Although the median time from human immunodeficiency virus (HIV) infection to the development of malignancies or opportunistic infections defined as AIDS is 7 to 10 years (4), AIDS may develop within a year of infection or it may not develop for more than 20 years following documented HIV seroconversion. Numerous studies have identified host and viral factors that are associated with the HIV disease course, including host HLA types and genetic polymorphisms of genes encoding HIV coreceptor or coreceptor ligands (reviewed in reference 8). Recently, studies found that HIV-positive people who are coinfected with a common, nonpathogenic human flavivirus referred to as GB virus type C (GBV-C) survive significantly longer than do HIV-positive individuals without GBV-C infection (10).
HISTORY AND VIROLOGY
In 1995, researchers from Genelabs, Inc., reported the discovery of a new virus that they called hepatitis G virus (HGV), as it was isolated from the blood of subjects with chronic hepatitis who did not have evidence of other types of viral hepatitis (3). Shortly thereafter, researchers from Abbott Laboratories reported the discovery of a new virus that they called GBV-C (9), because it was closely related to two previously identified primate viruses (GBV-A and GBV-B) linked to the passage of an infectious agent in the serum of a surgeon with the initials G.B. Characterization of HGV and GBV-C found them to be members of the family Flaviviridae and to contain a positive-polarity, single-strand RNA genome predicted to encode a polyprotein of approximately 3,000 amino acids. A sequence comparison demonstrated that HGV and GBV-C are actually different isolates of the same virus. Because subsequent studies did not find an association between these viruses and hepatitis, most researchers do not use the term HGV but refer to the virus as GBV-C. Phylogenetically, GBV-C is the human virus most closely related to hepatitis C virus (HCV), and there is approximately 30% amino acid homology between HCV and GBV-C (2). Based on limited experimental data and sequence comparisons between HCV and other flaviviruses, the viral polyprotein is thought to be processed by cellular and viral proteases into at least eight proteins (Fig. 1) (reviewed in references 8 and 10).
FIG. 1.
The GBV-C genome is composed of a single-stranded, positive-polarity RNA molecule that encodes a long polyprotein. A nontranslated region at the 5′ end of the open reading frame (5′ntr) contains an internal ribosomal entry site, and there is another nontranslated region at the 3′ end of the genome (3′ntr). It is believed that the predicted viral envelope proteins (E1 and E2) are cleaved by cellular proteases but that the nonstructural proteins NS2, NS3, NS4A (*4A), NS4B (*4B), NS5A, and NS5B are cleaved by the viral protease (NS3) in reactions involving a protease cofactor (NS4A). The coding region for the nucleocapsid protein has not yet been identified.
Although there are many similarities between GBV-C and HCV, distinct differences exist (reviewed in reference 10). HCV replicates primarily, if not exclusively, in hepatocytes, whereas GBV-C appears to replicate in both T (CD4+ and CD8+) and B lymphocytes (14; S. L. George, L. Katz, and J. T. Stapleton, Prog. Abstr. 10th Conf. Retrovir. Opportun. Infect., abstr. 847, 2003). Another difference is that the coding region for the core protein of GBV-C has not yet been identified, and the GBV-C major envelope glycoprotein (E2) is not glycosylated as extensively as is HCV (there are three potential sites for N-linked glycosylation for GBV-C compared to 11 for HCV E2). Unlike HCV, GBV-C E2 does not contain a hypervariable region, and people with active GBV-C viremia usually do not have detectable antibodies to viral proteins. Antibody against the GBV-C E2 protein is usually found after clearance of infection, and anti-E2 antibody is associated with some, although not complete, protection against reinfection with GBV-C (reviewed in reference 10). From a clinical standpoint, the majority of immunocompetent individuals who become infected with GBV-C clear the virus, while fewer than 25% of HCV-infected people spontaneously clear infection.
INFECTION AND TRANSMISSION
As noted above, the detection of GBV-C RNA in serum is indicative of active GBV-C infection, and the detection of E2 antibody is usually associated with prior GBV-C infection. It appears that GBV-C is more efficiently transmitted by sexual and vertical exposure than is HCV (reviewed in references 6 and 10). Because GBV-C is transmitted sexually, vertically, and by exposure to contaminated blood, populations with high rates of sexually transmitted or blood-borne infections have high prevalence rates of active or prior GBV-C infection. For example, 15 to 20% of individuals with chronic HCV infection have active GBV-C infection, and another 25 to 70% have evidence of prior GBV-C (E2 antibody) (reviewed in references 1, 6, 7, and 10). Also, due to shared modes of transmission, the rate of GBV-C infection among HIV-positive people is extremely high, with 86% of men in the Multicenter AIDS Cohort Study (MACS) group having active (40%) or prior (46%) GBV-C infection (12).
