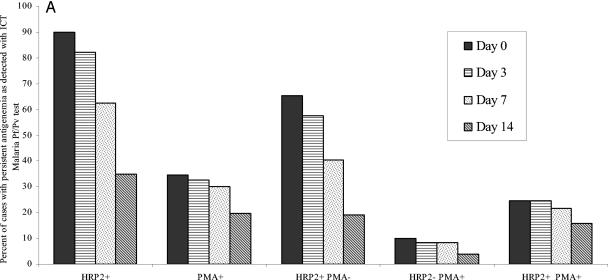
All 240 patients were screened for antigenemia with the ICT Malaria Pf/Pv assay at the time of admission (day 0). All 240 patients showed positive reactivity with the ICT assay; 216 (90%) showed reactivity for HRP2 antigen, and 83 (34.6%) reacted positively for PMA. The majority of patients 157 (65.4%) were positive for HRP2 antigen only (Fig. 1A). On day 3 after antimalarial therapy, 11 of the 240 patients were positive for asexual-stage peripheral parasitemia on microscopy and thus were not followed up further for persistent antigenemia. Of the 229 cases with negative parasitemia on day 3, persistent antigenemia was documented in 207 (90.4%) of the cases; 188 (82.1%) for HRP2 and 75 (32.8%) for PMA. There was a gradual decrease in the number of cases that reacted for both and/or either of the antigens on follow-up to day 14; however, the drop in reactivity to PMA was less than that for HRP2 antigen. The persistent antigenemia was observed in 62.4% (HRP2) and 30.1% (PMA) on day 7; the level of antigenemia dropped further to 34.9 and 19.7%, respectively, on day 14 (Fig. 1A). The difference in the percent decrease in HRP2 and PMA reactivity after therapy was significant (31% versus 13% on day 7 [P < 0.04] and 60% versus 43% on day 14 [P < 0.05], respectively). The proportionate reactivity for both antigens on follow-up is shown in Fig. 1A.
FIG. 1.
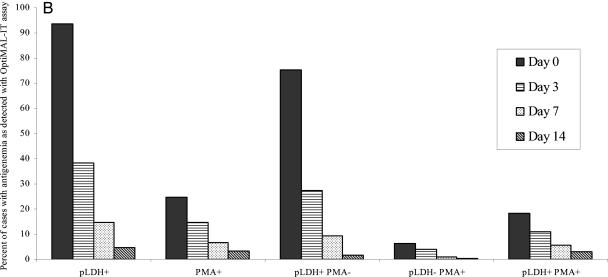
Percentage of cases with antigenemia as detected by ICT Malaria Pf/Pv assay (A) and OptiMAL-IT assay (B) on days 0, 3, 7, and 14 after antimalarial therapy. The cases were negative for asexual-stage parasites by microscopy of Giemsa-stained blood films. The ratio was expressed as the number of cases with persistent antigenemia for each antigen to the total number of cases (i.e., 229) negative for asexual-stage parasitemia.
The persistent antigen reactivity with the ICT Malaria Pf/Pv test was further evaluated by detecting pLDH and PMA reactivity with the OptiMAL-IT assay. On admission, all 240 patients were determined to be positive by the OptiMAL-IT assay; 225 (93.8%) of the patients reacted for pLDH, and 59 (24.6%) of the patients reacted for PMA. Most of the cases (181 [75.4%]) were positive for pLDH only (Fig. 1B). There was a significant decrease (P < 0.03) in antigenemia to 38.4% (pLDH) and 14.8% (PMA) on day 3. The antigenemia dropped further to 14.8% (pLDH) and 6.6% (PMA) on day 7 (Fig. 1B). The proportionate reactivity for both the antigens showed that the drop in pLDH+ PMA− reactivity was significantly higher than that for pLDH− PMA+ activity on day 3 (64% versus 38%, respectively [P < 0.03]) (Fig. 1B).
