Abstract
Nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in 226 children in different settings (in a crèche [day care center], in an orphanage, and at home) during two seasons (winter and spring) was studied. The rates of carriage of S. pneumoniae and H. influenzae were markedly higher in the crèche and in the orphanage than in the home setting (e.g., 56.5, 63.3, and 25.9%, respectively, for S. pneumoniae in winter). Approximately 80% of the S. pneumoniae isolates identified in the crèche and in the orphanage belonged to the serotypes represented in the seven-valent pneumococcal vaccine, and 4.4% of the children were colonized by H. influenzae type b. Almost all H. influenzae isolates were fully susceptible to the antimicrobial agents tested, and only five (3.6%) produced β-lactamase; in contrast, 100% of the M. catarrhalis isolates were β-lactamase positive. Among S. pneumoniae isolates, 36.2% were nonsusceptible to penicillin (PNSP) and 11.8% were fully resistant to penicillin (PRP). All PNSP isolates were obtained from children at the crèche and at the orphanage but not among children brought up at home, and all PRP isolates showed a multiresistant phenotype. Colonization by PRP isolates correlated well with prior treatment with β-lactams. For the majority of children colonized at both sampling times, strain replacement of S. pneumoniae and H. influenzae was observed; long-term colonization by a single strain was rare.
The nasopharynx of children is colonized by a broad variety of microorganisms, including such potential pathogens as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Well-known risk factors that promote bacterial carriage include overcrowding, e.g., due to attending day care centers (DCCs) (crèches) or residing in orphanages; frequent viral infections; and an excessive use of antibiotics (12). DCCs and orphanages have also been identified as places that promote the clonal spread of multiresistant strains; thus, healthy children represent an important reservoir of bacterial strains, in particular, S. pneumoniae, that are resistant to many antimicrobial agents. These strains are subsequently disseminated in the community by other contacts of colonized children, e.g., their siblings and parents. It is possible to limit the spread of H. influenzae type b and some serotypes of S. pneumoniae by vaccination, but studies of the impact of immunization on colonization have yielded discrepant results (5, 11, 14, 22).
Colonization of mucosal surfaces in the human respiratory tract represents a dynamic process in which bacteria are acquired, eliminated, and reacquired many times in a lifetime. Extensive and often excessive use of antibiotics can promote the replacement of strains susceptible to antimicrobial agents by resistant ones (12, 28). Moreover, the human nasopharynx is also often viewed as a place where frequent genetic exchange takes place due to such processes as transformation with foreign DNA, bacterial intra- and interspecies conjugation, and transduction by phages. Thus, in this niche, bacterial strains gain novel properties with respect to resistance and virulence.
The aims of this study were to analyze the risk factors for nasopharyngeal carriage of potential respiratory pathogens in children under the age of 5 years in three selected settings in Warsaw and to determine the susceptibilities of bacterial isolates to antimicrobial agents and the serotype distributions among S. pneumoniae and H. influenzae isolates. Moreover, we monitored the dynamics of S. pneumoniae and H. influenzae carriage by obtaining samples from some children during two seasons (winter and spring) and using molecular typing methods for the analysis of isolates.
MATERIALS AND METHODS
Investigated population.
Nasopharyngeal samples were collected over the winter and spring seasons in the period between November 2000 and May 2001 from 226 children between 6 months and 5 years old and belonging to three different social groups in Warsaw. Sixty children were from a crèche, 108 children were from an orphanage, and 58 children were brought up at home and had neither attended a DCC nor lived in an orphanage. Seventy-seven children (34 from the crèche and 43 from the orphanage) were examined twice, once in the winter and again in the following spring, and 149 children were examined only once (75 in the winter and 74 in the spring). In total, 303 samples were obtained, 152 in the winter and 151 in the spring. For all children taking part in the study, an acute respiratory tract infection (RTI) was excluded on the basis of their medical history and by physical examination by pediatricians.
Questionnaire.
The collection of demographic data and information on previous antibiotic therapy, preceding RTIs (for 3 months before sampling), and vaccination was performed by way of a special questionnaire. Appropriate parts of the questionnaire were completed by parents, nurses in the orphanage, and pediatricians who examined children before samples were obtained.
Isolation and identification of strains.
Material was collected from the nasopharynx by pediatricians using sterile swabs and was transferred on Stuart's medium (EUROTUBO collection swab) to the Department of Epidemiology and Clinical Microbiology at the National Institute of Public Health within 4 h of collection. The swabs were used to inoculate (i) Columbia agar plates with 5% sheep blood and (ii) chocolate agar plates with bacitracin (bioMérieux, Marcy l'Etoile, France). The plates were incubated in air with 5% CO2 at 35°C overnight. Identification was performed by conventional microbiological methods as follows: for S. pneumoniae—colony morphology, susceptibility to optochin (bioMérieux), and bile solubility; for H. influenzae—colony morphology, growth on chocolate agar with bacitracin, and requirement for X (hemin) and V (NAD) factors (Oxoid, Basingstoke, United Kingdom); and for M. catarrhalis—colony morphology and positive oxidase (BBL, Le Pont de Claix, France) and tributyrin (A/S Rosco, Taastrup, Denmark) tests. For S. pneumoniae and H. influenzae, four colonies were propagated separately per sample, and if any differences in colony morphology were observed, all such variants were tested further (susceptibility testing, serotyping, and pulsed-field gel electrophoresis [PFGE] typing).
Susceptibility testing.