Evidence of GBV-C is also found among people who have no acknowledged risk of blood-borne infections (e.g., healthy blood donors). Infection rates among Iowa blood donors who passed all donor screening procedures and tests reflect this finding, as 1.8% of Iowa blood donors had GBV-C viremia and anti-GBV-C E2 antibody was detected in the sera of 12.8% of the donors (J. T. Stapleton and L. Katz, unpublished data). This rate of viremia is similar to that seen in other blood donor populations (1 to 3%; reviewed in references 1, 3, 7, and 9). It is likely that this background level of GBV-C infection is due to sexual transmission, although GBV-C RNA is found in saliva, and alternative routes of transmission may exist.
GBV-C, HUMAN DISEASE, AND HIV
To determine if GBV-C infection is associated with hepatitis or other diseases, numerous studies of blood recipients, hepatitis patients, transplant recipients, and individuals with a variety of clinical diseases were undertaken in the first several years following discovery (1). All prospective studies failed to identify an association between GBV-C with either acute or chronic hepatitis, and no carefully controlled study has demonstrated an association between GBV-C and any human disease (reviewed in reference 1). Because GBV-C is not associated with a disease, blood products are not screened for this virus (1), and approximately 1 of 70 units of blood products in the United States contains GBV-C RNA.
Because GBV-C was not found to be pathogenic, research interest in the virus waned significantly in the late 1990s. However, studies of the pathogenesis of GBV-C in HIV-infected patients yielded surprising results. Ten separate studies published or presented since 1998 found that GBV-C infection in HIV-positive people was associated with either a decrease in mortality or improved clinical outcomes (i.e., increase in CD4, decreased need for antiretrovirals, etc.) compared to mortality or clinical outcomes for HIV-positive people without GBV-C infection (reviewed in reference 10). Nevertheless, four studies did not demonstrate a beneficial association between GBV-C and HIV disease, and the idea of a potential beneficial role for GBV-C in HIV-infected people has not been widely accepted (10). A common flaw of the initial studies of GBV-C's impact on HIV progression was that they were conducted with populations with unknown dates of HIV acquisition. This shortcoming made it impossible to account for the potential differences in survival prior to entry into the GBV-C-HIV study. To address this issue, we analyzed GBV-C infection in men with known dates of HIV acquisition who participated in the ongoing MACS. The MACS was established in 1983 to study the emerging epidemic of immune suppression in men self-identified as men who have sex with men. Individuals from four cities (Baltimore, Md., Pittsburgh, Pa., Chicago, Ill., and Los Angeles, Calif.) were recruited and have been evaluated at 6-month intervals for the past 20 years. Detailed clinical, laboratory, and behavioral data are collected at each visit. Serum is obtained at each visit, and samples are stored for future analysis. To study people with a known duration of HIV infection, we studied individuals who were HIV antibody negative when they enrolled in the MACS and for whom the time of HIV seroconversion was known within 1 year. The median date of HIV seroconversion was 1985 for the men studied in this subanalysis.
Sera obtained at an early (12 to 18 months; n = 271) and a late (5 to 6 years; n = 138) visit following HIV seroconversion were tested for the presence of GBV-C viremia (RNA detection) and E2 antibody (12). At the early visit, GBV-C viremia was detected in 39.6% of the HIV seroconverters, and 46% had E2 antibody (two individuals had both GBV-C viremia and E2 antibody). Clinical data, including survival, were analyzed, and it was found that the GBV-C status at the early visit did not predict survival (12). However, MACS participants who were GBV-C RNA positive at the late visit were 2.78 times more likely to survive than those who were GBV-C RNA negative (P = 0.006) (12). When results from the GBV-C RNA-negative subjects were stratified by E2 antibody status, those subjects with E2 antibody appeared to have improved survival rates compared to those with neither GBV-C RNA nor E2 antibody, although this benefit waned 9 years after HIV seroconversion (12). All analyses were censored on 1 January 1996 to avoid confounding the data by the introduction of effective, combination antiretroviral therapy.