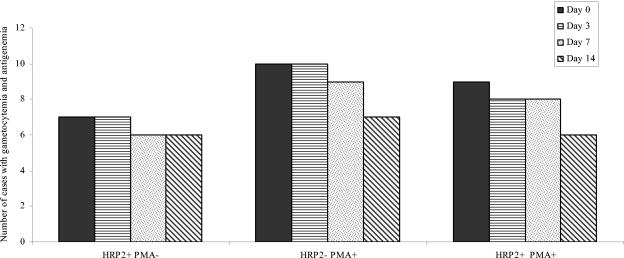
In order to investigate the relationship between gametocytes and antigen persistence after treatment, all cases with gametocytes were also enrolled irrespective of their antigen reactivity status. A total of 27 of 229 (12%) cases were determined to be positive for gametocytes only by microscopy. The ICT Malaria Pf/Pv assay detected antigenemia in 16 (59.3%) cases (HRP2) and in 19 (70.1%) cases (PMA) on day 0 (Fig. 2). One case with gametocytemia was negative for both the antigens. There was no significant drop in antigenemia on follow-up to day 14 (Fig. 2). Very few cases with gametocytes showed reactivity for pLDH (five cases) and PMA (two cases) when tested with the OptiMAL-IT assay on day 0. On day 7 only one case showed reactivity for pLDH.
FIG. 2.
Relationship between gametocytemia and HRP2/PMA antigenemia. The cases were negative for asexual-stage parasites and positive for gametocytes by microscopy. The data present the numbers of cases with gametocytemia for each antigen on days 0, 3, 7, and 14 after antimalarial treatment.
Of the 240 patients admitted, 21 were given quinine as they acquired the infection in areas (Gujrat, India, and Thailand) where chloroquine resistance was well documented. Of these 21 patients, 4 tested positive for asexual-stage parasites on microscopy on day 3 after therapy and thus were excluded from further analysis of persistent antigenemia. The status of antigen persistence in chloroquine versus quinine-treated patients could not be compared statistically since only 17 quinine-treated patients were monitored up to day 14.
DISCUSSION
In the present study we screened 240 patients with P. falciparum monoinfection for persistent antigenemia after antimalarial therapy to day 14. Clinical evaluation, microscopy, and ICT antigen testing were performed on days 3, 7, and 14 after antimalarial chemotherapy. All 240 patients showed positive reactivity with both ICT assays. The ICT Malaria Pf/Pv assay detected antigenemia in 90% of cases (HRP2) and 34.6% of cases (PMA). The reactivity to both or either of the antigens dropped gradually on follow-up to day 14. The drop in reactivity to PMA was less evident than that for HRP2 antigen. However, in contrast to persistent HRP2 antigenemia, the OptiMAL-IT assay detected a significant and sharp drop in antigenemia (pLDH) after antimalarial therapy.
HRP2 antigen has been shown to persist and is detectable after clinical symptoms of malaria have disappeared and the parasites have apparently been cleared from the circulation (11, 17). Humar et al. detected circulating HRP2 antigen in 68% of treated malaria patients on day 7 and in 27% of treated malaria patients on day 28 (6). Tjitra et al. documented persistent FP antigenemia after antimalarial therapy in 29% (HRP2) and 42% (PMA) of patients on day 7 and in 10% (HRP2) and 23% (PMA) of patients on day 14 (16). However, in the present study we observed persistent antigenemia in 62.4% of cases (HRP2) and in 30.1% (PMA) of cases on day 7; the antigenemia levels dropped to 34.9% (HRP2) and 19.7% (PMA) on day 14. We observed more cases with persistent HRP2 antigenemia than reported earlier (6, 16, 17). We also documented here the proportionate persistent antigen reactivity for HRP2 and/or PMA. We documented antigenemia in a significantly higher number of patients with parasitemia 65.4% (HRP2) and 10% (PMA). Furthermore, the drop in reactivity to PMA was less evident than that for HRP2 antigen on follow-up to day 14.
The causes of persistent antigenemia after malaria therapy are unclear. Potential causes include persistent viable asexual-stage parasitemia below the detection limit of microscopy, delayed clearance of circulating antigen (free or in antigen-antibody complex) (16), rheumatoid factor (13), and detection of circulating sexual stages in convalescence (4, 16). Furthermore, the action of antimalarial treatment on the parasites may also influence the persistence of HRP2. Eisen and Saul studied posttreatment response in P. falciparum malaria patients admitted to the Royal Brisbane Hospital (Australia) and found that HRP2 levels never disappeared during the study period despite clearance of visible parasites (3). Karbwang et al. also detected persistent HRP2 antigen during and after artemether therapy, acknowledging that the HRP2 signal was of no value during the first week of treatment but appeared to be a precise indicator of treatment failure under field conditions, when it was detected on day 14 posttreatment (10). However, we detected persistent HRP2 antigenemia in 80 (34.9%) of the cases on day 14 posttreatment.