MICs of the following antimicrobial agents were determined according to National Committee for Clinical Laboratory Standards (NCCLS) methodology (25): for S. pneumoniae—penicillin, cefuroxime, and erythromycin (Sigma-Aldrich, Steinheim, Germany), amoxicillin (SmithKline Beecham, Worthing, West Sussex, United Kingdom), cefotaxime (ICN Biomedicals, Eschwege, Germany), imipenem (Merck Sharp & Dohme, Haarlem, Holland), trimethoprim-sulfamethoxazole (Roche, Basel, Switzerland), clindamycin (Pharmacia, Kalamazoo, Mich.), doxycycline (Pfizer, Groton, Conn.), rifampin (Lepetit, Anagni, France), and vancomycin (Lilly, Indianapolis, Ind.); and for H. influenzae—ampicillin and amoxicillin-clavulanic acid (SmithKline Beecham), cefuroxime, cefotaxime, trimethoprim-sulfamethoxazole, azithromycin (Pliva, Cracow, Poland), rifampin, doxycycline, and chloramphenicol (Fournier Pierrel Farma, Milano, Italy). Standard type strains recommended by NCCLS were included for quality control purposes. For all M. catarrhalis isolates and ampicillin-resistant H. influenzae isolates, the production of a β-lactamase was tested with the nitrocefin test (BBL) by following the manufacturer's instructions.
Serotyping.
Serotyping of S. pneumoniae isolates was performed by capsular swelling at Statens Serum Institute, Copenhagen, Denmark. All H. influenzae isolates were tested for the presence of capsule and type-specific genes by PCR (9, 15).
Molecular analysis of S. pneumoniae and H. influenzae isolates.
DNA isolation and PFGE of SmaI-digested DNA were performed as described by Luna and Roberts (20) and by Tarasi et al. (35) for S. pneumoniae and H. influenzae, respectively. To assign PFGE patterns to particular types, the criteria proposed by Tenover et al. were applied (36).
For multilocus sequence typing (MLST), the original procedure of Enright and Spratt (8) was followed. Allele numbers and sequence types (ST) of isolates were identified by using the MLST database (www.mlst.net).
Statistical analysis.
Demographic data, previous antibiotic therapy, RTIs, and other tested variables were analyzed by using frequency tables. For the interpretation of data and for comparison of distributions and differences between studied populations, the chi-square test (χ2) with v-square or Yates' correction as appropriate was used with 95% confidence intervals. For assessment of the incidence of nasopharyngeal carriage, each population in different settings was analyzed twice—excluding or including children with samples collected during winter and spring. Because there was no significant difference between these analyses, children with repeated samplings were included in the whole group for calculation of the primary carriage frequency index.
RESULTS
Study population.
This study involved 226 children less than 5 years old. Of the total number of 303 samples examined, 52.8% were obtained from boys and 47.2% were obtained from girls. During the winter sampling, 83 samples were obtained from boys and 69 samples were obtained from girls; in the spring sampling, 86 and 65 samples were obtained from boys and girls, respectively. Overall, 44.6% of samples were derived from children less than 2 years old (74 of 152 samples in winter and 61 of 151 samples in spring).
RTI, prior antibiotic treatment, and vaccination.
During the 3 months before the winter sampling 72.1% of children in the crèche, 88.3% in the orphanage, and 48.1% in the home setting had presented with symptoms of RTIs; 48.4, 66.2, and 30.8% of these children, respectively, had received antibiotics. Before the spring sampling, the percentages of children presenting with RTIs were 51.2% in the crèche, 90.9% in the orphanage, and 66.7% in the home setting; the percentages of these children who had received antibiotics were 36.4, 71.7, and 55.5%, respectively. For children in the orphanage, which was the most homogeneous and numerous group in our study, similar high levels of RTIs and antibiotic usage before both samplings were observed. In the crèche, the frequencies of RTIs and antibiotic usage were higher before the winter sampling. In the home setting, we observed significant increases in RTIs and antibiotic usage before the spring sampling.
β-Lactams, including amoxicillin, amoxicillin-clavulanic acid, penicillin, cefuroxime, cefadroxil, and cefaclor, were the antibiotics most frequently prescribed (90 children), followed by trimethoprim-sulfamethoxazole (15 children) and macrolides (13 children).
None of the children in the orphanage was vaccinated against H. influenzae type b. In the home setting, six children were vaccinated against H. influenzae type b, but the data concerning vaccination against H. influenzae type b for the children in the crèche were not complete. None of the children included in the study was vaccinated against S. pneumoniae.
Incidence of nasopharyngeal colonization.
The percentages of carriers of S. pneumoniae, H. influenzae, and M. catarrhalis in the investigated centers in the two seasons (winter and spring) are shown in Table 1. Significant differences in levels of carriage of S. pneumoniae were observed both in winter and in spring between the crèche and the home setting (P < 0.05) and between the orphanage and the home setting (P < 0.01) but not between the crèche and the orphanage. Similar differences were observed for H. influenzae in the spring; however, in the winter, significant differences in carriage levels were found between the crèche and the orphanage (P < 0.01). We did not find any significant differences in the levels of carriage of M. catarrhalis between centers (P > 0.05) or between investigated seasons (P > 0.05), except for the orphanage, where we observed an increase in levels of carriage in the spring. A total of 123 children were colonized at the same time by more than one pathogen, including 51 colonized by S. pneumoniae and H. influenzae, 12 colonized by H. influenzae and M. catarrhalis, 24 colonized by S. pneumoniae and M. catarrhalis, and 36 colonized by all three species.
TABLE 1.