The natural history of GBV-C infection among these HIV-positive men revealed that acquisition of GBV-C infection between the early and late visits occurred with only one subject; however, 12 of 61 subjects (20%) lost GBV-C viremia (RNA) between the early and late visits. Taking into account the GBV-C RNA and E2 antibody status at both visits, the group of men who were persistently GBV-C RNA positive survived significantly longer than those who were persistently GBV-C RNA negative. Surprisingly, the group that cleared GBV-C viremia fared the worst, and 8 of those 12 men died. Only 3 of the 12 men who cleared viremia developed E2 antibody, suggesting that clearance of GBV-C was not immunologically mediated but that it may reflect the loss of critical lymphocytic cells (or a cellular milieu) necessary to maintain GBV-C replication. Only one of eight men who cleared GBV-C RNA without E2 antibody survived, compared to two of three men who cleared GBV-C with E2 antibody, again suggesting that there may be a difference in survival for men who clear GBV-C via an immune response versus those who do not form antibodies at the time of GBV-C clearance.
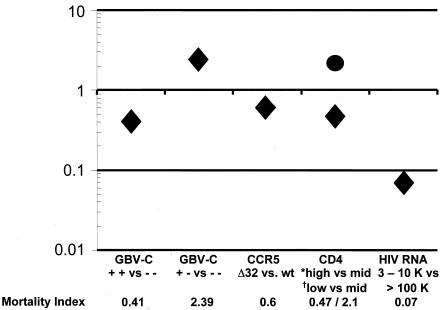
Among MACS HIV seroconverters who were studied for more than 5 years following HIV seroconversion, 75% of the men who were positive for GBV-C RNA at the late visit survived at the 11-year time point, compared to 39% of those negative for GBV-C RNA at both visits and 16% of those who cleared viremia. This result was in the absence of highly active, combination antiretroviral therapy. Not surprisingly, changes in other markers of HIV disease progression among GBV-C-positive and -negative individuals were consistent with the mortality findings. Specifically, CD4 cell counts fell by 26 cells/mm3 per year between early and late visits for the persistently GBV-C RNA positive group, compared to an annual fall of 60 cells/mm3 (E2 antibody negative) or 70 cells/mm3 (E2 antibody positive) per year in the persistently RNA-negative group and 107 cells/mm3 per year for the group that cleared viremia. Similarly, the extent of increase in HIV RNA between early and late visits was approximately 0.5 log10 greater among individuals without GBV-C RNA at the late visit than among those with GBV-C viremia (12). In summary, the MACS seroconverters study confirmed that prolonged viremia with GBV-C is highly predictive of prolonged survival among HIV-positive individuals. To put these findings in context with other known predictors of HIV survival, the relative benefit of persistent GBV-C infection was slightly greater than that found in MACS participants who were heterozygous for the Δ32 mutation of their CCR5 receptor gene (Fig. 2).
FIG. 2.
Comparison of the relative impacts of different predictors of HIV disease progression. Different mortality indexes of death for persons with HIV are compared for those persons with and without GBV-C, those persons with and without the protective CCR5 allele, and by stage of HIV disease, CD4 cell counts, and HIV RNA levels. An index value of greater than 1 indicates a higher risk of death, and an index value of less than 1 demonstrates a protective effect. As described in the text, men who were GBV-C RNA positive at both the early and late visits (+ +) were more than twice as likely to survive compared to those who were GBV-C RNA negative on both visits (− −) (index = 0.41), whereas those who cleared viremia between visits were more than twice as likely to die (index = 2.39). By comparison, those heterozygous for the CCR5 HIV coreceptor gene (Δ32) were also protected from death, but this protection was less than that seen for individuals with persistent GBV-C infection (index = 0.63). Another way to conceptualize the magnitude of this protection is to note that it is as large as the difference between a patient having a CD4 cell count of 500 to 750 (*high) and a patient with only a mid-range CD4 count (mid; 200 to 350 cells; mortality index = 0.47). Conversely, the negative impact of losing GBV-C infection is the same as the risk of death among persons with mid-range CD4 cell counts compared to those with high CD4 cell counts (index = 2.1). It is of note that while these protective effects are strong, HIV viral load (HIV RNA) is one of the strongest predictors of survival and that when those with low (3,000 to 10,000 [3-10 K] genome equivalents per milliliter) versus high (>100,000) viral loads are compared, the effect is considerably greater than that of the presence of GBV-C or CCR5 polymorphisms or a high versus a moderate CD4 count. wt, wild type.
