Abstract
We report, to our knowledge, on the first case of a woman suffering stillbirth due to Streptococcus porcinus on the basis of microbiologic and histologic data.
A 22-year-old woman, gravida 1, came for her first visit to the obstetric clinical service to confirm pregnancy. The obstetric exam was normal, and an ultrasound scan confirmed the presence of a 10.5-week intrauterine fetus of normal appearance.
Subsequent prenatal visits revealed no particular problems. The clinical examination performed at 22 weeks was normal, a digital cervical examination exhibited an elongate cervix closed at the external os, and investigation by ultrasound also showed no signs of fetal or ovarian abnormalities. However, 2 days later (i.e., at 22 weeks and 2 days), the patient came in urgently complaining about a strange intravaginal discomfort. Her general appearance and vital signs were normal, with a temperature of 37.4°C, blood pressure of 125/75 mm Hg, and a peripheral leukocyte count of 6,300 per mm3 (range, 4,000 to 9,000).
Active fetal movements were readily detectable, fundal height was 18 cm, and the patient felt no uterine contractions. Bidigital examination revealed complete cervical dilation with bulging membranes extending almost to the vulva. Cardiotocography detected normal fetal cardiac rhythm and numerous contractions that were not felt by the patient. That evening, the patient expulsed a nonviable fetus.
Laboratory evaluation of the patient was carried out, including screening of the expulsed placenta products for infection. Questioning the patient failed to identify any abnormal factors in the preceding days or any recent infections. Three days after samples were taken; amniotic fluid, fetal gastric fluid, and endocervical swabs yielded heavy growth of Streptococcus porcinus.
The patient, who had been empirically placed on amoxicillin-clavulanic acid up to that point, was placed on a 10-day course of amoxicillin on the second day after her admission. She was discharged on the third day after her stillbirth, having remained afebrile during her hospitalization and suffered no further clinical complications.
Direct examinations after Gram staining, performed on the amniotic liquid punctured before the rupture of the membranes and on vagina and cervix swabs, demonstrated numerous gram-positive cocci. Rectal, nose, and ear swabs from the fetus were also cultured, yielding a luxuriant growth of nonpigmented beta-hemolytic colonies on Columbia agar (Becton Dickinson, Le Pont de Claix, France) when incubated at 35°C in the presence of 5% CO2. Gram-positive cocci arranged in pairs associated with a negative catalase reaction and the absence of motility at 25 and 35°C rule out the possibility of a Listeria monocytogenes isolate. No reaction was observed in tests with enzyme extraction agglutination kit for group A, B, C, D, F, or G Streptococcus (bioMérieux, Marcy L'Etoile, France). The data for the utilization of carbon sources and enzyme profile obtained with Rapid ID32Strep and API 20Strep microplate assays, (bioMérieux) provided an excellent identification to the S. porcinus species. The phenotypic characteristics of our isolate were given and compared with those of 13 human S. porcinus strains previously published (7), as shown in Table 1. To confirm the phenotypic identification of this strain, an amplified ribosomal DNA restriction analysis based on PCR amplification of a fragment of RNA genes including 16S and 23S genes and the intergenic spacer (13) was performed. The combination of the three restriction patterns H-H2 (HhaI), M-A1 (MboII), and S-K1a (Sau3A) was identical to that obtained with the reference strain S. porcinus ATCC 43138 and confirmed that this isolate belonged to the species S. porcinus. The determination of antimicrobial susceptibilities was performed by the disk diffusion procedure on Mueller-Hinton agar supplemented with 5% sheep blood. The isolate was susceptible to penicillin, ampicillin, cephalothin, erythomycin, trimethoprim-sulfamethoxazole, rifampin, and vancomycin. Resistance to tetracycline was observed.
TABLE 1.
Comparison of phenotypic characteristics of 13 clinical (human) strains of S. porcinus and our isolate
| Test | Reaction (%)
|
|
|---|---|---|
| S. porcinusa | Our isolatec | |
| Voges-Proskauer | Vb (77) | + |
| Pyrrolidonyl arylamidase | + | − |
| Growth: | ||
| Bile-esculin | − | − |
| 6.5% NaCl broth | + | − |
| Brain heart infusion broth at 45°C | V (38) | − |
| Brain heart infusion broth at 10°C | V (15) | − |
| Hydrolysis of: | ||
| Arginine | + | + |
| Esculin | + | + |
| Hippurate | V (54) | + |
| Leucine aminopeptidase | + | + |
| Starch | − | − |
| Urea | − | − |
| Acid formation: | ||
| Arabinose | − | − |
| Inulin | − | − |
| Lactose | − | − |
| Maltose | + | + |
| Mannose | + | + |
| Melibiose | − | − |
| Raffinose | − | − |
| Ribose | + | + |
| Sorbitol | + | + |
| Sucrose | − | − |
| Trehalose | + | + |
Data from 13 strains isolated from human sources (7).
