Abstract
In the south of France, leishmaniasis due to Leishmania infantum occurs in the following five foci of endemicity (from west to east): Pyrénées-Orientales, Cévennes, Provence, Côte d'Azur, and Corsica. Between 1981 and 2002, 712 Leishmania strains obtained from humans, dogs, cats, and sand flies were studied by isoenzyme analysis. In total, seven zymodemes were identified: MON-1, MON-11, MON-24, MON-29, MON-33, MON-34, and MON-108. The Pyrénées-Orientales focus is characterized by a predominance of human cutaneous leishmaniasis and a high enzymatic polymorphism (five zymodemes). In the other foci, where human visceral leishmaniasis is predominant, only two zymodemes are present. L. infantum MON-1 is the parasite most frequently found, in patients both with and without concomitant human immunodeficiency virus infection. MON-1 is the only zymodeme present in dogs, which act as the reservoir host in all of the foci. In Cévennes, where the complete life cycle of zymodeme MON-1 has been identified, Phlebotomus perniciosus and Phlebotomus ariasi are vectors. The enzymatic polymorphism is compared to that of neighboring countries (Spain and Italy). In Pyrénées-Orientales, small variant zymodemes with electromorphs of heterozygote-like and homozygotic patterns can be explained by different genetic hypotheses.
Visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL) occur in the Mediterranean regions of the south of France, where they are common to dogs and humans. Canine leishmaniasis, human VL, and human CL have been known since 1914, 1918, and 1920, respectively (18, 37, 39), but it was only in 1981 that isoenzymatic identification showed the parasite to be Leishmania infantum (21, 41). Classically, five main areas of endemicity are recognized in the south of France. VL occurs in all of these foci, with the dog as a reservoir host, while CL is present mainly in Pyrénées-Orientales. The responsible vectors are Phlebotomus ariasi and/or Phlebotomus perniciosus.
Between 1981 and 2002, 712 strains from humans, dogs, cats, and sand flies were studied at the Centre National de Référence des Leishmania by isoenzymatic analysis. We report the enzymatic polymorphism in relation to the foci studied and its relationships to clinical and epidemiological features.
MATERIALS AND METHODS
Strains studied.
The 712 strains were isolated in the five foci of endemicity (Fig. 1): Pyrénées-Orientales (département, Pyrénées-Orientales), Cévennes (départements, Ardèche, Aveyron, Gard, Hérault, and Lozère), Provence (départements, Bouches-du-Rhône and Vaucluse), Côte d'Azur (départements, Alpes-Maritimes and Var), and Corsica (départements, Corse du Sud and Haute-Corse).
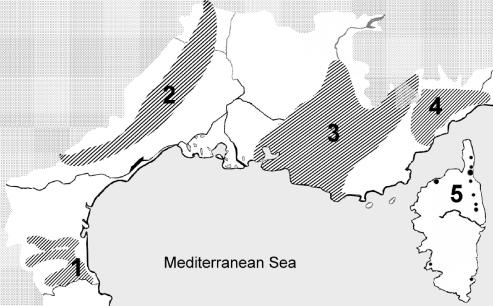
FIG. 1.
Geographical locations of the leishmaniasis foci of southern France. 1, Pyrénées-Orientales; 2, Cévennes; 3, Provence; 4, Côte d'Azur; 5, Corsica. The Mediterranean climate extension is in white. For Corsica, the localities of origin of the strains are shown as dots, due to the small sample size.
The strains were obtained from human leishmaniasis cases (for VL, n = 366 [of which 159 were human immunodeficiency virus {HIV}-positive patients]; for CL, n = 84; for mucosal leishmaniasis [ML], n = 1), from mammals (dogs [VL, n = 208; CL, n = 24] and cats [VL, n = 2]), and from phlebotomine sand flies (P. perniciosus, n = 2; P. ariasi, n = 25) (Table 1). The isolates were obtained from bone marrow or blood for human and cat VL, from skin for CL, from mucous lesions for ML, from popliteal lymph nodes for canine VL, and from the intestinal tract for sand flies.
TABLE 1.
