Abstract
Enteroaggregative Escherichia coli (EAEC) is an important diarrheal enteropathogen defined by aggregative adherence to cultured epithelial cells. We have detected EAEC from 121 (6.6%) of 1,826 hospitalized patients admitted with diarrhea to the Infectious Diseases Hospital in Kolkata, India. Watery diarrhea was recorded significantly (P = 0.0142) more often in children. The majority of the EAEC isolates were not serotypeable (62%) and showed resistance to five or more antibiotics (76%). We studied different virulence genes and the molecular epidemiology of 121 EAEC isolates recovered from diarrheal patients. A PCR assay for detection of virulence genes, an assay for determination of clump formation in liquid culture, and a HeLa cell adherence assay were carried out to characterize the EAEC isolates. Investigations were also conducted to correlate the virulence gene profiles with diarrheal symptoms and molecular epidemiology by pulsed-field gel electrophoresis (PFGE). Two or more virulence genes were detected in 109 (90.1%) EAEC isolates. In the cluster analysis, some isolates with specific gene profiles and phenotypes formed a group or subcluster. This study highlights the comparative distributions of three fimbrial adhesins and other virulence genes among EAEC isolates. The diverse virulence gene and PFGE profiles, along with the existence of diverse serotypes and antibiograms, suggests that the EAEC isolates are genetically heterogeneous in Kolkata.
Enteroaggregative Escherichia coli (EAEC) has been implicated as an emerging cause of persistent and acute diarrhea in both developing and developed countries (5, 18, 21, 26, 32, 39). Several epidemiological investigations of diarrhea suggest that EAEC is an important etiologic agent (25, 31, 33). The defining feature of EAEC is its ability to elicit characteristic stacked brick-like aggregative adherence (AA) to HEp-2 or HeLa cells. It has recently been shown that EAEC can induce growth impairment and malnutrition among children, even in children without diarrhea (50). Thus, the long-term effects of this pathogen in developing countries may be more threatening than the short-term self-limiting diarrhea.
The majority of EAEC isolates harbor a 60- to 65-MDa plasmid (pAA2) that encodes several putative virulence factors, including one of three fimbrial adhesins, designated AA fimbria I (AAF/I), AAF/II, and AAF/III (4, 10, 34, 35), which are responsible for the AA phenotype. In the operon for AAF/I, the gene encoding the major structural pilin subunit has been designated aggA, whereas the corresponding genes involved in AAF/II and AAF/III are aafA and agg3A, respectively. The biogenesis of both AAF/I and AAF/II require the activation of the transcriptional activator aggR, whereas its role in the biogenesis of AAF/III has not been proven (4). The other pAA2 plasmid-borne virulence factors include the EAEC heat-stable enterotoxin 1 (EAST 1) (44); Aap, a novel antiaggregation protein (dispersin) (48); and a heat-labile enterotoxin, Pet (plasmid-encoded toxin) (15).
Some of the EAEC isolates express chromosome-encoded virulence markers, such as the 116-kDa secreted mucinase, Pic (a protein involved in intestinal colonization) (20), and a protein responsible for yersiniabactin biosynthesis, designated Irp2 (iron-repressible high-molecular-weight protein 2) (47). It has recently been shown that a chromosomal locus from AAF/II-harboring strain 042 encodes the flagellin protein, FliC, that interacts with the epithelial cells, leading to the secretion of an intestinal inflammation marker, the cytokine interleukin-8 (50).
Despite the voluminous information available on the possible roles of different virulence factors for EAEC-mediated diarrhea, studies on the prevalence of these factors among EAEC isolates are scanty (31, 59). In the present study, investigations were carried out to compare the distribution of virulence genes in a collection of EAEC isolates recovered over a period of 2 years from hospitalized patients with diarrhea in Kolkata, India. A comparison was also made to determine whether a correlation between the virulence gene profiles of isolates that cause different diarrheal symptoms and molecular epidemiology by pulsed-field gel electrophoresis (PFGE) exists.
MATERIALS AND METHODS
Study design.
This study was conducted from January 2000 to December 2001 by use of an active surveillance program in which stool specimens were systematically collected from every fifth hospitalized patient on two randomly selected days per week at the Infectious Diseases Hospital, Kolkata. The passage of three or more loose stools in 24 h, in addition to one or more clinical symptoms of an enteric disorder (nausea, vomiting, abdominal pain or cramps, fecal urgency, or dysentery), was regarded as diarrhea. Stool specimens were collected in sterile McCartney bottles and were examined for characteristics of stool consistency (watery, loose, or formed) and other characteristics, like the presence of blood, a mucoid appearance, or positivity for occult blood. Rectal swab specimens were taken with sterile cotton-tipped swabs from patients from whom stool specimens could not be obtained at the time of collection.
Bacteriology.
Stool or rectal swab specimens were directly streaked onto MacConkey agar (Difco, Detroit, Mich.) for isolation of E. coli. The identities of these isolates as E. coli were confirmed by different biochemical tests by standard procedures (56). In this study, three to five E. coli isolates from each hospitalized patient were tested and were assigned to specific pathotypes by specific virulence gene-targeted PCR assays. All the isolates were cryopreserved at −70°C. Each stool specimen was also tested for other common enteric pathogens, such as Vibrio spp., Salmonella spp., Shigella spp., Aeromonas spp., Entamoeba histolytica, Giardia lamblia, and rotavirus (56). The E. coli isolates found to be of specific pathotypes were subjected to serological tests by characterization of the O antigen with 8 different polyvalent antisera and 43 monovalent antisera (Denka-Seiken Co. Ltd., Tokyo, Japan).
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed by the disk diffusion method (3) with commercially available disks (HiMedia, Mumbai, India). Isolates were considered susceptible, reduced susceptible, or resistant to a particular antimicrobial agent on the basis of the diameters of the inhibitory zones that matched the criteria of the manufacturer's interpretive table, which followed the recommendations of the National Committee for Clinical Laboratory Standards (36). The American Type Culture Collection (ATCC) strains E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used for quality control.
PCR-based virulence gene identification.
Initial screening for diarrheagenic E. coli was performed by either a simplex or a multiplex PCR with primer pairs specific for the elt and est genes for enterotoxigenic E. coli, the eae and bfpA genes and EAF for enteropathogenic E. coli, the stx1 and stx2 genes for Shiga toxin-producing E. coli (6), and plasmid pCVD432 for EAEC (46). The isolates were evaluated for the presence of EAEC plasmid virulence genes by PCR assays for astA (58), aap (29), shf (10), aggR (35), aggA (45), aggC (45), agg3A (4), agg3C (4), and afaBC (24). The chromosomal virulence genes were screened for the pic (10) and irp2 (47) genes. The primer pairs used to detect the aafA, aafC, and fliC genes were designed from published sequences (GenBank nucleotide accession numbers AF012835, F114828, and AF194946, respectively). Descriptions of the primer pairs, the sizes of the expected amplicons, and the amplification cycles are listed in Table 1.
TABLE 1.