V, variable reaction; +, positive reaction; −, negative reaction.
Obtained with the Rapid ID 32 Strep and the API 20 Strep microplate assays (bioMérieux).
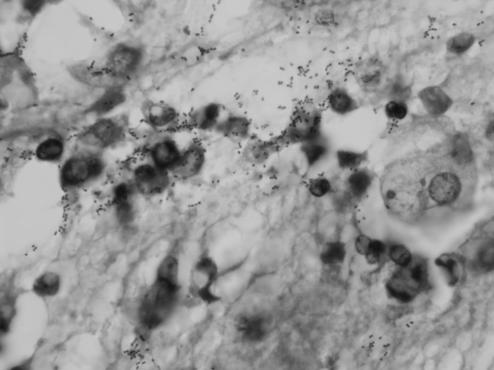
Postmortem, gross, and microscopic examinations of the fetus and placenta were carried out. No morphological abnormality of the fetus was found. Histological examination of the placenta and fetus viscera revealed evidence of bacterial chorioamnionitis associated with the presence of pulmonary microabscesses and therefore indicative of a fetal infection. Microscopic observation, after hematoxylin-eosin staining, showed neutrophilic polynuclear infiltration focused on the canalicular cavities of the lung and in the luminal cavity of the gastric sections (Fig. 1), associated with the presence of numerous gram-positive cocci (Gram and Giemsa stains) localized in extracellular spaces. These elements strongly indicated a fetal sepsis, confirmed by the identification of S. porcinus when cultured from samples of the gastric lumen fluid and of amniotic fluid. Similarly to the finding in the pulmonary tissues, polynuclear leukocyte infiltration was consistently observed in the subchorionic membranes of the placenta and as far as Wharton's jelly, associated with zones of necrotic and calcified debris. No villitis or abscesses were visible. Evidence of histological chorioamnionitis supports the hypothesis of an ascending route despite the absence of bacteriological proof of chorioamnion infection (defined as a positive culture); no sample was cultured. This hypothesis is also substantiated by the fact that the main organs affected were the lung and the gastrointestinal tract, suggesting that the organism entered by the way of the amniotic fluid.
FIG. 1.
Presence of numerous diplococci and inflammatory cells in the luminal gastric mucus (Giemsa stain; original magnification, ×1,000).
S. porcinus is the etiologic agent of various pathological situations in swine, including lymphadenitis, endocarditis (15), and abortion (11). An increased incidence of S. porcinus strains mainly isolated from the female genital tract has been previously reported (7). Two of 13 strains described were associated with complications of pregnancy, but the very little clinical data available did not permit appreciation of the pathogenicity of these isolates (7). However, S. porcinus still remains relatively infrequent and has been implicated as a human pathogen in only a few cases (septicemia, pelvic disease, and pregnancy) (7). Here, we report, to our knowledge, on the first case of a woman suffering stillbirth due to S. porcinus.
Human and swine strains of S. porcinus have been studied phenotypically (7, 9, 14) and genotypically (1). S. porcinus strains which exhibit common biochemical characteristics and cell wall composition and thought to belong to various Lancefield serogroups (E, P, U, and V) are clustered in a single genetic group (4). Phenotypic identification of streptococci is based on the determination of hemolytic activity on blood agar plates and nutritional requirements (12). The most common genitourinary pathogen in pregnant women is serogroup B beta-hemolytic Streptococcus (6). In the present case, the key factors in identification were the presence of a large and highly pronounced zone of beta-hemolysis and the presence of colonies smaller than those observed with group B streptococci. Our strain failed to react with the Lancefield group antigens commercially available. The biochemical profile of the isolates (manufactured microplates; API20 Strep and Rapid ID 32 Strep) associated with two additional reactions (positive CAMP [Christie-Atkins-Munch-Peterson] test and susceptibility to bacitracin) enables clear identification of the S. porcinus strain, which, like group B streptococci, is known to display resistance to tetracycline (7). The exact serogroup of the strain remains to be determined. Indeed, 9 of the 13 clinical isolates recently studied (7) belonged to the new group C1. The significance of the serological specificity and the identification of virulence factors (adhesion antigens and toxins) are determinants for understanding the mechanism of colonization and genitourinary tract infection in women. Any eventual epidemiological links between this patient or her physician and the presence of swine or relevant culinary customs were established.