Percentages of carriers among investigated groups of children colonized by various species
| Speciesa | % (no.) of carriers in the following season and settingb:
|
|||||
|---|---|---|---|---|---|---|
| Winter (n = 152)
|
Spring (n = 151)
|
|||||
| Crèche (n = 46) | Orphanage (n = 79) | Home (n = 27) | Crèche (n = 48) | Orphanage (n = 72) | Home (n = 31) | |
| S. pneumoniae | 56.5 (26)A | 63.3 (50)A | 25.9 (7)AA | 54.2 (26)D | 51.4 (37)E | 19.3 (6)DE |
| PSP | 34.8 (16) | 36.7 (29) | 25.9 (7) | 39.6 (19) | 27.8 (20) | 19.3 (6) |
| PISP | 21.7 (10) | 26.6 (21) | 0 | 6.25 (3) | 4.2 (3) | 0 |
| PRP | 0 | 0 | 0 | 8.3 (4) | 19.4 (14) | 0 |
| H. influenzae | 23.9 (11)CH | 58.2 (46)BC | 11.1 (3)B | 50.0 (24)FH | 69.4 (50)G | 3.2 (1)FG |
| Type b | 0 | 7.6 (6) | 0 | 0 | 0 | 0 |
| Non-type b | 23.9 (11) | 50.6 (40) | 11.1 (3) | 50.0 (24) | 69.4 (50) | 3.2 (1) |
| M. catarrhalis | 36.9 (17) | 27.8 (22)I | 11.1 (3) | 31.2 (15) | 41.7 (30)I | 29.0 (9) |
PSP and PISP, S. pneumoniae isolates that were susceptible and intermediate to penicillin, respectively.
Pairs of values in which significant relationships were observed upon comparison by the chi-square test are labeled with the same letters; roman type represents a P value of <0.01, italic type represents a P value of <0.05.
Colonies showing two different morphologies were found for five S. pneumoniae and four H. influenzae isolations. The PFGE analysis confirmed the heterogeneity of one S. pneumoniae and two H. influenzae isolates, thus revealing simultaneous colonization by two different strains of the same species.
Susceptibilities to antimicrobial agents.
Altogether, 152 isolates of S. pneumoniae, 136 isolates of H. influenzae, and 96 isolates of M. catarrhalis were collected. Data on the susceptibilities of S. pneumoniae and H. influenzae to antibiotics are shown in Table 2. In the spring, a decrease in pneumococci intermediately resistant to penicillin was observed along with a concomitant increase in isolates resistant to penicillin (PRP). Overall, 55 isolates of S. pneumoniae (36.2%) were nonsusceptible to penicillin (PNSP), and of these, 18 (11.8% of all isolates) were PRP isolates. They were isolated in the orphanage (14 isolates) and the crèche (4 isolates). Penicillin MICs for PRP isolated in the orphanage were 4 to 8 mg/liter; these isolates were also resistant to other β-lactams, such as amoxicillin, cefuroxime, and cefotaxime, and intermediately resistant to imipenem. For PRP isolated in the crèche, the penicillin MIC was 2 mg/liter, and these isolates showed resistance to cefuroxime and intermediate resistance to imipenem. All of the PRP isolates displayed a multiresistant phenotype, with the isolates from the orphanage being resistant to trimethoprim-sulfamethoxazole, erythromycin, clindamycin, chloramphenicol, and doxycycline and the crèche isolates being resistant to trimethoprim-sulfamethoxazole, chloramphenicol, and doxycycline. Thirteen PRP isolates were recovered from children who had been treated with β-lactams shortly before the study started, and two PRP isolates were recovered from a child who had not been treated with any antimicrobial agents during the 3-month period before sampling. No data on antibiotic prescriptions were available for three of the children investigated. No PNSP isolates were recovered from the group of children brought up at home (Table 1), despite the fact that eight of these children (13.8%) had been treated with β-lactams.
TABLE 2.
Susceptibilities to antimicrobial agents of S. pneumoniae and H. influenzae strains isolated from the nasopharynges of children
| Antibiotic | No. (%) of susceptible strains of the following species in the indicated settinga:
|
|||||
|---|---|---|---|---|---|---|
|
S. pneumoniae
|
H. influenzae
|
|||||
| Crèche (n = 52) | Orphanage (n = 87) | Home (n = 13) | Crèche (n = 35) | Orphanage (n = 96) | Home (n = 4) | |
| Penicillin | 35 (67.3)A | 49 (56.3)B | 13 (100)AB | NT | NT | NT |
| Amoxicillin | 52 (100)C | 73 (83.9)C | 13 (100) | NT | NT | NT |
| Ampicillin | NT | NT | NT | 33 (94.3) | 93 (96.9) | 5 (100) |
| Amoxicillin-clavulanic acid | NT | NT | NT | 35 (100) | 96 (100) | 5 (100) |
| Cefuroxime | 48 (92.3) | 73 (83.9) | 13 (100) | 35 (100) | 96 (100) | 5 (100) |
| Cefotaxime | 52 (100)D | 73 (83.9)D | 13 (100) | 35 (100) | 96 (100) | 5 (100) |
| Imipenem | 48 (92.3) | 74 (85.1) | 13 (100) | NT | NT | NT |
| Erythromycin | 38 (73.1)E | 35 (40.2)EF | 12 (92.3)F | NT | NT | NT |
| Azithromycin | NT | NT | NT | 35 (100) | 96 (100) | 5 (100) |
| Clindamycin | 38 (73, 1)G | 35 (40.2)GH | 13 (100)H | NT | NT | NT |
| Trimethoprim-sulfamethoxazole | 10 (19.2)I | 11 (12.6)J | 7 (53.8)IJ | 34 (97.1)M | 62 (64.6)M | 5 (100) |
| Doxycycline | 27 (51.9) | 40 (46)K | 10 (76.9)K | 35 (100) | 96 (100) | 5 (100) |
| Chloramphenicol | 29 (55.8)L | 64 (73.6)L | 8 (61,5) | NT | NT | NT |
| Rifampin | 52 (100) | 87 (100) | 13 (100) | 35 (100) | 96 (100) | 5 (100) |
| Vancomycin | 52 (100) | 87 (100) | 13 (100) | NT | NT | NT |
Pairs of values in which significant relationships were observed upon comparison by the chi-square test are labeled with the same letters (P < 0.05). NT, not tested.