PCR primer sequences for detection of different virulence-associated genes in EAEC
| Target gene | Description of encoded protein | Primer pairc | PCR condition for 30 cyclesa | Control strain(s) | Amplicon size (bp) | Reference (GenBank accession no.) |
|---|---|---|---|---|---|---|
| pCVD432 | Unknown function | CTGGCGAAAGACTGTATCAT and CAATGTATAGAAATCCGCTGTT | 1 min 94°C, 1 min 55°C, 1 min 72°C | 17-2, 042 | 630 | 46 |
| aggR | AAF/I and AAF/II transcriptional activator | CTAATTGTACAATCGATGTA and ATGAAGTAATTCTTGAAT | 1 min 94°C, 1 min 42°C, 1 min 72°C | 17-2, 042 | 308 | 35 |
| aggA | AAF/I fimbrial subunit | TTAGTCTTCTATCTAGGG and AAATTAATTCCGGCATGG | 1 min 94°C, 1 min 55°C, 1 min 72°C | 17-2 | 450 | 45 |
| aafA | Fimbrial subunit of AAF/II | ATGTATTTTTAGAGGTTGAC and TATTATATTGTCACAAGCTC | 1 min 94°C, 1 min 60°C, 1 min 72°C | 042 | 518 | This study (AF012835) |
| aggC | Usher subunit of AAF/I | TATTAAACCATGGTAGCG and GCCAAGATCCGAGATTGA | 1 min 94°C, 1 min 45°C, 1 min 72°C | 17-2 | 538 | 45 |
| aafC | Usher subunit of AAF/II | CTTCAGTTTTCCAGTTTCAG and CAGCAGAAGGGATATAATTA | 1 min 94°C, 1 min 48°C, 1 min 72°C | 042 | 1,160 | This study (F114828) |
| agg3A | Fimbrial subunit of AAF/III | GTATCATTGCGAGTCTGGTATTCAG and GGGCTGTTATAGAGTAACTTCCAG | 1 min 94°C, 1 min 50°C, 1 min 72°C | 55989 | 462 | 4 |
| agg3C | Usher subunit of AAF/III | GTTTGGAACCGGGAATTAACATTG and ATACTTTAGATACCCCTCACGCAG | 1 min 94°C, 1 min 50°C, 1 min 72°C | 55989 | 485 | 4 |
| aap | Antiaggregation protein (dispersin) | CTTTTCTGGCATCTTGGGT and GTAACAACCCCTTTGGAAGT | 1 min 94°C, 1 min 52°C, 1 min 72°C | 042 | 232 | 29 |
| afaBC | DAEC factor, Chaperone and Usher subunit of AFAb | GCTGGGCAGCAAACTGATAACTCTC and CATCAAGCTGTTTGTTCGTCCGCCG | 1 min 94°C, 1 min 65°C, 1 min 72°C | A30 | 750 | 24 |
| astA | Aggregative stable toxin 1 (EAST 1) | CCATCAACACAGTATATCCGA and GGTCGCGAGTGACGGCTTTGT | 1 min 94°C, 1 min 55°C, 1 min 72°C | 17-2, 042 | 111 | 58 |
| shf | Cryptic open reading frame | ACTTTCTCCCGAGACATTC and CTTTAGCGGGAGCATTCAT | 1 min 94°C, 1 min 50°C, 1 min 72°C | 042 | 613 | 10 |
| pic | Protein involved in colonization | GGGTATTGTCCGTTCCGAT and ACAACGATACCGTCTCCCG | 1 min 94°C, 1 min 52°C, 90 s 72°C | 042 | 1,175 | 10 |
| irp2 | Yersiniabactin biosynthesis gene | AAGGATTCGCTGTTACCGGAC and TCGTCGGGCAGCGTTTCTTCT | 1 min 94°C, 1 min 55°C, 1 min 72°C | 042 | 264 | 47 |
| fliC | Gene encoding flagellin | CATTAATACCAACAGCCTCT and ACTAGTAATAGTGCCATCAG | 1 min 94°C, 1 min 58°C, 90 s 72°C | 042 | 1,290 | This study (AF194946) |
For all PCR conditions, a final extension step of 7 min at 72°C was performed. The final reaction volume for all PCRs was 25 μl.
DAEC, diffusely adhering E. coli; AFA, afimbrial adhesin of Dr family.
Sequences are given in the 5′ to 3′ direction.
Each PCR assay was performed in a final reaction volume of 25 μl, which contained 2.5 mM each deoxynucleoside triphosphate, 1 pmol each primer, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 1.25 U of r-Taq DNA polymerase (Takara Shuzo, Otsu, Japan), and 2.5 μl of boiled bacterial cell lysate as the DNA template. Amplifications were performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, Calif.). PCR products were electrophoresed onto gels with 1% Seakem agarose (FMC Bioproducts, Rockland, Maine) and stained with ethidium bromide, and the gel image was captured digitally with a gel documentation system (Gel-Doc 2000; Bio-Rad, Hercules, Calif.). The EAEC control isolates used in this study were 17-2, which harbors AAF/I (53); 042, which harbors AAF/II (28); and 55989, which harbors AAF/III (4). E. coli K-12 C600 was used as a negative control in all PCR assays.
Colony hybridization.
A colony blot hybridization assay was performed with the 1.7-kb PCR-amplified fragment harboring the 3′ end of the pet gene from strain 042. Cultures of EAEC isolates were spotted onto a nylon membrane (Hybond-N+; Amersham Pharmacia Biotech, Little Chalfont, England) and hybridized under high-stringency conditions (68°C for 18 h) with a pet gene probe by using a nonradioactive digoxigenin labeling and detection kit (Boehringer, Mannheim, Germany). The membranes were first washed with 2× SSC (1× SSC is 0.15 M NaCl plus 15 mM sodium citrate)-0.1% sodium dodecyl sulfate (SDS) at ambient temperature and twice with 0.5× SSC-0.1% SDS at 54°C with constant agitation. Detection of hybrids was done according to the instructions of the manufacturer (Boehringer).
Clump formation test.
The clump formation test, which is specific for EAEC, was performed as described by Albert et al. (2). Briefly, the isolates were inoculated into Mueller-Hinton broth (Difco) and incubated for 20 h at 37°C under either stationary or shaking conditions. The formation of a clump or a pellicle as a ring at the side of the test tube was regarded as a positive result.
Adhesion assay.
All the EAEC isolates were examined by the adherence assay with the HeLa cell line by the method described by Cravioto et al. (8), with little modification. The HeLa cells were maintained at 37°C with humidified 5% CO2 in Eagle's minimal essential medium (MEM; Gibco/BRL, Gaithersburg, Md.) with 10% fetal bovine serum (FBS; Gibco/BRL), penicillin (100 U/ml), and streptomycin (100 μg/ml) in 25-cm2 tissue culture flasks (Nunc, Roskilde, Denmark). The HeLa cells were grown to 50 to 70% confluence as monolayers on glass coverslips in a 24-well flat-bottom tissue culture plate (Nunc). The EAEC isolates were grown overnight at 37°C without shaking in Trypticase soy broth (Difco). The HeLa cells were washed three times with phosphate-buffered saline (PBS) and 1 ml of fresh MEM with 2% FBS and 0.5% d-mannose without antibiotics. Twenty-five microliters of the bacterial suspension was added to each well, and the mixture was incubated at 37°C for 3 h. The cells were washed three times with PBS, fixed with 100% methanol, and stained with 10% Giemsa for 15 min. The adherence patterns were examined under ×40 magnification with a light microscope (model IX70; Olympus Optical Co. Ltd., Tokyo, Japan).