Although the frequency of GBV-C viremia in HIV-positive people is high, which argues against a protective effect, the potential role of GBV-C viremia or E2 antibody for protecting people exposed to HIV via sexual or parenteral exposure has not been reported, although there are several studies under way to address this question. However, GBV-C viremia does not appear to decrease vertical transmission of HIV, as neither GBV-C viremia nor GBV-C E2 antibody detection was associated with a lower rate of vertical HIV transmission in a group of HIV-positive Tanzanian mothers (11).
CAUSE OR EFFECT?
The epidemiological studies summarized above clearly demonstrate an association between GBV-C infection and delayed mortality among HIV-positive individuals. However, these studies do not provide evidence that GBV-C is causally related to improved survival, and it is possible that GBV-C infection is not the reason that HIV-positive people live longer but, rather, that it serves as a marker of another factor related to HIV disease progression. The epidemiological research has demonstrated a temporal relationship between GBV-C infection and improved survival. Future, ongoing research will determine if there are plausible biological mechanisms by which GBV-C interacts with the host or virus to slow progression.
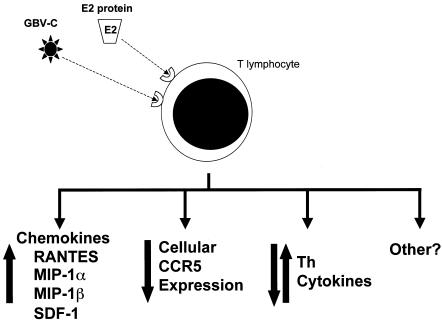
Because both GBV-C and HIV replicate in CD4+ T lymphocytes (15), it is reasonable to speculate that viral interference between GBV-C and HIV might occur. To address this question, an in vitro model of GBV-C and HIV coinfection using primary phytohemagglutinin-interleukin-2 (IL-2)-stimulated human peripheral blood mononuclear cells (PBMCs) was developed (14). In that coinfection model, GBV-C infection did not cause toxicity to PBMCs but reproducibly led to inhibition of HIV replication (13, 14). The inhibition of HIV replication was increased when the cells were infected with GBV-C ≥24 h prior to HIV infection, suggesting that GBV-C replication induces cellular factors that might inhibit HIV (14). An evaluation of PBMCs infected with GBV-C compared to mock-infected controls revealed an increase in the cellular mRNA levels and protein levels in cell culture supernatants for the natural ligands for the two major HIV coreceptors (13). Specifically, levels of the chemokines RANTES, MIP-1α, MIP-1β (CCR5), and SDF-1 (CXCR4) all increased in GBV-C-infected cells compared to levels in mock-infected cells. The inhibitory effect of GBV-C on HIV was completely abolished when cells were incubated with antibodies that neutralize the binding of these four chemokines to their receptors, confirming that, at least in vitro, GBV-C inhibits HIV by inducing these chemokines (13).
In addition, the HIV coreceptor CCR5 was down-regulated during in vitro infection (13), a finding supported by Nattermann and colleagues, who demonstrated that CCR5 levels on CD4+ T cells in vivo were significantly lower in GBV-C RNA-positive (HIV-positive) individuals than in those from the GBV-C RNA-negative (HIV-positive) control group (5). Several previous studies of HIV-positive, long-term nonprogressors found elevated levels of RANTES and SDF-1 and decreased surface expression of CCR5 on PBMCs; thus, the effects of GBV-C on PBMCs are consistent with results of other studies of HIV disease progression. It is possible that the chemokine and receptor effects are mediated at least in part by the GBV-C E2 protein, as Nattermann et al. (5) found that CD4+ T cells incubated with a cell lysate containing GBV-C E2 protein resulted in increased levels of RANTES production. Incubation of CD4 cells with E2 also led to down-regulation of CCR5 and decreased detection of CD81, suggesting that CD81 may serve as a cell attachment molecule. This is of interest since CD81 has been shown to bind HCV E2 protein.