A high prevalence of isolates resistant to erythromycin and clindamycin was also observed both in the orphanage setting and in the crèche setting. A high percentage of pneumococci from all three investigated centers was resistant to trimethoprim-sulfamethoxazole, but the percentage was markedly higher in the orphanage and in the crèche (the P value for the orphanage versus the home setting and for the crèche versus the home setting was <0.05). However, the number treated with macrolides or trimethoprim-sulfamethoxazole in the respective centers, in particular, children colonized by macrolide-resistant or trimethoprim-sulfamethoxazole-resistant strains, was not high. For the remaining antibiotics, decreased susceptibility to doxycycline and chloramphenicol was also observed in the crèche, the orphanage, and the home setting, despite the fact that no child had been treated with these antibiotics.
Almost all isolates of H. influenzae were fully susceptible to the antimicrobial agents tested. Significant differences were observed between orphanage and crèche isolates only in susceptibility to trimethoprim-sulfamethoxazole (P < 0.05). Out of 136 isolates of H. influenzae, only 5 (2 from the crèche and 3 from the orphanage) were resistant to ampicillin, and all of these produced β-lactamase. In this study, 100% of M. catarrhalis isolates were β-lactamase positive.
Serotypes of carried bacteria.
Data concerning pneumococcal serotypes, which were partially published previously (33), showed that in 152 isolates of S. pneumoniae, the most frequent serotypes were 6B (62 isolates), 23F (36 isolates), 19F (22 isolates), and 6A (11 isolates). The remaining serotypes, represented by one or two isolates each, were diverse (3, 4, 7C, 9N, 10A, 14, 15C, 18C, 20, 24F, 33F, 34, and 38). Five strains were nontypeable (rough). All of the PRP isolates (both from the orphanage and from the crèche) belonged to serotype 23F and were isolated in the spring. The pneumococcal serotypes in the crèche and in the orphanage are shown in Table 3. Thirteen isolates from children brought up at home belonged to various serotypes (3, 6B, 14, 15C, 18C, 19F, 20, 24F, 33F, 34, and 38) and were mostly represented by one or two isolates each.
TABLE 3.
Pneumococcal serotype distributions in the crèche and in the orphanage
| Serotype | No. of pneumococcal isolates represented by the given serotype in the following setting and season:
|
|||
|---|---|---|---|---|
| Crèche
|
Orphanage
|
|||
| Winter (n = 26) | Spring (n = 26) | Winter (n = 50) | Spring (n = 37) | |
| 4 | 0 | 0 | 1 | 0 |
| 6A | 1 | 1 | 6 | 3 |
| 6B | 10 | 11 | 28 | 12 |
| 7C | 0 | 2 | 0 | 0 |
| 9N | 1 | 0 | 0 | 0 |
| 10A | 0 | 0 | 0 | 1 |
| 14 | 1 | 0 | 0 | 0 |
| 19F | 11 | 3 | 2 | 4 |
| 23F | 1 | 7 | 12 | 16 |
| Rough | 1 | 2 | 1 | 1 |
Out of 136 isolates of H. influenzae, 6 (4.4%) were found to be capsule positive by PCR with cap-specific primers. All of these isolates belonged to capsular serotype b (H. influenzae type b) and were isolated in the orphanage in the winter.
Dynamics of S. pneumoniae and H. influenzae carriage in the nasopharynx.
Seventy-seven children (34 from the crèche and 43 from the orphanage) were examined twice. The majority (66 in winter and 71 in spring) were colonized by one or more of the investigated species. There were no significant differences in the frequencies of colonization by one (32 children in winter and 33 in spring), two (27 children in winter and 28 in spring), or three (7 children in winter and 10 in spring) species between the winter and the spring seasons. Out of the 77 children, 21 (9 from the crèche and 12 from the orphanage) were colonized by S. pneumoniae both at the first and at the second samplings and yielded 43 isolates (due to the fact that two morphologically distinguishable isolates were obtained from the same child in the spring).
Analyses of antimicrobial susceptibility phenotypes, serotyping data, and molecular typing data (PFGE and MLST) were performed to study the dynamics of pneumococcal carriage in these children (Table 4 and Fig. 1). Among the 43 pneumococcal isolates, 11 PFGE types and 10 different STs, including 9 known STs and 1 novel ST (ST1021), were detected. Three PFGE clones were predominant, represented by isolates with A (eight isolates), B (nine isolates), and J (seven isolates) profiles. Isolates with profile A were isolated both from the orphanage and from the crèche. All members of this clone expressed capsular polysaccharide 19F and belonged to ST423. However, the orphanage isolates exhibited a susceptibility pattern different from that of the crèche isolates. Isolates with PFGE profile B were isolated in the winter both from the orphanage and from the crèche. All of these isolates belonged to serotype 6B and had similar susceptibility patterns. There were four different PFGE subtypes (B1 to B4) of this clone. All of these isolates belonged to international clone Poland6B-20 (ST315) (30). The third widespread clone, with PFGE profile J and ST490, was found only in the orphanage but in both winter and spring. There were two PFGE subtypes of clone J. All of these isolates belonged to serotype 6A. However, the J2 subtype, which was isolated in the spring, was intermediately susceptible to trimethoprim-sulfamethoxazole; in contrast, the J1 subtype, which was isolated in the winter, was susceptible to all investigated antimicrobial agents. All of the PRP isolates were represented by clones with PFGE profiles C and I and ST81 (characteristic for global clone Spain23F-1) and ST272 (clone Poland23F-16), respectively. One of these clones was spread in the orphanage, and the other was spread in the crèche. Although determined by PFGE and MLST analyses to be unrelated, both clones expressed capsular polysaccharide 23F and were multiresistant (Table 4).
TABLE 4.