PFGE.
PFGE was performed according to the guidelines of the Centers for Disease Control and Prevention, Atlanta, Ga. (60). The results were interpreted as described previously (51).
Cluster analysis.
PFGE gel images were retrieved and aligned to generate a composite image containing the banding profiles of all the isolates. The image was analyzed with the Diversity Database fingerprinting software (version 2.2.0; Bio-Rad) to detect the relatedness of the isolates. Bands ranging from 48.5 to 438.5 kb were considered for the construction of a dendrogram. Degrees of homology were determined by comparison by use of the Dice coefficient, and clustering correlation coefficients were calculated by an unweighted pair-group method with arithmetic means (UPGAMA). A dendrogram showing the hierarchical representation of the levels of linkage between the isolates was drawn to predict the degree of clonal relatedness.
Statistical analysis.
Clinical data and the virulence features of the EAEC isolates were compared by Fisher's exact test (Epi-Info, version 6.02, software; World Health Organization, Geneva, Switzerland, and Centers for Disease Control and Prevention), and a P value <0.05 was considered statistically significant. The virulence gene profile results were entered into a database (Microsoft Excel) in a personal computer and converted into Epi-Info, version 6.02, software as a record file to match the data to determine their consistency and validity. The validated data were compiled and analyzed with the SPSS, version 4.0, software package (SPSS Inc., Chicago, Ill.). The virulence gene profile of each EAEC isolate was considered in the cluster analysis. To assess the relatedness between isolates on the basis of the overall gene profile, a data matrix in which a score of 1 was given for presence of a gene and a score of 0 was given for the absence of a gene was first compiled for each isolate. In the cluster analysis, isolates were grouped on the basis of similarity and were depicted in a dendrogram for correlation with other traits, such as serogroup, severity of diarrhea, and age group. Similarities between isolates were based on the squared Euclidean distance, and grouping was obtained by the UPGAMA clustering criterion (49) with the SPSS software package (27).
RESULTS
Prevalence and characteristics of EAEC-associated clinical symptoms.
In this study, a total of 4,996 E. coli isolates from 1,826 hospitalized patients with diarrhea were analyzed for EAEC by a pCVD432-specific PCR assay. The clinical features of the 121 (6.6%) EAEC-infected patients includes watery diarrhea (75.2%), vomiting (64.5%), and abdominal pain (45.5%) with either moderate (53.7%) or severe dehydration (43.0%) (Table 2). In most of the cases (85.1%), acute diarrhea was recorded and the patients were admitted up to 24 h after the onset of the symptoms. The duration of diarrhea was recorded for more than 2 days for only six patients (Table 2). Stratification of the diarrheal patients by age revealed that the rate of isolation of EAEC was highest (51.2%) among children <5 years of age. In children <5 years of age, the rate of EAEC-mediated watery diarrhea was statistically significantly (P = 0.0142) greater than that among the other age groups. The other pathogenic organisms identified among the EAEC-infected diarrheal patients are listed in Table 3. EAEC was identified as the sole pathogen in 90 cases (74.4%). Mixed infections with Vibrio cholerae O1 and EAEC were more common than other mixed infections (Table 3).
TABLE 2.
clinical Characteristics of EAEC-infected diarrheal cases
| Characteristic | No. (%) of hospitalized patients |
|---|---|
| Stool consistency | |
| Watery | 91 (75.2) |
| Mucoid | 5 (4.1) |
| Bloody | 4 (3.3) |
| Loose | 22 (18.2) |
| Clinical symptoms | |
| Vomiting | 78 (64.5) |
| Fever (temp, >38.8°C) | 24 (19.8) |
| Abdominal pain | 55 (45.5) |
| Dehydration status | |
| Severe | 52 (43.0) |
| Moderate | 65 (53.7) |
| None | 4 (3.3) |
| Duration of diarrhea before admission to the hospital | |
| Up to 24 h | 103 (85.1) |
| >24 to 48 h | 12 (9.9) |
| >48 h | 6 (5.0) |
TABLE 3.
Frequencies of EAEC and with other pathogens among diarrheal patients
| Pathogen | No. of patients |
|---|---|
| EAEC alone | 90 |
| EAEC + V. cholerae O1 | 16 |
| EAEC + V. cholerae O139 | 3 |
| EAEC + V. cholerae non O1, O139 | 4 |
| EAEC + Vibrio parahaemolyticus | 3 |
| EAEC + Shigella spp. | 1 |
| EAEC + rotavirus | 4 |
The EAEC organisms isolated in this study belonged to 13 different O serogroups (Table 4), with O86a (9.1%) being the dominant serogroup. Other frequently identified serogroups were O44, O15, and O153. However, 62% of EAEC isolates remained untypeable, as they did not agglutinate with any of the O antisera.
TABLE 4.
Frequency of different serogroups associated with EAEC
| Serogroup | No. (%) of isolates (n = 121) |
|---|---|
| ONTa | 75 (62.0) |
| O86a | 11 (9.1) |
| O44 | 6 (5.0) |
| O153 | 5 (4.1) |
| O15 | 5 (4.1) |
| O63 | 3 (2.5) |
| O166 | 3 (2.50) |
| Rough | 3 (2.5) |
| O114 | 2 (1.7) |
| O20 | 2 (1.7) |
| O128 | 2 (1.7) |
| O25 | 1 (0.82) |
| O1 | 1 (0.82) |
| O111 | 1 (0.82) |
| O55 | 1 (0.82) |
ONT, O nontypeable.
Antibiotic sensitivities of EAEC isolates.
Antibiotic sensitivity testing performed with 15 antimicrobial agents revealed that majority of the EAEC isolates were resistant to ampicillin, nalidixic acid, tetracycline, cephalothin, and co-trimoxazole (Table 5). The relative frequencies of resistance to other antimicrobials are shown in Table 5. Considerable numbers of isolates were also resistant to ciprofloxacin (41.3%) and norfloxacin (39.7%). Multidrug resistance (MDR) was demonstrated in almost every isolate, and 76% of the isolates were found to be resistant to five or more antibiotics (data not shown). The most common MDR profiles encountered in this study were ampicillin, cephalothin, tetracycline, co-trimoxazole, nalidixic acid, and streptomycin resistance and ampicillin, cephalothin, tetracycline, chloramphenicol, co-trimoxazole, nalidixic acid, and streptomycin resistance (six isolates each); ampicillin, cephalothin, ciprofloxacin, ceftazidime, tetracycline, chloramphenicol, co-trimoxazole, nalidixic acid, norfloxacin, and streptomycin resistance (four isolates); and ampicillin, cephalothin, gentamicin, amikacin, ciprofloxacin, ceftriaxone, kanamycin, tetracycline, chloramphenicol, ceftazidime, co-trimoxazole, nalidixic acid, norfloxacin, and streptomycin resistance and ampicillin, cephalothin, tetracycline, chlorampenicol, and nalidixic acid resistance (three isolates each). Very few isolates exhibited identical resistance profiles (data not shown).