GBV-C might also influence HIV disease by causing alterations in cytokine production by PBMCs. It was found that GBV-C infection of PBMCs in vitro led to a reduction in the levels of mRNA for several Th2 cytokines (J. Xiang, S. L. George, S. Wuenschmann, D. Klinzman, and J. T. Stapleton, Prog. Abstr. 10th Conf. Retrovir. Opportun. Infect., abstr. 156, 2003). Consistent with this finding, Nunnari et al. demonstrated that HIV-positive people with GBV-C viremia maintained serum Th1 and Th2 cytokine levels during longitudinal follow-up but that HIV-positive, GBV-C-negative subjects had a fall in serum Th1 cytokine concentrations (IL-2 and IL-12) and a rise in serum Th2 cytokine concentrations (IL-4 and IL-10) over time (6). Thus, GBV-C may help maintain cytokine profiles associated with long-term nonprogression among HIV-positive people. Also, since Th cytokines are involved in the pathogenesis of a variety of diseases, GBV-C potentially may influence other comorbid conditions. In summary, GBV-C may influence HIV disease by inhibiting HIV by inducing chemokines, down-regulating an HIV coreceptor(s), influencing cytokine profiles, and having other yet-undefined effects on the host lymphocytes (summarized in Fig. 3).
FIG. 3.
Potential effects of GBV-C that may alter HIV disease. GBV-C must interact with a receptor (or receptors) on lymphocytes to initiate infection. (Bottom) GBV-C replication (i) increases chemokine expression and down-regulates the expression of CCR5 (13) and (ii) alters Th cytokines in vitro (13; J. Xiang et al., Prog. Abstr. 10th Conf. Retrovir. Opportun. Infect., abstr. 156), both of which lead to decreased HIV replication. Similarly, GBV-C viremia is associated with decreased CCR5 expression and a modulation of Th cytokines in clinical studies (5, 6). These effects may be mediated by the GBV-C E2 protein via interactions with CD81 on the surfaces of CD4 cells (5). In addition to the effects of GBV-C on chemokine and cytokine expression, other undefined effects of GBV-C may also influence HIV disease progression.
REMAINING QUESTIONS
Numerous questions related to GBV-C and HIV interactions remain to be studied. The epidemiology of GBV-C infection in HIV-positive populations with different ethnicity, gender, and HIV transmission profiles needs to be studied. Further analysis of the characteristics associated with the clearance of GBV-C infection is also needed. Careful characterization of the temporal relationship between GBV-C clearance and HIV disease progression is necessary in order to understand whether GBV-C clearance (with or without E2 antibody) precedes the immunological decline or if the clearance of GBV-C occurs as a result of an HIV-mediated functional and quantitative decline in CD4+ T cells. Is GBV-C RNA replication required for the effects of GBV-C on chemokine and cytokines? Is a viral protein responsible for the cellular effects of GBV-C that lead to improved HIV outcomes? If so, which viral protein(s)? Why do some individuals with GBV-C E2 antibody (but without active infection) appear to progress more slowly than those without E2 antibody? Much remains to be learned about this common, nonpathogenic virus, and identification of the mechanism(s) by which GBV-C influences HIV disease progression may lead to novel therapeutic strategies in the fight against HIV infection. However, an increasing number of studies indicate that this previously unrecognized virus is associated with prolonged survival, a delay in CD4 decline, and lower HIV RNA levels in HIV-positive individuals.
Acknowledgments
This work was funded in part by Merit Review Grants from the Veterans Administration (J.T.S. and J.X.) and by NIH grant AI 058740 (J.T.S.).