Characteristics of pneumococcal strains isolated from children colonized both in winter and in spring
| Penicillin MIC (mg/liter) | Resistance profilea | Serotype | PFGE type | MLST allelic profile
|
ST | Season in which the indicated children in the following setting were colonized by pneumococcic:
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crèche
|
Orphanage
|
|||||||||||||||||||||||||||||||
| aroE | gdh | gki | recP | spi | xpt | doll | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |||||
| 0.015 | T Cl D | 19F | A | 1 | 5 | 4 | 12 | 5 | 3 | 8 | 423 | S | S | W | W | |||||||||||||||||
| 0.015 | E C Cl D | 19F | A | 1 | 5 | 4 | 12 | 5 | 3 | 8 | 423 | S | S | S | S | |||||||||||||||||
| 0.12 | T E C D | 6B | B1 | 20 | 28 | 1 | 1 | 15 | 14 | 14 | 315 | W | W | W | ||||||||||||||||||
| 0.12 | T E C D | 6B | B2 | 20 | 28 | 1 | 1 | 15 | 14 | 14 | 315 | W | ||||||||||||||||||||
| 0.12-0.25 | T E C D | 6B | B3 | 20 | 28 | 1 | 1 | 15 | 14 | 14 | 315 | W | W | W | W | |||||||||||||||||
| 0.12 | T E C D | 6B | B4 | 20 | 28 | 1 | 1 | 15 | 14 | 14 | 315 | W | ||||||||||||||||||||
| 2 | Cr I T Cl D | 23F | C | 4 | 4 | 2 | 4 | 4 | 1 | 1 | 81 | S | S | S | ||||||||||||||||||
| 0.015 | T | 6B | D1 | 7 | 13 | 8 | 6 | 10 | 6 | 14 | 176 | S | B | S | ||||||||||||||||||
| 0.015 | T | 6B | D2 | 7 | 13 | 8 | 6 | 10 | 6 | 14 | 176 | S | ||||||||||||||||||||
| 0.015 | T | 6B | D3 | 7 | 13 | 8 | 6 | 10 | 6 | 14 | 176 | S | ||||||||||||||||||||
| 0.015 | T | 9N | E1 | 2 | 8 | 2 | 4 | 6 | 1 | 1 | 66 | W | ||||||||||||||||||||
| 0.015 | T | 23F | E2 | 2 | 8 | 2 | 4 | 6 | 1 | 1 | 66 | W | ||||||||||||||||||||
| 0.03 | All | 7C | F | 7 | 10 | 1 | 8 | 10 | 28 | 8 | 1021 | S | S | |||||||||||||||||||
| 0.015 | D | 23F | G1 | 7 | 5 | 1 | 1 | 13 | 31 | 14 | 440 | S | ||||||||||||||||||||
| 0.06 | D | 23F | G2 | 7 | 5 | 1 | 1 | 13 | 31 | 14 | 440 | W | ||||||||||||||||||||
| 0.015 | All | 6B | H | 7 | 13 | 8 | 6 | 10 | 6 | 14 | 176 | W | ||||||||||||||||||||
| 8 | A Cr Cf I T E C Cl D | 23F | I | 7 | 13 | 8 | 1 | 10 | 6 | 37 | 272 | S | S | |||||||||||||||||||
| 0.015 | All | 6A | J1 | 2 | 13 | 9 | 1 | 6 | 19 | 14 | 490 | W | W | W | W | W | ||||||||||||||||
| 0.015 | T | 6A | J2 | 2 | 13 | 9 | 1 | 6 | 19 | 14 | 490 | S | S | |||||||||||||||||||
| 0.015 | T E C | Rough | K | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | |||||||||||||||||||||
T, trimethoprim-sulfamethoxazole; Cl, chloramphenicol; D, doxycycline; E, erythromycin; C, clindamycin; Cr, cefuroxime; I, imipenem; A, amoxicillin; Cf, cefotaxime. All, strains were susceptible to all antibiotics tested. Bold type indicates that strains were intermediately susceptible.
Numbers are alleles.
W, winter; S, spring; B, both winter and spring (one child).
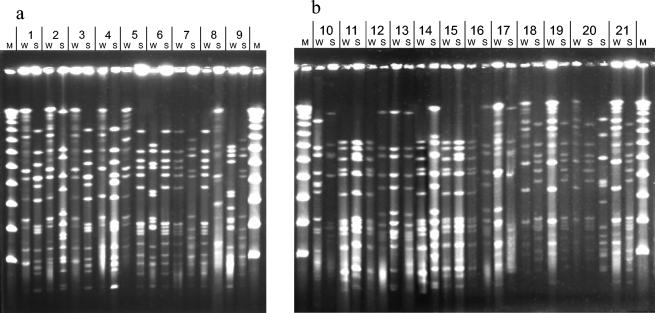
FIG. 1.
PFGE patterns of S. pneumoniae isolates cultured in winter (W) and spring (S) from the children who were investigated twice. The isolates are grouped in pairs collected from different children and are designated with numbers corresponding to the numbers used in Table 4. (a) Isolates from children at the crèche. (b) Isolates from children at the orphanage. M, λ ladder DNA molecular size marker (New England BioLabs Inc.).
In most cases, pneumococci isolated from the same child in two different seasons (winter and spring) were unrelated. Out of 21 children, only 3 were colonized by the same or a closely related strains during the first and second samplings (PFGE types D1-D1, J1-J2, and J1-J2). The first pair of these strains belonged to serotype 6B, and the two other pairs belonged to serotype 6A. Five children were colonized in winter by a strain that was susceptible or intermediately susceptible to penicillin (PFGE types G2, H, B2, B1, and B1) and by a resistant strain in spring (PFGE types I, I, C, C, and C). Molecular analysis of these strains showed that this pattern was due to the acquisition of a new strain in the nasopharynx.
The H. influenzae isolates collected in both seasons from the same children were analyzed by PFGE as well. From the 24 pairs of isolates from 24 children, 2 pairs were excluded because DNA preparations of the winter isolates could not be digested by SmaI (32). Of the remaining pairs, only 3 consisted of indistinguishable isolates, whereas 19 pairs were heterogeneous.