TABLE 5.
Antibiotic susceptibilities of EAEC strains isolated from patients with acute diarrhea
| Antibiotic | No. (%) of isolates (n = 121)
|
||
|---|---|---|---|
| Susceptible | Reduced susceptible | Resistant | |
| Amikacin | 95 (78.5) | 18 (14.8) | 8 (6.6) |
| Ampicillin | 7 (5.8) | 9 (7.4) | 105 (86.8) |
| Ceftazidime | 66 (54.5) | 15 (12.4) | 40 (33.1) |
| Ceftriaxone | 83 (68.6) | 21 (17.4) | 17 (14.0) |
| Cephalothin | 15 (12.4) | 12 (9.9) | 94 (77.7) |
| Chloramphenicol | 66 (54.5) | 1 (0.8) | 54 (44.6) |
| Ciprofloxacin | 62 (51.2) | 9 (7.4) | 50 (41.3) |
| Co-trimoxazole | 35 (28.9) | 1 (0.8) | 85 (70.2) |
| Gentamycin | 109 (90.1) | 5 (4.1) | 7 (5.8) |
| Kanamycin | 61 (54.4) | 16 (13.2) | 44 (36.4) |
| Nalidixic acid | 14 (11.6) | 5 (4.1) | 102 (84.3) |
| Neomycin | 57 (47.1) | 53 (43.8) | 11 (9.1) |
| Norfloxacin | 67 (55.4) | 6 (4.9) | 48 (39.7) |
| Streptomycin | 36 (29.8) | 13 (10.7) | 72 (59.5) |
| Tetracycline | 20 (16.5) | 5 (4.1) | 96 (79.3) |
Adherence patterns and clump formation.
The typical AA (AAt) pattern was grouped into three types of adherence patterns, namely, a stacked brick (AA sb) pattern, a honeycomb-like (AA hc) pattern, and chain-like adherence (CLA) pattern (17). The AAt pattern was noticed among 103 (85.1%) EAEC isolates. The AA sb pattern was observed among 69 (57%) isolates, followed by the AA hc pattern among 28 (23.1%) isolates and the CLA pattern among 6 (5%) isolates. Six (5%) isolates adhered only to coverslips (AA cs pattern), and with 11 (9.1%) isolates, adherence to either HeLa cells or coverslips was not observed (nonadherent). Only one EAEC isolate displayed diffuse adherence. Clump or pellicle formation was noticed among 94 (77.7%) EAEC isolates.
Prevalence of fimbrial and other virulence genes.
The relative frequencies of chromosomal and plasmid-encoded virulence genes among the EAEC isolates are shown in Table 6. One hundred seventeen (96.7%) isolates had one or more virulence gene marker and were more frequently recovered from children than from adults (data not shown). aap was the gene most commonly identified and was detected in 92 (76%) isolates, followed by aggR and shf, each of which was identified in 79 (65.3%) isolates. Among the fimbrial genes identified, the accessory gene for AAF/I, aggC, was the most commonly encountered and was detected in 45 isolates (37.2%). The accessory genes of other fimbrial subtypes (aafC and agg3C for AAF/II and AAF/III, respectively) were detected in 4 (3.3%) and 27 (22.3%) isolates, respectively. Genes encoding the structural subunits of AAF/I (aggA), AAF/II (aafA), and AAF/III (agg3A) were found in 11 (9.1%), 2 (1.7%), and 17 (14.1%) isolates, respectively. Among the toxin genes, pet, which encodes heat-labile toxin, was identified in 37 (30.6%) isolates by colony hybridization, while astA, which encodes EAST 1 was detected in 36 isolates (29.8%). The chromosomal virulence gene marker irp2 was identified in 58 isolates (47.9%), followed by pic in 26 (21.5%) isolates. The gene encoding the flagellin that promotes interleukin-8 release was rarely encountered (3.3%).
TABLE 6.
Frequency of plasmid and chromosomal gene markers among EAEC isolates
| Gene location and virulence gene | No. (%) of isolates (n = 121) |
|---|---|
| Plasmid | |
| aafA | 2 (1.7) |
| aggA | 11 (9.1) |
| agg3A | 17 (14.1) |
| aggR | 79 (65.3) |
| aafC | 4 (3.3) |
| aggC | 45 (37.2) |
| agg3C | 27 (22.3) |
| aap | 92 (76.0) |
| shf | 79 (65.3) |
| pet | 37 (30.6) |
| astA | 36 (29.8) |
| afaBC | 2 (1.7) |
| Chromosome | |
| pic | 26 (21.5) |
| irp2 | 58 (47.9) |
| fliC | 4 (3.3) |
PFGE and cluster analysis.
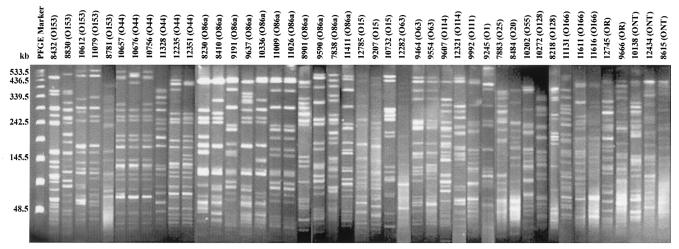
All 43 EAEC isolates typeable with O antisera were tested by PFGE to assess their clonal relatedness. Three isolates could not be typed by PFGE due to endonuclease activity. Three O-untypeable EAEC isolates and two O rough EAEC isolates were also included in the PFGE analysis. The PFGE profiles of the EAEC isolates showed 38 different patterns (Fig. 1). These patterns were distinguished by more than three bands. Three serogroup O44 isolates (isolates 10657, 10676, and 10756), two serogroup O86a isolates (isolates 11009 and 10026), and two serogroup O63 isolates (isolates 9464 and 9554) were closely related to each other, as they shared maximum similarities in their banding patterns (Fig. 1).
FIG. 1.
XbaI-restricted PFGE profiles of the 45 EAEC isolates from hospitalized patients with diarrhea. The identity of the isolate and its serogroup are indicated above each lane. The bacteriophage lambda DNA ladder standard for PFGE (New England Biolabs, Beverly, Mass.) was used in the lane labeled PFGE Marker as a DNA molecular size marker. ONT, O nontypeable.
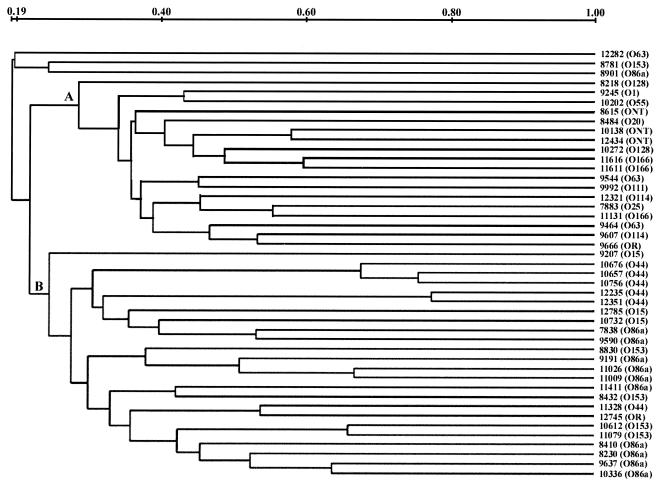
Dendrogram analysis showed two well-separated clusters, clusters A and B (Fig. 2). Cluster A comprised 18 EAEC isolates, in which serogroups O166, O128, O114, O63, O55, O111, O20, O25, and O1 and the three O-untypeable serotypes were clustered. Cluster B represents the major one, with 24 EAEC isolates, in which all the EAEC isolates of serogroups O44 and O15 were grouped. Except for one isolate each of serogroups O86a and O153, the rest of the isolates in the different serogroups fell into cluster B.