REFERENCES
- 1.Alter, H. J. 1997. G-pers creepers, where'd you get those papers? A reassessment of the literature on the hepatitis G virus. Transfusion 37:569-572. [DOI] [PubMed] [Google Scholar]
- 2.Leary, T. P., A. S. Muerhoff, J. N. Simons, T. J. Pilot-Matias, J. C. Erker, M. L. Chalmers, G. G. Schlauder, G. J. Dawson, S. M. Desai, and I. K. Mushahwar. 1996. Sequence and genomic organization of GBV-C: a novel member of the Flaviviridae associated with human non-A-E hepatitis. J. Med. Virol. 48:60-67. [DOI] [PubMed] [Google Scholar]
- 3.Linnen, J., J. Wages, Z.-Y. Zhang-Keck, K. E. Fry, K. Z. Krawczynski, H. Alter, E. Koonin, M. Gallagher, M. Alter, S. Hadziyannis, P. Karayiannis, K. Fung, Y. Nakatsuji, J. W. K. Shih, M. Piatak, C. Hoover, J. Fernandez, S. Chen, J.-C. Zou, T. Morris, K. C. Hyams, S. Ismay, J. D. Lifson, G. Hess, S. K. H. Foung, H. Thomas, D. Bradley, H. Margolis, and J. P. Kim. 1996. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science 271:505-508. [DOI] [PubMed] [Google Scholar]
- 4.Lui, K. J., W. W. Darrow, and G. W. Rutherford. 1988. A model-based estimate of the mean incubation period for AIDS in homosexual men. Science 240:1333-1335. [DOI] [PubMed] [Google Scholar]
- 5.Nattermann, J., H. D. Nischalke, B. Kupfer, J. Rockstroh, L. Hess, T. Sauerbruch, and U. Spengler. 2003. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS 17:1457-1462. [DOI] [PubMed] [Google Scholar]
- 6.Nunnari, G., L. Nigro, F. Palermo, M. Attanasio, A. Berger, H. W. Doerr, R. J. Pomerantz, and B. Cacopardo. 2003. Slower progression of HIV-1 infection in persons with GB virus C co-infection correlates with an intact T-helper 1 cytokine profile. Ann. Intern. Med. 139:26-30. [DOI] [PubMed] [Google Scholar]
- 7.Polgreen, P. M., J. Xiang, Q. Chang, and J. T. Stapleton. 2003. GB virus type C/hepatitis G virus: a nonpathogenic flavivirus associated with prolonged survival in HIV-infected individuals. Microbes Infect. 5:1255-1261. [DOI] [PubMed] [Google Scholar]
- 8.Rowland-Jones, S. 1999. Long-term non-progression in HIV infection: clinico pathological issues. J. Infect. 38:67-70. [DOI] [PubMed] [Google Scholar]
- 9.Simons, J. N., T. P. Leary, G. J. Dawson, T. J. Pilot-Matias, A. S. Muerhoff, G. G. Schlauder, S. M. Desai, and I. K. Mushahwar. 1995. Isolation of novel virus-like sequences associated with human hepatitis. Nat. Med. 1:564-569. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton, J. T. 2003. GB virus type C/hepatitis G virus. Semin. Liver Dis. 23:137-148. [DOI] [PubMed] [Google Scholar]
- 11.Weintrob, A. C., J. D. Hamilton, C. Hahn, D. Klinzman, G. Moyo, D. Zdunek, G. Hess, D. K. Benjamin, Jr., and J. T. Stapleton. 2004. Active or prior GB virus C infection does not protect against vertical transmission of HIV in co-infected women from Tanzania. Clin. Infect. Dis. 38:e46-e48. [Online.] http://www.journals.uchicago.edu/CID/journal/issues/v38n6/32236/32236.html. [DOI] [PubMed] [Google Scholar]
- 12.Williams, C. F., D. Klinzman, T. E. Yamashita, J. Xiang, P. M. Polgreen, C. Rinaldo, C. Liu, J. Phair, J. B. Margolick, D. Zdunek, G. Hess, and J. T. Stapleton. 2004. Persistent GB virus C infection and survival in HIV-infected men. N. Engl. J. Med. 350:981-990. [DOI] [PubMed] [Google Scholar]
- 13.Xiang, J., S. L. George, S. Wunschmann, Q. Chang, D. Klinzman, and J. T. Stapleton.2004. GB virus C infection inhibits HIV-1 replication by increasing RANTES, MIP-1α, MIP-1β, and SDF-1. Lancet 363:2040-2046. [DOI] [PubMed] [Google Scholar]
- 14.Xiang, J., S. Wunschmann, D. J. Diekema, D. Klinzman, K. D. Patrick, S. L. George, and J. T. Stapleton. 2001. Effect of coinfection with GB virus C (hepatitis G virus) on survival among patients with HIV infection. N. Engl. J. Med. 345:707-714. [DOI] [PubMed] [Google Scholar]
- 15.Xiang, J., S. Wünschmann, W. Schmidt, J. Shao, and J. T. Stapleton. 2000. Full-length GB virus C (hepatitis G virus) RNA transcripts are infectious in primary CD4-positive T cells. J. Virol. 74:9125-9133. [DOI] [PMC free article] [PubMed] [Google Scholar]