DISCUSSION
Soon after birth, the nasopharynx of children becomes colonized by a variety of microorganisms, including commensal bacteria that may be pathogenic, such as S. pneumoniae, H. influenzae, and M. catarrhalis. Several different factors are known to affect the frequency of carriage of these species. Increased carriage rates are often observed in settings such as DCCs and orphanages, as these are efficient environments for the transmission of bacteria due to the often crowded conditions and frequent contact among children for long periods of time (27, 29, 39). In this study, we found that the nasopharyngeal carriage rates were markedly higher both in the crèche and in the orphanage settings than in the home setting. We demonstrated that there were significant differences in the levels of carriage of S. pneumoniae and H. influenzae for the orphanage and the crèche settings versus the home setting, whereas there were no such differences between the crèche and the orphanage, except for the higher rate of carriage of H. influenzae in the orphanage in winter. However, for M. catarrhalis, we found no significant differences between the centers, and the level of colonization by M. catarrhalis in the crèche was lower than that in other studies (7, 34).
Respiratory pathogens are recovered from the nasopharynx of healthy children throughout the year (33); however, some authors (37, 39) have reported seasonal differences in the rates of colonization, with a peak in the winter. In other studies (21, 34), the seasonal effect for colonization by S. pneumoniae, H. influenzae, and M. catarrhalis has been shown to be insignificant or absent. In this study, we made a similar observation between two seasons, winter and spring, and only in the cases of H. influenzae in the crèche and M. catarrhalis in the orphanage we did observe an increase in colonization during the spring (the P value for the crèche in the winter versus the crèche in the spring was <0.05). It is well established that high antibiotic usage exerts on the nasopharyngeal flora a selective pressure that favors the acquisition of resistant strains and hence is a risk factor for the clonal spread of such bacteria, with greater pathogenic potential (6, 18). This phenomenon occurred both in the crèche and in the orphanage facilities in this study, where the spread of two unrelated pneumococcal clones clearly correlated with prior treatment with β-lactams. Similar associations were reported by other researchers in relation to macrolides and trimethoprim-sulfamethoxazole (1, 19). In this study, these antimicrobial agents were prescribed only occasionally in both centers. However, we found a significant percentage of pneumococcal isolates that were resistant to macrolides and trimethoprim-sulfamethoxazole; this finding may have been due in part to the clonal dissemination of multiresistant PRP isolates, as previously discussed.
Data from the beginning of the 1990s in Poland showed that pneumococci isolated from the nasopharynx were fully susceptible, while resistance to this drug had already occurred in other countries in Central Europe and Eastern Europe (2). Since then, a gradual increase in penicillin resistance has been noted among pneumococci isolated from various sources in Poland. In a recent study, 14.4% of isolates from RTIs and 13% from meningitis were reported to be PNSP isolates (10, 31); in this study, 36.2% of pneumococci from the nasopharynx were PNSP and, of these, almost one-third, or 11.8% of the total, were fully resistant to penicillin.
The incidences of β-lactamase-positive H. influenzae strains isolated from the nasopharynx vary greatly among countries, from 1.8% in The Netherlands (26) to 20% in Portugal (7) and 47.4% in France (4). In this study, only five isolates of H. influenzae (3.7%) produced β-lactamase. Despite the high consumption of β-lactams in Poland, the frequency of β-lactamase-positive H. influenzae strains isolated from lower RTIs remains low (16). The data from a recent multicenter study showed that 0% of encapsulated and 4.4% of nontypeable H. influenzae strains isolated between 1996 and 2001 from cases of community-acquired lower RTIs in Poland were β-lactamase positive (A. Skoczyñska, M. Lewandowska, A. Klarowicz, and W. Hryniewicz, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-1586, 2003). However, the percentage of β-lactamase-positive strains isolated from cerebrospinal fluid between 1997 and 2002 in Poland was found to be as high as 14% (17). This epidemiological trend concerning a lower frequency of β-lactamase-positive H. influenzae strains isolated from the respiratory tract is characteristic of the situation in Poland.
H. influenzae recovered from cases of carriage typically lacks a capsule (4, 26). In a previous study in England (14), the overall H. influenzae type b nasopharyngeal carriage rate among children 1 to 4 years old was 3.97% before routine vaccination was introduced, but following the introduction of vaccination, the rate of H. influenzae type b (Hib) isolation decreased to 0.7%. In this study, 4.4% of children were colonized with Hib. As vaccination of children with the H. influenzae vaccine is not routinely implemented in Poland, the observed level of Hib carriage is not surprising.
Like the decrease in Hib carriage, a decrease in the carriage of S. pneumoniae strains representing the pneumococcal vaccine serotypes is known to occur in children immunized with conjugate vaccines (5). The facts that approximately 90% of the isolates identified both in the crèche and in the orphanage settings belonged to the serotypes included in the seven-valent pneumococcal conjugate vaccine and that all penicillin-resistant and multiresistant isolates in this study belonged to serotype 23F, which is present in the vaccine, strongly suggest that vaccination could interrupt the transmission of these isolates in such high-risk communities.
It is known that colonization with pneumococci can last for up to 4 months and that strains colonizing the nasopharynx for longer periods of time usually belong to serotypes 6B and 6A, which are known to be weak inducers of immunity (13, 29). This finding was also confirmed in this study. Out of 77 children investigated twice over a 4-month period, only 3 were colonized at both sampling times with the same or closely related pneumococcal isolates belonging to serotype 6A or 6B.
In a number of cases, susceptibility patterns and/or serotypes of pneumococci colonizing the nasopharynx of a given child varied at different points in time. Using two molecular typing methods, PFGE and MLST, we showed that these differences were basically due to the replacement of susceptible pneumococcal strains with unrelated resistant ones and not to the rapid acquisition of resistance genes. A similar observation was made by Meats et al. (24) with respect to pneumococcal serotypes. Strain replacement was observed in general in most of the cases of repeated isolation of S. pneumoniae and/or H. influenzae from a single child, regardless of the eventual differences in susceptibility. However, the simultaneous presence of two (or more) strains cannot be completely excluded, since the PFGE analysis was performed with a limited number of double isolates collected from the same child at the same time point.