FIG. 2.
The XbaI restriction patterns were digitized and analyzed by using the Diversity Database fingerprinting software (version 2.2.0; Bio-Rad) to calculate the similarity matrix by use of Dice coefficients of correlation, and clustering correlation coefficients were determined by the UPGAMA method. The scale indicated at the top collates the levels of pattern similarity. ONT, O nontypeable.
Virulence gene profile analysis.
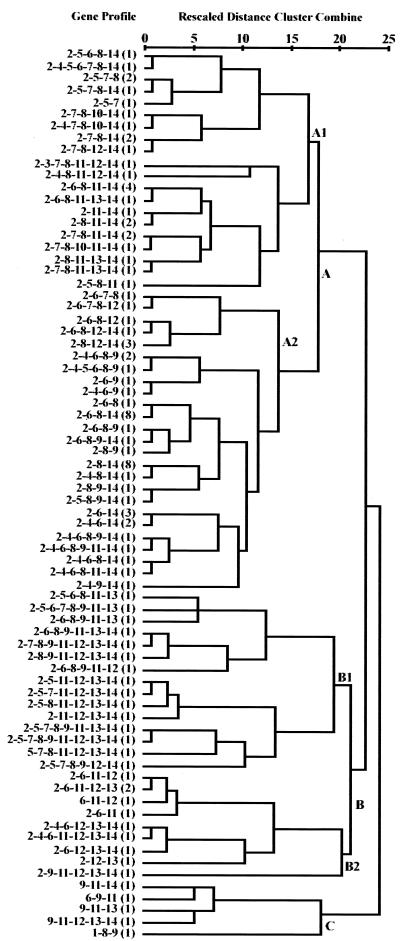
In the virulence gene profile analysis, we have considered the EAEC isolate recovered with multivirulence gene markers to be the sole pathogen from each patient. A total of 74 gene profiles shared by 102 EAEC isolates from 90 patients were recorded. The UPGAMA method of dendrogram analysis, which reflected different lineages on the basis of the similarity matrices of the gene profiles of the EAEC isolates, formed clusters A and B, with 72 and 25 EAEC isolates, respectively (Fig. 3). The isolates with identical gene profiles were confined predominantly in the A2 subcluster. Profiles aap aggC aggR shf and aap aggR shf were each shared by eight EAEC isolates in this cluster. Within subcluster A1, EAEC isolates harboring AAF/III-related genes were found more than EAEC isolates harboring AAF/I-related genes (16 isolates with agg3C versus 6 isolates with aggC and 7 isolates with agg3A versus 2 isolates with aggA). However, the pet gene was found in only three isolates, and astA was not detected in any of the isolates in this cluster. Isolates harboring the chromosomal fliC gene were grouped in subcluster A1 along with isolates harboring irp2 and pic. aggC and its corresponding structural aggA genes were detected less frequently than the agg3-related genes (agg3C and agg3A). Subcluster A2, which represented the major subcluster, consisted of 45 EAEC isolates. The conspicuous feature of the isolates in subcluster A2 was the abundance of plasmid-encoded virulence genes and the almost complete absence of the chromosomal virulence genes (irp2 in two isolates). In subcluster A2, AAF/I-related genes were detected more frequently than AAF/III-related genes (27 isolates with aggC versus 2 isolates with agg3C and 12 isolates with aggA versus 1 isolate with agg3A). Similarly, among the isolates in subcluster A2, the astA gene was present in more isolates than the pet gene. The aap gene was distributed among isolates in subclusters A1 through B2 but not among those in cluster C. aggR and shf were found in isolates in all clusters.
FIG. 3.
Virulence gene profile relationships of the EAEC isolates recovered as the sole pathogens from diarrheal patients. The five major phylogenetic clusters (clusters A1, A2, B1, B2, and C) are labeled. The dendrogram was obtained by cluster analysis (by the UPGAMA method) of the virulence gene profiles shared by 102 EAEC isolates. The average distance between clusters was determined by the cluster analysis, based on the similarity matrices of the EAEC isolates exhibiting diversified gene profiles. The virulence genes were coded 1 through 14 for aafA, aap, afaBC, aggA, agg3A, aggC, agg3C, aggR, astA, fliC, irp2, pet, pic, and shf, respectively. The number of EAEC isolates harboring the respective virulence profile is indicated in parentheses. The similarity scale is shown above the dendrogram.
irp2 and pic were the dominant genes among the isolates in subcluster B1, and agg3 genes were detected more often than agg genes among the isolates in that cluster. Toxin genes astA and pet were distributed equally in subcluster B1. The agg3 genes were completely absent from the isolates in cluster B2, and the pet gene was detected more frequently than the astA gene (eight and one isolates, respectively). Virulence genes of chromosomal origin were more frequently detected than plasmid-encoded genes among the isolates in subclusters B1 and B2. Isolates harboring the aap gene were not detected in cluster C.
DISCUSSION
EAEC has been considered a heterogeneous pathogen, but EAEC isolates share a common character, i.e., expression of the AA phenotype on HEp 2 or HeLa cells (30). Although in many instances EAEC strains have been isolated from asymptomatic individuals at a frequency similar to that from which they are isolated from patients with diarrhea (9, 18), their association with diarrhea in several outbreaks and in volunteer studies has unquestionably confirmed the role of EAEC as an important etiologic agent (25, 33). The discrimination factor used in the initial detection of the EAEC isolates was the PCR assay with primers derived from a probe for AA. Some reports have shown that the correlation of results obtained by the PCR- and probe-based assay and assays for AA may vary by location (11, 37, 52, 55). However, compared to the assay for AA, the PCR- and probe-based detection of EAEC has been found to be simple and specific in many epidemiological studies (12, 46). In this study, 85% of the EAEC isolates exhibited the AAt pattern, and 78% were positive by the clump testing.
The incidence rate of EAEC among diarrheal patients in our study was 6.6%, which closely matches that found in a previous hospital-based surveillance study conducted in Kolkata (39). However, the detection rate was 9.8% among pediatric cases (12). Critical analysis of EAEC-infected patients with stratification by age revealed a unique association of EAEC with children younger than 10 years of age (60.3%), which was statistically significant (P < 0.05). EAEC infections have generally been reported to be unique because of the relatively long duration of symptoms (31). However, in this study we encountered only six patients who had diarrheal episodes for more than 2 days. Since most of the patients had watery diarrhea (75.2%) with severe (43%) or moderate (53.7%) dehydration and received medical treatment, the observed duration was much less in this study. Even though EAEC infections persist in the presence of infections with other pathogens, none of the patients had infections caused by other pathotypes of diarrheagenic E. coli. As reported by many investigators (22, 38, 40, 53), the majority of the EAEC isolates in our study remained O-antigen untypeable (62%). Similar to a recent report from Brazil (59), the prevalence of serogroup O44 was comparatively high in this study.