Numbers of strains studied according to host and area
| Host | No. of strains
|
|||||
|---|---|---|---|---|---|---|
| Pyrénées- Orientales | Cévennes | Provence | Côte d'Azur | Corsica | Total | |
| Human | 72 | 153 | 75 | 138 | 13 | 451 |
| Dog | 81 | 116 | 5 | 26 | 4 | 232 |
| Cat | 0 | 0 | 0 | 2 | 0 | 2 |
| Sand fly | 2 | 25 | 0 | 0 | 0 | 27 |
| Total | 155 | 294 | 80 | 166 | 17 | 712 |
Human strains were obtained from diagnostic investigations within laboratories or clinical services of hospitals in France (Centres Hospitaliers Universitaires and Centre Hospitaliers Généraux) for VL and from dermatologists for tegumentary cases. The majority of canine and sand fly strains came from field epidemiological investigations. A few other canine and sand fly strains and the feline strains were from veterinary diagnostic services.
Reference strains.
Seven reference strains of L. infantum were used in this study: MHOM/FR/78/LEM 75, zymodeme MON-1; MHOM/FR/80/LEM 189, MON-11; MHOM/DZ/82/LIPA 59, MON-24; MHOM/ES/81/BCN1, MON-29; MHOM/FR/82/LEM 356, MON-33; MHOM/FR/84/LEM 538, MON-34; and MCAN/FR/87/RM1, MON-108.
Methods.
Starch gel electrophoresis was performed as described by Rioux et al. (44), using the following 15 enzymatic systems: malate dehydrogenase (MDH), EC 1.1.1.37; malic enzyme, EC 1.1.1.40; isocitrate dehydrogenase, EC 1.1.1.42; 6-phosphogluconate dehydrogenase (PGD), EC 1.1.1.44; glucose-6-phosphate dehydrogenase, EC 1.1.1.49; glutamate dehydrogenase, EC 1.4.1.3; NADH diaphorase (DIA), EC 1.6.2.2; purine nucleoside phosphorylase 1 (NP1), EC 2.4.2.1; NP2, EC 2.4.2.*; glutamate-oxaloacetate transaminases 1 and 2 (GOT1 and GOT2), EC 2.6.1.1; phosphoglucomutase, EC 5.4.2.2; fumarate hydratase, EC 4.2.1.2; mannose phosphate isomerase, EC 5.3.1.8; and glucose phosphate isomerase, EC 5.3.1.9. Isoelectrofocusing was used as a technique with greater resolving power for NP1 (29).
RESULTS
Between 1981 and 2002, 712 strains from the south of France were identified by isoenzyme analysis. All were L. infantum. In total, seven zymodemes were present (Table 2). The zymodeme MON-1 was predominant (n = 630; 88.48%). The six other zymodemes represent 11.52% of the sample: MON-11 (n = 11; 1.55%), MON-24 (n = 4; 0.56%), MON-29 (n = 44; 6.18%), MON-33 (n = 18; 2.53%), MON-34 (n = 1; 0.14%), and MON-108 (n = 4; 0.56%). Compared to L. infantum MON-1, these six other zymodemes differ by only 1 to 3 enzymatic systems out of the 15 enzymes studied (Table 2). In Table 3 the results are expressed according to foci, from west to east.
TABLE 2.
Enzymatic profiles of the seven zymodemes found in southern France
| Zymodeme | Electromorph valuea of the following enzyme:
|
|||
|---|---|---|---|---|
| MDH | Glucose-6-phosphate dehydrogenase | DIA | NP1 | |
| MON- 1 | 100 | 100 | 100 | 100 |
| MON- 11 | 104 | 105 | 100 | 130 |
| MON- 24 | 104 | 100 | 100 | 140 |
| MON- 29 | 104 | 105 | 100 | 140 |
| MON- 33 | 104 | 105 | 100 | 100 |
| MON- 34 | 104 | 100 | 100 | 100 |
| MON- 108 | 100 | 100 | 120 | 100 |
The electromorph value is the mobility of the migration band of a studied strain relative to that of the reference strain, MON-1 (distance from the start of the studied strain band/distance from the start of the reference band × 100). Only the electromorph values of the four enzymes that vary from those of the reference zymodeme MON-1 are presented. The electromorph values of the 11 other enzymes (malic enzyme, isocitrate dehydrogenase, glutamate dehydrogenase, phosphoglucomutase, PGD, NP2, GOT1, GOT2, fumarate hydratase, mannose phosphate isomerase, and glucose phosphate isomerase) are equal to 100.