The two molecular typing methods used in this study showed similar discriminatory powers (11 PFGE types versus 10 STs due to the fact that two isolates of ST176 were unrelated in terms of PFGE types). Of the STs present in the studied group, only ST176 was related to ST272, representing its double-locus variant. These two clones, however, differed in serotype (6B and 23F, respectively) and resistance phenotype. MLST is particularly useful for determining the overall clonal relationships between isolates, as it allows comparison with all isolates published and/or reported to the MLST database (www.mlst.net). Except for two isolates of serotype 7C, which showed a novel allelic profile (ST1021), the remaining isolates represented known STs (3, 8, 30); many of these belonged to the internationally distributed pneumococcal clones, including Spain23F-1 (ST81), Poland23F-16 (ST272), and Poland6B-20 (ST315), that have been isolated worldwide from cases of carriage as well as various infections (23, 30). Isolates of ST66 differed at a single locus from international clone Tennessee14-19 but showed different serotypes (9N and 23F instead of 14) and resistance patterns. ST423 represented a double-locus variant of international clone England14-9 but showed serotype 19F. Isolates of four remaining STs (ST344, ST440, ST490, and ST1021) were unrelated to the international pneumococcal clones described so far. Our observations clearly indicate that under the conditions characteristic for the crèche and the orphanage settings, which greatly facilitate the transmission of nasopharyngeal bacteria, the appearance of epidemic resistant pneumococcal clones in association with greater antibiotic pressure leads to their efficient spread in these settings.
Acknowledgments
We acknowledge the use of the pneumococcal MLST database, which is located at Imperial College, London, United Kingdom, and is funded by the Wellcome Trust. This study was supported by the State Committee for Scientific Research (grant number 3 PO5E 03822).
We thank S. Murchan for critical reading of the manuscript.
REFERENCES
- 1.Abel-Haq, N., W. Abuhammour, B. Asmar, R. Thomas, S. Dabbagh, and R. Gonzales. 1999. Nasopharyngeal colonization with Streptococcus pneumoniae in children receiving trimethoprim-sulfamethoxazole prophylaxis. Pediatr. Infect. Dis. J. 18:647-649. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C., C. Gladkowa, W. Hryniewicz, B Kojouharov, D. Kotulova, F. Mihalcu, J. Schnider, L. Setchanoa, N. Semina, J. Trupl, S. Tyski, P. Urbaskova, and M. R. Jacobs. 1996. Carriage of antibiotic-resistant Streptococcus pneumoniae by children in Eastern and Central Europe—a multicenter study with use of standardized methods. Clin. Infect. Dis. 23:712-717. [DOI] [PubMed] [Google Scholar]
- 3.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 4.Dabernat, H., M. A. Plisson-Saune, C. Delmas, M. Seguy, G. Faucon, R. Pelissier, H. Carsenti, C. Pradier, M. Roussel-Delvallez, J. Leroy, M. J. Dupont, F. De Bels, and P. Dellamonica. 2003. Haemophilus influenzae carriage in children attending French day care centers: a molecular epidemiological study. J. Clin. Microbiol. 41:1664-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan, R., R. Melamed, M. Mullaem, L. Piglansky, D. Greenberg, O. Abramson, P. M. Mendelman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 174:1271-1278. [DOI] [PubMed] [Google Scholar]
- 6.De Lencastre, H., and A. Tomasz. 2002. From the ecological reservoir to disease: the nasopharynx, day care centers and drug-resistant clones of Streptococcus pneumoniae. J. Antimicrob. Chemother. 50(Suppl. S2):75-81. [DOI] [PubMed] [Google Scholar]
- 7.De Lencastre, H., K. G. Kristinsson, A. Brito-Avo, I. Santos Sanches, R. Sa-Leao, J. Saldanha, E. Sigvaldadottir, S. Karlsson, D. Oliveira, R. Mato, M. Aires de Sousa, and A. Tomasz. 1999. Carriage of respiratory tract pathogens and molecular epidemiology of Streptocccus pneumoniae colonization in healthy children attending day care centers in Lisbon, Portugal. Microb. Drug Resist. 5:19-29. [DOI] [PubMed] [Google Scholar]
- 8.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 9.Falla, T. J., D. W. Crook, L. N. Brophy, D. Maskell, J. S. Kroll, and E. R. Moxon. 1994. PCR for capsular typing of Haemophilus influenzae. J. Clin. Microbiol. 32:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felmingham, D., R. N. Gruneberg, et al. 2000. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J. Antimicrob. Chemother. 45:191-203. [DOI] [PubMed] [Google Scholar]
- 11.Galil, K., R. Singleton, O. S. Levine, M. A. Fitzgerald, L. Bulkow, M. Getty, B. A. Perkins, and A. Parkinson. 1999. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. J. Infect. Dis. 179:101-106. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rodrigues, J. A., and M. J. Fresnadillo Martinez. 2002. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J. Antimicrob. Chemother. 50(Suppl. S2):59-73. [DOI] [PubMed] [Google Scholar]
- 13.Ghaffaar, F., I. R. Friedland, and G. H. McCracken, Jr. 1999. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 18:638-646. [DOI] [PubMed] [Google Scholar]
- 14.Handford, S., A. Rushdy, and M. Ramsay. 1998. An evaluation of Haemophilus influenzae type b (Hib) vaccination and description of risk factors for Hib vaccine failure in Europe 1996-1999. Mid-study report. PHLS Communicable Disease Surveillance Centre, Colindale, London, United Kingdom.