The results of the clump formation test correlated with positive PCR results for EAEC and the AAt pattern of adherence (data not shown). In comparison to detection of the pattern of adherence and PCR assays, the clump formation test (2) facilitates the simple and rapid detection of EAEC. The AAt patterns were mostly associated with the AAF-related gene sequences or their regulator, the aggR gene. Analysis of different adherence patterns showed that the AA sb pattern was the most dominant AAt pattern. EAEC isolates exhibiting other types of adherence, such as the AA cs pattern, diffuse adherence, and nonadherence, were found to be devoid of any of the AAF-related genes or other putative virulence genes. We did not notice any cell detachment or any cellular distention characteristic of cell-detaching E. coli isolates in the adherence assay that has previously been reported by others (T. A. Gomes, C. M. Abe, and L. R. M. Marques, Letter, J. Clin. Microbiol. 33:3364, 1995).
The frequency of occurrence of different plasmid-encoded and chromosomal virulence genes of EAEC showed that the gene profiles varied depending upon the age of the patients. EAEC isolates with multiple virulence genes were more frequently detected in children than in adults. Children had watery diarrhea significantly more often. EAEC isolates with one or more or two or more virulence gene markers were detected in 117 (96.7%) and 109 (90.1%) patients, respectively. In our study, the most frequent plasmid-borne genes detected among the EAEC isolates were aap, shf, and aggR. This finding is in agreement with those of a previous study with a worldwide collection of EAEC isolates (10) and a recent study conducted in Brazil (59).
The prevalence of the AAF/II operon is generally reported to be low (13, 41, 52), and the results of our study are consistent with that finding. Bernier et al. (4) have recently reported on the existence of a high level of heterogeneity in the AAF/II operon and demonstrated the use of an aafDA-specific probe for the detection of AAF/II. The use of primers designed from the conserved region of the aafDA sequence might have improved the level of detection of AAF/II in the present study. Only 30 (24.8%) isolates harbored any of the structural genes of the three AAFs. The higher frequency of the accessory genes (aggC, aafC, and agg3C) in comparison to the lower frequency of the corresponding structural genes encoding pilin subunit provide evidence for the heterogeneity in the latter. These results are in agreement with those of the earlier studies on the higher prevalence of aggC genes (10, 16, 41). The presence of agg3C in 22.3% of the EAEC isolates indicated that the agg3 gene or a variant agg3 gene was the second most frequent adhesin after agg. From this study, it is evident that the maximum heterogeneity exists in the agg operon rather than the agg3 operon. We presume that although the accessory genes for all three adhesins are conserved, the low prevalence of pilin-encoding genes is due to the remarkable variations in the structural gene sequences. Thus, primers designed from the regions encompassing the conserved flanking regions of the fimbrial operons would certainly be useful for the detection of all three variant AAF operons. The recently identified AAF/III operon was from human immunodeficiency virus-infected patients with persistent diarrhea (4), and the prevalence of this fimbria in our study (14.1%) matched that (13.8%) described in a recent report from Mongolia (43). This fimbria might play an important role in acute diarrhea in different regions of the world. However, our findings indicate that considerable numbers of EAEC isolates did not harbor any of the three adhesins; thus, future investigations should target the identification of other variant fimbrial types.
Chromosomal virulence genes were also sought in this study to determine their epidemiological significance in patients with diarrhea. The low incidence of fliC (3.3%), as detected by the PCR assay, might be due to variations in the sequence of this gene. Diverse H types were shown to be associated with EAEC, and it has been shown that not all the flagellins are responsible for an inflammatory response (38). Since H typing was not done in this study, we are unable to correlate our findings with the findings presented previously. The frequencies of pic (21.6%) and irp2 (47.9%) were comparatively less than those reported elsewhere (10, 14, 40). Compared to the findings described in other reports (25, 41), we have encountered only two EAEC isolates that harbored the uropathogenic E. coli-specific afaBC gene. Similar to the findings of other studies (14, 37, 40), we have detected the astA and the pet genes at almost the same frequency. To our knowledge, this is the first report from the Indian subcontinent on the distribution of three adhesins, the diffusely adhering E. coli factor, and the gene encoding the interleukin-8 secreting factor, fliC, among EAEC isolates from hospitalized diarrheal patients.
Dendrogram analysis based on the virulence gene profiles indicated that all EAEC isolates in subclusters A1 through B2 harbored diversified gene profiles. Plasmid-borne genes aap, aggR, and shf were dispersed almost evenly in all clusters. Many isolates with several similar gene profiles were clustered in subcluster A2. The EAEC isolates with the aap aggC aggR shf and aap aggR shf virulence gene profiles were from children with watery diarrhea and severe dehydration. The AAF/I genes were specifically clustered in subcluster A1, whereas subcluster A2 showed a specific association with AAF/III genes. Chromosomal virulence genes were generally absent from the EAEC isolates in subcluster A2, although they were clustered in subclusters B1 and B2. Our results confirm the presence of more than two putative virulence factors among 90% of the EAEC isolates tested, which follows the proposed criterion for EAEC as a potential pathogen (37).
Other investigators (1, 23) have previously determined the genetic diversity of EAEC by using PFGE. In this study, all except seven EAEC isolates had distinct PFGE patterns. These isolates exhibited identical PFGE patterns, serotypes, antibiograms, and virulence gene profiles. The clinical data indicated that the patients from whom these isolates were sequestered were children younger than 5 years of age who had watery diarrhea and who were hospitalized on the same day.
In concurrence with the findings presented in other reports (7, 19, 42), the majority of the EAEC isolates evaluated in this study exhibited MDR. In one report it was shown that the antibiotic resistance might be linked to EAEC virulence (57). EAEC isolates with five or more MDR properties were frequently encountered. We have detected EAEC resistance to fluoroquinolones, which is the drug of choice for the treatment of diarrhea in India (54).
The identification of EAEC alone from 90 patients with other accompanying symptoms, such as fever, vomiting, severe diarrhea, and watery manifestations of stools, in more than 80% of the cases explains the etiological importance of EAEC in this region where diarrhea is endemic. Therefore, continuous monitoring for the prevalence, epidemiology, and appropriate treatment by consideration of the MDR properties of this important etiologic agent is needed.
In conclusion, EAEC-mediated diarrhea in Kolkata is mostly associated with children. The diverse PFGE profiles along with the existence of different serotypes, antibiograms, and virulence gene profiles in the majority of the isolates show that EAEC isolates are highly heterogeneous in their genetic makeups.
Acknowledgments
We are indebted to Chantal LeBouguénec, Institut Pasteur, Paris, France, for providing bacterial strains 55989 and A30 used in this study.