Evidence of infection of the placenta (chorioamnionitis) and of the fetus (recovery of inflammatory elements associated with the presence of gram-positive cocci in lung tissue and in gastric lumen) was strongly supported by anatomical pathological observations and bacterial cultures (gastric aspirate, amniotic fluid, peripheral cutaneous tissues, endocervix, and vagina samples). The maternal leukocyte count was not an antenatal predictive test; the normal peripheral leukocyte count observed was consistent with the critical review appraisal of antenatal predictive tests of chorioamnionitis in preterm premature rupture of the membranes (10). Infection of membranes, placenta, and fetus was strongly corroborated by the pathological observations, bacterial data, and premature rupture of membranes resulting in stillbirth. The absence of placental villitis and abscesses ruled against a hematogenous infection with L. monocytogenes. By analogy with neonatal group B streptococcal infections with a history of intact membrane (5, 8) at the time of delivery and taking into account data for S. porcinus, the route of fetal infection was probably an ascendant one and infection caused premature delivery. Indeed, the main etiologies of preterm labor with intact membranes such as uterine malformation or multiparity were excluded. The effect of digital cervical examination 2 days prior to abortion could be a possible explanation for the rapid delivery following preterm rupture of the membranes (2, 3). However, it is more likely that the virulence of this strain (capsular polysaccharide or surface proteins) caused loss of the fetus, rather than a side effect of digital examination (3).
Intact membranes at the time of delivery should not exclude diagnosis of an intrauterine infection, thus underlining the importance of including routine bacteriological studies in the pathological examination of all perinatal deaths.
Acknowledgments
We thank Jonathan Bass for his contribution to improvement of the final version of the manuscript.
REFERENCES
- 1.Abdulmawjood, A., R. Weiβ, and C. Lämmler. 1998. Species identification of Streptococcus porcinus by restriction fragment length polymorphism analysis of 16S ribosomal DNA. Res. Vet. Sci. 65:85-86. [DOI] [PubMed] [Google Scholar]
- 2.Adoni, A., A. Ben Chetrit, D. Zacut, Z. Palti, and A. Hurwitz. 1990. Prolongation of the latent period in patients with premature rupture of the membranes by avoiding digital examination. Int. J. Gynecol. Obstet. 32:19-21. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, J. M., B. M. Mercer, M. Miodovnik, G. R. Thurnau, R. L. Goldenberg, A. F. Das, P. J. Meis, A. H. Moawad, J. D. Iams, J. P. Vandorsten, R. H. Paul, M. P. Dombrowski, J. M. Roberts, and D. McNellis. 2000. The impact of digital cervical examination on expectantly managed preterm rupture of membranes. Am. J. Gynecol. Obstet. 183:1003-1007. [DOI] [PubMed] [Google Scholar]
- 4.Collins, M. D., J. A. E. Farrow, V. Kati, and O. Kandler. 1984. Taxonomic studies on streptococci of serological groups E, U, V, and P: description of Streptococcus porcinus sp. nov. Syst. Appl. Microbiol. 5:402-413. [Google Scholar]
- 5.Desa, D. J., and C. L. Trevenen. 1984. Intrauterine infections with group B β-hemolytic streptococci. Br. J. Obstet. Gynaecol. 91:237-239. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, M. S., and C. J. Baker. 2001. Group B streptococcal infections, p. 1091-1156. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W. B. Saunders Company, Philadelphia, Pa.
- 7.Facklam, R., J. Elliott, N. Pigott, and R. Franklin. 1995. Identification of Streptococcus porcinus from human sources. J. Clin. Microbiol. 33:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz, V., and W. A. Bowes. 1998. Perinatal group B streptococcal infections across intact amniotic membranes. J. Rep. Med. 33:445-449. [PubMed] [Google Scholar]
- 9.Lämmler, C., N. Cirak, and J. Smola. 1998. Studies on biochemical, serological and further characteristics of Streptococcus porcinus. J. Vet. Med. B 45:235-243. [DOI] [PubMed] [Google Scholar]
- 10.Ohlsson, A., and E. Wang. 1990. An analysis of antenatal tests to detect infection in preterm premature rupture of the membranes. Am. J. Obstet. Gynecol. 162:809-818. [DOI] [PubMed] [Google Scholar]
- 11.Plagemann, O. 1988. Streptococcus porcinus as a cause of abortion in swine. Zentbl. Vetmed. B 35:770-772. [PubMed] [Google Scholar]
- 12.Ruoff, K. L., R. A. Whiley, and D. Beighton. 2003. Streptococcus, p 405-421. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 13.Schlegel, L., F. Grimont, P. A. D. Grimont, and A. Bouvet. 2003. Identification of major streptococcal species by rrn-amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 41:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson, T., and R. Facklam. 1997. Cross-reactions of reagents from streptococcal grouping kits with Streptococcus porcinus. J. Clin. Microbiol. 35:1885-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessman, G. E. 1986. Biology of the group E streptococci. Vet. Microbiol. 12:297-328. [DOI] [PubMed] [Google Scholar]