Abstract
We describe a fatal case of Clostridium symbiosum bacteremia in a 70-year-old man with metastatic colon cancer. Our report is the first, in the world literature, of human infection caused by this microorganism.
CASE REPORT
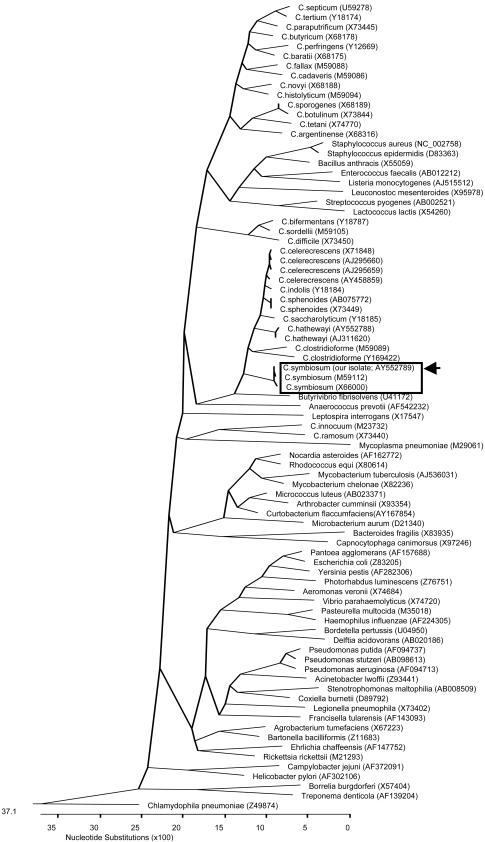
A 70-year-old man with recently diagnosed metastatic cancer of the colon, a history of prostate cancer in remission for 3 years since radiation and hormonal therapy, and a pacemaker insertion 5 years previously for third degree heart block was brought by ambulance to the hospital because of acute-onset altered level of consciousness, shortness of breath, and inability to cope at home. He was found to be cachectic, dehydrated, febrile (38.8°C), hypoxic, tachycardic, and hypotensive on admission. His Glasgow coma score was 13. Chest exam revealed decreased air entry bilaterally, with bibasilar inspiratory crackles. His abdomen was slightly distended but diffusely tender, with no audible bowel sounds. Chest X rays revealed normal lungs and a normal-size heart with a visible pacemaker. Abdominal films demonstrated the presence of dilated small bowel loops but no obstruction or free air. He was found to have severe metabolic acidosis and acute renal failure, although a complete blood count was within normal limits. He received supplemental oxygen, analgesics, and other supportive measures, and a diagnosis of sepsis with multiorgan dysfunction was made. He received intravenous piperacillin-tazobactam therapy after two sets of BacT/Alert FAN (bioMerieux Inc., Durham, N.C.) aerobic and anaerobic blood cultures were drawn. After 18 h of incubation in the BacT/Alert 3D system (bioMerieux Inc.), the anaerobic bottles of both blood culture sets grew what initially appeared to be gram-negative rod-shaped bacteria. The organism failed to grow aerobically and anaerobically on initial subculture with solid agar media, although a second subculture from the anaerobic bottles resulted in dusty growth on anaerobic brucella blood agar media (PML Microbiologicals, Wilsonville, Oreg.) after 72 h of incubation. The organism repeatedly stained gram negative although results of special potency (5 μg) vancomycin antibiotic disk testing (based on the presence of any zone of inhibition around the disk after overnight incubation on brucella blood agar) suggested it was a gram-positive bacterium. Additional phenotypic identification tests were unsuccessful due to poor growth of the organism. The isolate was subsequently identified by partial 16S rRNA gene sequencing using MicroSeq 500 kits (Applied Biosystems, Foster City, Calif.) and an ABI Prism 3100 genetic analyzer (Applied Biosystems). A GenBank BLAST search revealed an almost perfect (99%) match with a previously sequenced Clostridium symbiosum strain (GenBank accession no. M59112). Further phylogenetic analysis indicated that our sequence clustered tightly with the two C. symbiosum 16S rRNA sequences currently available in the GenBank database (Fig. 1) and helped confirm the species identification of the present isolate. Susceptibility testing could not be performed due to the fastidious nature of the organism. Despite all appropriate measures, the patient's condition worsened, resulting in death 1 week after admission. An autopsy was not performed.
FIG. 1.
Phylogenetic tree showing the 16S rRNA relationships of our C. symbiosum isolate with various Clostridium species and other medically important bacteria. The tree was constructed by ClustalW analysis, based on the first 475 nucleotides of the 16S rRNA gene. The sequences were obtained from the GenBank database; their nucleotide sequence accession numbers are in parentheses. Chlamydophila pneumoniae was used as the outgroup to root the tree. The locations within the tree of our C. symbiosum isolate (accession no. AY552789) along with the two previously published C. symbiosum isolates (accession no. M59112 and X66000) are boxed, with our isolate delineated with an arrow.