Compared to the ICT Malaria Pf/Pv test, the OptiMAL-IT assay detected significantly fewer cases with persistent antigenemia on days 3, 7, and 14. On day 3 after antimalarial therapy, antigenemia dropped significantly from 93.8% (pLDH) and 24.6% (PMA) to 38.4% (pLDH) and 14.8% (PMA) (P < 0.02). The antigenemia level dropped further to 15.7% (pLDH) and 5.2% (PMA) on day 7. The OptiMAL-IT assay does not detect “antigen” per se but detects intact and functional parasite-specific LDH (pLDH) (14, 16, 19). Thus, the level of pLDH declines in parallel with the clearance of asexual parasitemia, and it has been suggested that this lack of antigen persistence after treatment may make this test useful in predicting treatment failure.
The persistent HRP2 antigenemia has also been suggested to be associated with gametocytemia (16, 17). We detected persistent antigenemia in 15 of the 27 (55.6%, HRP2) cases and in 19 (70.1%, PMA) patients with gametocytemia on day 3 after antimalarial therapy. The antigenemia in most of these cases persisted by day 14. It is not clear to what extent HRP2 antigen is found in gametocytes. Recently, the HRP2 mRNA transcript and protein have both been demonstrated in late-stage gametocytes (17). A study conducted in India found a high frequency of persistent HRP2 antigen after treatment with sulfadoxine-pyrimethamine (42% on day 7), with half of these FP HRP2 results being gametocytogenic (13). Tjitra et al. found a strong association of persistent HRP2 antigenemia and PMA reactivity with gametocytemia (16). They concluded that gametocytes were the dominant cause of persistent PMA reactivity after treatment for malaria and that persistent PMA reactivity in convalescence did not appear to occur in patients who did not develop gametocytes after treatment. However, in the present study only 23 of the 160 (14%) patients with gametocytemia showed persistent antigenemia. No association with persistent HRP2 and/or PMA antigenemia and gametocytemia was observed in an Australian study since none of their patients had gametocytemia (3). In the present study, the proportionate reactivity for both antigens showed that the drop in pLDH+ PMA− reactivity was significantly higher than that for pLDH− PMA+ reactivity on day 3 (64% versus 38%, respectively [P < 0.03]).
In conclusion, the new generations of nonmicroscopic immunochromatographic assay offer a practical chance to move the diagnosis of malaria away from the laboratory and nearer to the patient. However, persistent HRP2 and PMA antigenemia beyond the clearance of peripheral parasitemia in certain cases reduces the usefulness of the ICT Malaria Pf/Pv test for monitoring the response to therapy. The levels of pLDH and PMA were shown to decline in parallel with the clearance of asexual parasitemia, and thus it was suggested that the disappearance of the parasite-specific enzyme pLDH after treatment may make the OptiMAL-IT assay useful in predicting treatment failure. Although improvements in quantification of current antigens may improve predictive ability of treatment failure, the use of alternative antigens with more rapid clearance and greater sensitivity and specificity for viable parasites is essential for reaching a higher level of clinical utility.
Acknowledgments
The financial support of Kuwait University (J.I. and P.R.H., MI 109) and Punjab Health Ministry, Pakistan (A.S. and M.J., Healthnet P764), is gratefully acknowledged.
We thank all technical staff and patients who participated in this study.
REFERENCES
- 1.Cho-Min, N., and M. L. Gatton. 2002. Performance appraisal of rapid on-site malaria diagnosis (ICT Malaria Pf/Pv test) in relation to human resources at village level in Myanmar. Acta Trop. 81:13-19. [DOI] [PubMed] [Google Scholar]
- 2.Dyer, M. E., E. Tjitra, B. J. Currie, and N. M. Anstey. 2000. Failure of “panmalarial” antibody of the ICT Malaria Pf/Pv immunochromatographic test to detect symptomatic Plasmodium malariae infection. Trans. R. Soc. Trop. Med. Hyg. 94:518. [DOI] [PubMed] [Google Scholar]
- 3.Eisen, D. P., and A. Saul. 2000. Disappearance of pan-malarial antigen reactivity using the ICT Malaria Pf/Pv kit parallels decline of patent parasitemia as shown by microscopy. Trans. R. Soc. Trop. Med. Hyg. 94:169-170. [DOI] [PubMed] [Google Scholar]