This work was supported in part by the Council of Scientific and Industrial Research [project no. 37 (1019)/99/EMRII] and the Japan International Cooperation Agency (JICA/NICED project no. 054.1061.E.O).
REFERENCES
- 1.Adachi, J. A., Z. D. Jiang, J. J. Mathewson, M. P. Verenkar, S. Thompson, F. Martinez-Sandoval, R. Steffen, C. D. Ericsson, and H. L. DuPont. 2001. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin. Infect. Dis. 32:1706-1709. [DOI] [PubMed] [Google Scholar]
- 2.Albert, M. J., F. Qadri, A. Haque, and N. A. Bhuiyan. 1993. Bacterial clump formation at the surface of liquid culture as a rapid test for identification of enteroaggregative Escherichia coli. J. Clin. Microbiol. 31:1397-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, A. W., W. M. Kirby, J. C. Sherris, and M. Turck. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493-496. [PubMed] [Google Scholar]
- 4.Bernier, C., P. Gounon, and C. Le Bouguenec. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70:4302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhan, M. K., P. Raj, M. M. Levine, J. B. Kaper, N. Bhandari, R. Srivastava, R. Kumar, and S. Sazawal. 1989. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J. Infect. Dis. 159:1061-1064. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, S., J. S. Deokule, P. Garg, S. K. Bhattacharya, R. K. Nandy, G. B. Nair, S. Yamasaki, Y. Takeda, and T. Ramamurthy. 2001. Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholerae O1 and O139 in Ahmedabad, India. J. Clin. Microbiol. 39:3241-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobeljic, M., B. Miljkovic-Selimovic, D. Paunovic-Todosijevic, Z. Velickovic, Z. Lepsanovic, N. Zec, D. Savic, R. Ilic, S. Konstantinovic, B. Jovanovic, and V. Kostic. 1996. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol. Infect. 117:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravioto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 9.Cravioto, A., A. Tello, A. Navarro, J. Ruiz, H. Villafan, F. Uribe, and C. Eslava. 1991. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 337:262-264. [DOI] [PubMed] [Google Scholar]
- 10.Czeczulin, J. R., T. S. Whittam, I. R. Henderson, F. Navarro-Garcia, and J. P. Nataro. 1999. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrer, P., R. Zbinden, F. Fleisch, M. Altwegg, B. Ledergerber, H. Karch, and R. Weber. 2000. Intestinal infection due to enteroaggregative Escherichia coli among human immunodeficiency virus-infected persons. J. Infect. Dis. 182:1540-1544. [DOI] [PubMed] [Google Scholar]
- 12.Dutta, S., S. Pal, S. Chakrabarti, P. Dutta, and B. Manna. 1999. Use of PCR to identify enteroaggregative Escherichia coli as an important cause of acute diarrhoea among children living in Calcutta, India. J. Med. Microbiol. 48:1011-1016. [DOI] [PubMed] [Google Scholar]
- 13.Elias, W. P., S. Suzart, L. R. Trabulsi, J. P. Nataro, and T. A. Gomes. 1999. Distribution of aggA and aafA gene sequences among Escherichia coli isolates with genotypic or phenotypic characteristics, or both, of enteroaggregative E. coli. J. Med. Microbiol. 48:597-599. [DOI] [PubMed] [Google Scholar]
- 14.Elias, W. P., A. P. Uber, S. K. Tomita, L. R. Trabulsi, and T. A. Gomes. 2002. Combinations of putative virulence markers in typical and variant enteroaggregative Escherichia coli strains from children with and without diarrhoea. Epidemiol. Infect. 129:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forestier, C., M. Meyer, S. Favre-Bonte, C. Rich, G. Malpuech, C. Le Bouguenec, J. Sirot, B. Joly, and C. De Champs. 1996. Enteroadherent Escherichia coli and diarrhea in children: a prospective case-control study. J. Clin. Microbiol. 34:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioppo, N. M., W. P. Elias, Jr., M. C. Vidotto, R. E. Linhares, H. O. Saridakis, T. A. Gomes, L. R. Trabulsi, and J. S. Pelayo. 2000. Prevalence of HEp-2 cell-adherent Escherichia coli and characterisation of enteroaggregative E. coli and chain-like adherent E. coli isolated from children with and without diarrhoea, in Londrina, Brazil. FEMS Microbiol. Lett. 190:293-298. [DOI] [PubMed] [Google Scholar]
- 18.Gomes, T. A., P. A. Blake, and L. R. Trabulsi. 1989. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J. Clin. Microbiol. 27:266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haider, K., S. M. Faruque, N. S. Shahid, M. J. Albert, S. Nahar, A. Malek, S. Tzipori, and A. N. Alam. 1991. Enteroaggregative Escherichia coli infections in Bangladeshi children: clinical and microbiological features. J. Diarrhoeal Dis. Res. 9:318-322. [PubMed] [Google Scholar]
- 20.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huppertz, H. I., S. Rutkowski, S. Aleksic, and H. Karch. 1997. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in Western Europe. Lancet 349:1660-1662. [DOI] [PubMed] [Google Scholar]
- 22.Jalaluddin, S., P. de Mol, W. Hemelhof, N. Bauma, D. Brasseur, P. Hennart, R. E. Lomoyo, B. Rowe, and J. P. Butzler. 1998. Isolation and characterization of enteroaggregative Escherichia coli (EAggEC) by genotypic and phenotypic markers, isolated from diarrheal children in Congo. Clin. Microbiol. Infect. 4:213-219. [DOI] [PubMed] [Google Scholar]
- 23.Keskimaki, M., M. Eklund, H. Pesonen, T. Heiskanen, A. Siitonen, and Study Group. 2001. EPEC, EAEC and STEC in stool specimens: prevalence and molecular epidemiology of isolates. Diagn. Microbiol. Infect. Dis. 40:151-156. [DOI] [PubMed] [Google Scholar]
- 24.Le Bouguenec, C., M. Archambaud, and A. Labigne. 1992. Rapid and specific detection of the Pap, AFA, and SFA adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 30:1189-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathewson, J. J., P. C. Johnson, H. L. DuPont, D. R. Morgan, S. A. Thornton, L. V. Wood, and C. D. Ericsson. 1985. A newly recognized cause of travelers' diarrhea: enteroadherent Escherichia coli. J. Infect. Dis. 151:471-475. [DOI] [PubMed] [Google Scholar]
- 26.Morelli, R., L. Baldassarri, V. Falbo, G. Donelli, and A. Caprioli. 1994. Detection of enteroadherent Escherichia coli associated with diarrhoea in Italy. J. Med. Microbiol. 41:399-404. [DOI] [PubMed] [Google Scholar]
- 27.Narusis, M. J. 1988. SPSS/PC+ advanced statistics. version 2.0, B-71-B/01. SPSS Inc., Chicago, Ill.