The genus Clostridium is comprised of at least 150 species of obligately anaerobic, endospore-forming, usually gram-positive, rod-shaped bacteria, the majority of which have not been implicated in human disease (1). Members of this genus are widely distributed in the environment, with a few species being found as part of the normal gastrointestinal and vaginal bacterial flora of humans and animals (1, 6). Although most clostridial species maintain a saprophytic existence, a number of infectious and toxin-mediated illnesses due to these organisms have been described in humans (1, 6). Toxin-mediated diseases such as food poisoning, botulism, and tetanus occur secondary to exogenous acquisition of C. perfringens, C. botulinum, and C. tetani, respectively, the last two species never occurring as part of the normal human bacterial flora (1). True clostridial infections, however, typically arise from endogenously colonizing strains that cause localized disease or which may disseminate to various anatomic sites due to perturbations in normal host defense mechanisms as a result of various conditions including trauma, surgery, hypoxia, diabetes, alcoholism, chemotherapy- or radiation-induced tissue damage, malignancy, or bowel perforation (1, 6, 7). Disease may range from relatively mild skin and soft tissue infections to more serious illnesses associated with significant morbidity and/or mortality, such as suppurative intra-abdominal, respiratory, and central nervous system infections; septicemia; necrotizing fasciitis; and myonecrosis (gas gangrene) (1, 6). Most of these cases have been due to C. perfringens, an organism commonly found as part of the normal intestinal flora of humans and which possesses a number of potent tissue-damaging exotoxins, although infections due to other clostridial species are being reported with increasing frequency (1, 6, 7). Clostridium spp. are not infrequent causes of bacteremia, accounting for about 1% of all significant blood culture isolates (1, 5). The presence of clostridial bacteremia is often associated with an underlying malignancy, particularly colon cancer, but may also be secondary to skin contamination or transient bacteremia of no clinical significance in patients without a compatible underlying condition (2, 5, 6, 7). However, the mortality rate in case series of patients with clostridial bacteremia has been reported to be as high as 50% but may be explained, in part, by the presence of underlying medical conditions in the affected hosts (2, 7). As far as we are aware, there have been no previous case reports, in the world literature, of human infection due to C. symbiosum, although ours is not the first to mention recovery of the organism from a clinical source (1). This organism was first described almost 3 decades ago and was previously known as Fusobacterium symbiosum, given its gram-negative staining characteristics, but was later reassigned to the genus Clostridium by virtue of its ability to produce endospores (3). It may be found as part of the human intestinal bacterial flora (3, 4, 8). Phenotypically, C. symbiosum is a non-toxin-producing strict anaerobe that forms subterminal endospores and demonstrates flagellar motility (4). Like certain members of the genus Clostridium (e.g., C. clostridium and C. ramosum), it has a propensity to stain gram negative (3). Although a number of biochemical tests can be used to help differentiate this organism from other Clostridium spp. (4), these methods are cumbersome and time-consuming. Many laboratories are now resorting to the use of more-definitive identification methods, such as 16S rRNA gene sequencing, for characterizing phenotypically difficult-to-identify bacteria of potential medical importance, including anaerobic organisms (10). Partial 16S rRNA gene sequencing of our patient's blood culture isolates resulted in definitive species identification. Phylogenetically, C. symbiosum displays the closest relationships with C. hathewayi, C. clostridioforme, C. celerecrescens, and C. sphenoides (Fig. 1), all of which may be found as part of the normal human intestinal bacterial flora (9) and also have gram-negative staining properties (1, 4, 8, 9). Given our patient's predisposition to a clostridial infection, his clinical presentation, and the fact that two separate blood culture sets were positive for C. symbiosum, we conclude that infection with this organism was clinically significant, although we could not definitively prove that the infection was endogenous or whether or not it was the ultimate cause of his demise.
Nucleotide sequence accession number.
The sequence for the C. symbiosum isolate from this study was submitted to GenBank under accession number AY552789.
REFERENCES
- 1.Allen, S. D., C. L. Emery, and D. M. Lyerly. 2003. Clostridium, p. 835-856. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 2.Bodey, G. P., S. Rodriguez, V. Fainstein, and L. S. Elting. 1991. Clostridial bacteremia in cancer patients. A 12-year experience. Cancer 67:1928-1942. [DOI] [PubMed] [Google Scholar]
- 3.Johnson, M. J., E. Thatcher, and M. E. Cox. 1995. Techniques for controlling variability in Gram staining of obligate anaerobes. J. Clin. Microbiol. 33:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneuchi, C., K. Watanabe, A. Terada, Y. Benno, and T. Mitsuoka. 1976. Taxonomic study of Bacteroides clostridiiformis subsp. clostridiiformis (Burri and Ankersmit) Holdeman and Moore and of related organisms: proposal of Clostridium clostridiiformis (Burri and Ankersmit) comb. nov. and Clostridium symbiosum (Stevens) comb. nov. Int. J. Syst. Bacteriol. 26:195-204. [Google Scholar]
- 5.Lombardi, D. P., and N. C. Engleberg. 1992. Anaerobic bacteremia: incidence, patient characteristics, and clinical significance. Am. J. Med. 92:53-60. [DOI] [PubMed] [Google Scholar]
- 6.Lorber, B. 2000. Gas gangrene and other Clostridium-associated diseases, p. 2549-2561. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 7.Rechner, P. M., W. A. Agger, K. Mruz, and T. H. Cogbill. 2001. Clinical features of clostridial bacteremia: a review from a rural area. Clin. Infect. Dis. 33:349-353. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki, K., Y. Benno, T. Mitsuoka, S. Takebe, K. Kobashi, and J. Hase. 1979. Urease-producing species of intestinal anaerobes and their activities. Appl. Environ. Microbiol. 37:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson, K. H., and R. B. Blitchington. 1996. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 62:2273-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo, P. C., K. H. Ng, S. K. Lau, K. T. Yip, A. M. Fung, K. W. Leung, D. M. Tam, T. L. Que, and K. Y. Yuen. 2003. Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. J. Clin. Microbiol. 41:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]