- 4.Farcas, G. A., K. J. Zhong, F. E. Lovegrove, C. M. Graham, and K. C. Kain. 2003. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returning travelers. Am. J. Trop. Med. Hyg. 69:589-592. [PubMed] [Google Scholar]
- 5.Gatti, S., A. M. Bernuzzi, Z. Bisoffi, A. Raglio, M. Gulletta, and M. Scaglia. 2002. Multicentre study, in patients with imported malaria, on the sensitivity and specificity of a dipstick test (ICT Malaria Pf/Pv) compared with expert microscopy. Ann. Trop. Med. Parasitol. 96:15-28. [DOI] [PubMed] [Google Scholar]
- 6.Humar, A., M. A. Harrington, D. Pillai, and K. C. Kain. 1997. ParaSight-F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am. J. Trop. Med. Hyg. 56:44-48. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal, J., A. Sher, P. R. Hira, and R. Al-Owaish. 1999. Comparison of the OptiMAL test with PCR for diagnosis of malaria in immigrants. J. Clin. Microbiol. 39:3644-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal, J., P. R. Hira, A. Sher, and A. A. Al-Enezi. 2001. Diagnosis of imported malaria by Plasmodium lactate dehydrogenase (pLDH) and histidine-rich protein 2 (PfHRP-2)-based immunocapture assays. Am. J. Trop. Med. Hyg. 64:20-23. [DOI] [PubMed] [Google Scholar]
- 9.Jelinek, T., M. P. Grobusch, and G. Harms. 2001. Evaluation of a dipstick test for the rapid diagnosis of imported malaria among patients presenting within the Network TropNetEurop. Scand. J. Infect. Dis. 33:752-754. [DOI] [PubMed] [Google Scholar]
- 10.Karbwang, J., O. Tasanor, T. Kanda, Y. Wattanagoon, M. Ibrahim, K. Na-Bangchang, A. Thanavibul, and W. Rooney. 1996. ParaSight-F test for the detection of treatment failure in multidrug-resistant Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 90:513-515. [DOI] [PubMed] [Google Scholar]
- 11.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price, R., F. Nosten, J. A. Simpson, C. Luxemburger, L. Phaipun, F. ter Kuiler, M. ter Vugt, T. Chongsuphajaisiddhi, and N. J. White. 1999. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 60:1019-1023. [DOI] [PubMed] [Google Scholar]
- 13.Singh, N., A. Saxena, and N. Valecha. 2000. Field evaluation of the ICT malaria Pf/Pv immunochromatographic test for diagnosis of Plasmodium falciparum and P. vivax infection in forest village of Chindwara, central India. Trop. Med. Int. Health 5:765-770. [DOI] [PubMed] [Google Scholar]
- 14.Singh, N., N. Valecha, A. C. Nagpal, S. S. Mishra, H. S. Varma, and S. K. Subbarao. 2003. The hospital- and field-based performance of the OptiMAL test, for malaria diagnosis and treatment monitoring in central India. Ann. Trop. Med. Parasitol. 97:5-13. [DOI] [PubMed] [Google Scholar]
- 15.Tjitra, E., S. Suprianto, M. E. Dyer, B. J. Currie, and N. M. Anstey. 1999. Utility of the ICT Malaria Pf/Pv immunochromatographic test in diagnosing/predicting treatment outcome following treatment of uncomplicated falciparum malaria in Eastern Indonesia. Am. J. Trop. Med. Hyg. 61(Suppl.):S472. [Google Scholar]
- 16.Tjitra, E., S. Suprianto, J. McBroom, B. J. Currie, and N. M. Anstey. 2001. Persistent ICT Malaria Pf/Pv panmalarial and HRP2 antigen reactivity after treatment of Plasmodium falciparum malaria is associated with gametocytemia and results in false-positive diagnosis of P. vivax in convalescence. J. Clin. Microbiol. 39:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vakharia, S., N. Gopinathan, and N. A. Kshirsagar. 1997. The ParaSight-F test for detecting treatment failure. Trans. R. Soc. Trop. Med. Hyg. 91:490-491. [DOI] [PubMed] [Google Scholar]
- 18.Wongsrichanalai, C., I. Arevalo, A. Laoboonchai, K. Yingyuen, R. S. Miller, A. J. Magill, J. R. Forney, and R. A. Gasser, Jr. 2003. Rapid diagnostic devices for malaria: field evaluation of a new prototype immunochromatographic assay for the detection of Plasmodium falciparum and non-falciparum Plasmodium. Am. J. Trop. Med. Hyg. 69:26-30. [PubMed] [Google Scholar]
- 19.World Health Organization. 1996. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria: W.H.O. informal consultation on recent advances in diagnostic techniques and vaccines for malaria. Bull. W. H. O. 74:47-54. [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 2000. New perspectives: malaria diagnosis. Report of a joint WHO/USAID informal consultation. W.H.O./MAL/2000 1091. World Health Organization, Geneva, Switzerland.