- 28.Nataro, J. P., M. M. Baldini, J. B. Kaper, R. E. Black, N. Bravo, and M. M. Levine. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560-565. [DOI] [PubMed] [Google Scholar]
- 29.Nataro, J. P., Y. Deng, D. R. Maneval, A. L. German, W. C. Martin, and M. M. Levine. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nataro, J. P., J. B. Kaper, R. Robins-Browne, V. Prado, P. Vial, and M. M. Levine. 1987. Patterns of adherence of Escherichia coli to HEp-2 cells. Pediatr. Infect. Dis. J. 6:829-831. [DOI] [PubMed] [Google Scholar]
- 31.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nataro, J. P., T. Steiner, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli. Emerg. Infect. Dis. 4:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nataro, J. P., D. Yikang, S. Cookson, A. Cravioto, S. J. Savarino, L. D. Guers, M. M. Levine, and C. O. Tacket. 1995. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J. Infect. Dis. 171:465-468. [DOI] [PubMed] [Google Scholar]
- 34.Nataro, J. P., D. Yikang, J. A. Giron, S. J. Savarino, M. H. Kothary, and R. Hall. 1993. Aggregative adherence fimbria I expression in enteroaggregative Escherichia coli requires two unlinked plasmid regions. Infect. Immun. 61:1126-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nataro, J. P., D. Yikang, D. Yingkang, and K. Walker. 1994. AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J. Bacteriol. 176:4691-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing, 12th informational supplement. Approved standard M100-S12. National Committee for Clinical Laboratory Standard, Wayne, Pa.
- 37.Okeke, I. N., A. Lamikanra, J. Czeczulin, F. Dubovsky, J. B. Kaper, and J. P. Nataro. 2000. Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in southwest Nigeria. J. Infect. Dis. 181:252-260. [DOI] [PubMed] [Google Scholar]
- 38.Okeke, I. N., and J. P. Nataro. 2001. Enteroaggregative Escherichia coli. Lancet Infect. Dis. 1:304-313. [DOI] [PubMed] [Google Scholar]
- 39.Paul, M., T. Tsukamoto, A. R. Ghosh, S. K. Bhattacharya, B. Manna, S. Chakrabarti, G. B. Nair, D. A. Sack, D. Sen, and Y. Takeda. 1994. The significance of enteroaggregative Escherichia coli in the etiology of hospitalized diarrhoea in Calcutta, India and the demonstration of a new honey-combed pattern of aggregative adherence. FEMS Microbiol. Lett. 117:319-325. [DOI] [PubMed] [Google Scholar]
- 40.Piva, I. C., A. L. Pereira, L. R. Ferraz, R. S. Silva, A. C. Vieira, J. E. Blanco, M. Blanco, J. Blanco, and L. G. Giugliano. 2003. Virulence markers of enteroaggregative Escherichia coli isolated from children and adults with diarrhea in Brasilia, Brazil. J. Clin. Microbiol. 41:1827-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rich, C., S. Favre-Bonte, F. Sapena, B. Joly, and C. Forestier. 1999. Characterization of enteroaggregative Escherichia coli isolates. FEMS Microbiol. Lett. 173:55-61. [DOI] [PubMed] [Google Scholar]
- 42.Sang, W. K., J. O. Oundo, J. K. Mwituria, P. G. Waiyaki, M. Yoh, T. Iida, and T. Honda. 1997. Multidrug-resistant enteroaggregative Escherichia coli associated with persistent diarrhea in Kenyan children. Emerg. Infect. Dis. 3:373-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarantuya, J., J. Nishi, N. Wakimoto, S. Erdene, J. P. Nataro, J. Sheikh, M. Iwashita, K. Manago, K. Tokuda, M. Yoshinaga, K. Miyata, and Y. Kawano. 2004. Typical enteroaggregative Escherichia coli is the most prevalent pathotype among E. coli strains causing diarrhea in Mongolian children. J. Clin. Microbiol. 42:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savarino, S. J., A. Fasano, D. C. Robertson, and M. M. Levine. 1991. Enteroaggregative Escherichia coli elaborate a heat-stable enterotoxin demonstrable in an in vitro rabbit intestinal model. J. Clin. Investig. 87:1450-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savarino, S. J., P. Fox, Y. Deng, and J. P. Nataro. 1994. Identification and characterization of a gene cluster mediating enteroaggregative Escherichia coli aggregative adherence fimbria I biogenesis. J. Bacteriol. 176:4949-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt, H., C. Knop, S. Franke, S. Aleksic, J. Heesemann, and H. Karch. 1995. Development of PCR for screening of enteroaggregative Escherichia coli. J. Clin. Microbiol. 33:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schubert, S., A. Rakin, H. Karch, E. Carniel, and J. Heesemann. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheikh, J., J. R. Czeczulin, S. Harrington, S. Hicks, I. R. Henderson, C. Le Bouguenec, P. Gounon, A. Phillips, and J. P. Nataro. 2002. A novel dispersin protein in enteroaggregative Escherichia coli. J. Clin. Investig. 110:1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sneath, P. H., and R. R. Sokal. 1972. Computer taxonomy. Methods Microbiol. 74:28-98. [Google Scholar]
- 50.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tenover, F. C., R. D. Arbeit, R. V. Goering, et al. 1997. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect. Control Hosp. Epidemiol. 18:426-439. [DOI] [PubMed] [Google Scholar]
- 52.Tsai, C. C., S. Y. Chen, and H. Y. Tsen. 2003. Screening the enteroaggregative Escherichia coli activity and detection of the aggA, aafA, and astA genes with novel PCR primers for the Escherichia coli isolates from diarrhea cases in Taiwan. Diagn. Microbiol. Infect. Dis. 46:159-165. [DOI] [PubMed] [Google Scholar]
- 53.Vial, P. A., R. Robins-Browne, H. Lior, V. Prado, J. B. Kaper, J. P. Nataro, D. Maneval, A. Elsayed, and M. M. Levine. 1988. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J. Infect. Dis. 158:70-79. [DOI] [PubMed] [Google Scholar]
- 54.Vila, J., M. Vargas, J. Ruiz, M. Corachan, M. T. Jimenez De Anta, and J. Gascon. 2000. Quinolone resistance in enterotoxigenic Escherichia coli causing diarrhea in travelers to India in comparison with other geographical areas. Antimicrob. Agents Chemother. 44:1731-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wanke, C. A., J. B. Schorling, L. J. Barret, M. A. De Souza, and R. L. Guerrant. 1991. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr. Infect. Dis. J. 10:746-751. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. 1987. Manual for laboratory investigations of acute enteric infections, CDD/83.3. World Health Organization, Geneva, Switzerland.
- 57.Yamamoto, T., P. Echeverria, and T. Yokota. 1992. Drug resistance and adherence to human intestines of enteroaggregative Escherichia coli. J. Infect. Dis. 165:744-749. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto, T., and M. Nakazawa. 1997. Detection and sequences of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene in enterotoxigenic E. coli strains isolated from piglets and calves with diarrhea. J. Clin. Microbiol. 35:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamboni, A., S. H. Fabbricotti, U. Fagundes-Neto, and I. C. A. Scaletskey. 2004. Enteroaggregative Escherichia coli virulence factors are found to be associated with infantile diarrhea in Brazil. J. Clin. Microbiol. 42:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao, S., D. G. White, B. Ge, S. Ayers, S. Friedman, L. English, D. Wagner, S. Gaines, and J. Meng. 2001. Identification and characterization of integron-mediated antibiotic resistance among Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 67:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]