- 15.Hobson, R. P., A. Williams, K. Rawal, T. H. Pennington, and K. J. Forbes. 1995. Incidence and spread of Haemophilus influenzae on an Antarctic base determined using the polymerase chain reaction. Epidemiol. Infect. 114:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hryniewicz, W. 2003. Alexander Project—5 years in Poland. Merkuriusz Lekarski 79:5-8. [PubMed] [Google Scholar]
- 17.Kadłubowski, M., A. Skoczyñska, A. Klarowicz, and W. Hryniewicz. 2003. Molecular characteristics of Haemophilus influenzae type b responsible for meningitis in Poland, 1997-2002, p. 98. In Proceedings of the 6th International Meeting on Microbial Epidemiological Markers, Les Diablerets, Switzerland. American Society for Microbiology, Washington, D.C.
- 18.Kristinsson, K. G. 1997. Effect of antimicrobial use and other risk factors on antimicrobial resistance in pneumococci. Microb. Drug Resist. 3:117-123. [DOI] [PubMed] [Google Scholar]
- 19.Leach, A. J., T. M. Shelby-James, M. Mayo, M. Gratten, A. C. Laming, and B. J. Currie. 1997. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage of Streptococcus pneumoniae. Clin. Infect. Dis. 24:356-362. [DOI] [PubMed] [Google Scholar]
- 20.Luna, V. A., and M. C. Roberts. 1998. The presence of the tetO gene in a variety of tetracycline-resistant Streptococcus pneumoniae serotypes from Washington State. J. Antimicrob. Chemother. 42:613-619. [DOI] [PubMed] [Google Scholar]
- 21.Marchissio, P., S. Gironi, S. Esposito, G. C. Schito, S. Mannelli, N. Principi, et al. 2001. Seasonal variation in nasopharyngeal carriage of respiratory pathogens in healthy Italian children attending day-care centers or schools. J. Med. Microbiol. 50:1095-1099. [DOI] [PubMed] [Google Scholar]
- 22.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of nonvalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 23.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meats, E., A. B. Brueggemann, M. C. Enright, K. Sleeman, D. T. Griffiths, D. W. Crook, and B. G. Spratt. 2003. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. J. Clin. Microbiol. 41:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. NCCLS, Wayne, Pa.
- 26.Peerbooms, P. G. H., M. N. Engelen, D. A. J. Stokman, B. H. B. van Benthem, M. L. van Weert, S. M. Bruisten, A. van Belkum, and R. A. Coutinho. 2002. Nasopharyngeal carriage of potential bacterial pathogens related to day care attendance, with special reference to the molecular epidemiology of Haemophilus influenzae. J. Clin. Microbiol. 40:2832-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Principi, N., P. Marchisio, G. C. Schito, S. Mannelli, et al. 1999. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Pediatr. Infect. Dis. J. 18:517-523. [DOI] [PubMed] [Google Scholar]
- 28.Prodier, C., B. Dunais, R. Largillier, H. Carsenti-Eresse, E. Bernard, A. Scheimberg, and P. Dellamonica. 1997. Nasopharyngeal carriage of Streptococcus pneumoniae in children's day-care centers: 10-month follow-up study in Nice, France. Clin. Microbiol. Infect. 3:705-708. [DOI] [PubMed] [Google Scholar]
- 29.Raymond, J., I. Le Thomas, F. Moulin, A. Commeau, D. Gendrel, and P. Berche. 2000. Sequential colonization by Streptococcus pneumoniae of healthy children living in an orphanage. J. Infect. Dis. 181:1983-1988. [DOI] [PubMed] [Google Scholar]
- 30.Sadowy, E., J. Zhou, E. Meats, M. Gniadkowski, B. G. Spratt, and W. Hryniewicz. 2003. Identification of multidrug-resistant Streptococcus pneumoniae isolated in Poland by multilocus sequence typing. Microb. Drug Resist. 9:81-86. [DOI] [PubMed] [Google Scholar]
- 31.Skoczyñska, A., P. Kriz, H. Bossen Konradsen, and W. Hryniewicz. 2000. Characteristics of the major etiologic agents of bacterial meningitis isolated in Poland in 1997-1998. Microb. Drug Resist. 6:147-153. [DOI] [PubMed] [Google Scholar]
- 32.St. Sauver, J., C. F. Marrs, B. Foxman, P. Somsel, R. Madera, and J. R. Gilsdorf. 2000. Risk factors for otitis media and carriage of multiple strains of Haemophilus influenzae and Streptococcus pneumoniae. Emerg. Infect. Dis. 6:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulikowska, A., P. Grzesiowski, M. Taraszkiewicz, and W. Hryniewicz. 2003. The carriage of Streptococcus pneumoniae in the nasopharynx of children under 5 years of age in selected settings in Warsaw. Pediatr. Pol. 5:377-384. [Google Scholar]
- 34.Syrjanen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 35.Tarasi, A., F. D'Ambrosio, G. Perrone, and A. Pantosti. 1998. Susceptibility and genetic relatedness of invasive Haemophilus influenzae type b in Italy. Microb. Drug Resist. 4:301-306. [DOI] [PubMed] [Google Scholar]
- 36.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Hare, G. F., P. A. Shurin, C. D. Marchant, N. A. Cartelli, C. E. Johnson, D. Fulton, S. Carlin, and C. H. Kim. 1987. Acute otitis media caused by Branhamella catarrhalis: biology and therapy. Rev. Infect. Dis. 9:16-27. [DOI] [PubMed] [Google Scholar]
- 38.Verduin, C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagupsky, P., N. Porat, D. Fraser, F. Prajgrod, M., Merires, L. McGee, K. P. Klugman, and R. Dagan. 1998. Acquisition, carriage, and transmission of pneumococci with decreased antibiotics susceptibility in young children attending a day care facility in southern Israel. J. Infect. Dis. 177:1003-1012. [DOI] [PubMed] [Google Scholar]