TABLE 3.
Details of the zymodemes found, according to host, clinical form, and focus
| Focus (no. of strains) | Zymodeme(s) (no. of strains)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Human leishmaniasis
|
Canine leishmaniasis
|
Cat infection (VL) |
Phlebotomus infection
|
|||||
| VL | CL | VL-HIV coinfection | VL | CL | P. ariasi | P. perniciosus | ||
| Pyrénées-Orientales (155) | MON-1 (11) | MON-1 (11), MON-11 (11), MON-29 (14), MON-33 (16), MON-34 (1) | MON-1 (5), MON-29 (1), MON-33 (2) | MON-1 (74) | MON-1 (7 [associated with VL]) | MON-29 (2) | ||
| Cévennes (294) | MON-1 (84) | MON-1, (6) (+ 1 ML strain), MON-29 (14) | MON-1 (36), MON-29 (12) | MON-1 (99) | MON-1 (17 [5 associated with VL]) | MON-1 (23) | MON-1 (2) | |
| Provence (80) | MON-1 (26) | MON-1 (1) | MON-1 (46), MON-108 (2) | MON-1 (3), MON-108 (2) | ||||
| Côte d'Azur (166) | MON-1 (79) | MON-1 (7), MON-24 (2) | MON-1 (48), MON-24 (2) | MON-1 (26) | MON-1 (2) | |||
| Corsica (17) | MON-1 (7) | MON-29 (1) | MON-1 (5) | MON-1 (4) | ||||
(i) In Pyrénées-Orientales, 155 strains were studied, of which 72 were from humans, 81 were from dogs, and 2 were from sand flies. The human strains were obtained mainly from CL cases (n = 53; 73.6%). Out of the 19 strains isolated from VL cases, eight were from HIV-positive patients. The canine strains were obtained from VL cases, with some of the animals (n = 7) presenting cutaneous lesions associated with VL. Only two strains were isolated from the sand fly P. ariasi. Five zymodemes were found in this focus: MON-1, MON-11, MON-29, MON-33, and MON-34. They were all found in human CL cases. MON-1 was the only zymodeme found in canine leishmaniasis and in human VL in immunocompetent persons. MON-29 and MON-33 were found in humans, during both CL infection and VL-HIV coinfection. The sand fly strains belonged to the MON-29 zymodeme.
(ii) In Cévennes, 294 strains were studied, of which 153 were from humans, 116 were from dogs, and 25 were from sand flies. The human strains were obtained mainly from VL cases (n = 132, including 48 strains from VL-HIV coinfection). For tegumentary leishmaniasis, 20 strains involved CL and a single strain involved ML. The canine strains came mainly from VL cases (n = 99). Out of 17 canine CL cases, five were associated with VL. The strains obtained from sand flies were from P. ariasi (n = 23) and P. perniciosus (n = 2). Only two zymodemes were found: MON-1 and MON-29. As in Pyrénées-Orientales, MON-1 was the only zymodeme found in canine leishmaniasis and in VL cases in immunocompetent persons. This was also the only zymodeme found infecting sand flies. CL was due to both zymodemes, which were also responsible for VL in VL-HIV coinfection. The only ML case was due to zymodeme MON-1.
(iii) In Provence, 80 strains were studied, of which 75 were from human cases and 5 were from dogs. A single human strain was from a case of CL. Of the 74 human VL strains, 48 were obtained from VL-HIV coinfection patients. The five canine strains were from VL cases. Only two zymodemes were found: MON-1 and MON-108. MON-108 was found only in canine VL and in HIV-infected patients.
(iv) In Côte d'Azur, 166 strains were studied, of which 138 were from human cases, 26 were from dogs, and 2 were from cats. The human strains were obtained mainly from VL cases (n = 79 in immunocompetent patients and n = 50 in HIV-positive patients). Only nine strains were isolated from patients with CL. The canine strains were isolated from dogs with VL. Two zymodemes were found: MON-1 and MON-24. MON-24 was found in human CL cases and human VL-HIV coinfection.
(v) In Corsica, 17 strains were isolated, of which 13 were from humans (12 from VL cases and a single strain from a CL case) and 4 were from dogs (VL). Only two zymodemes were found: MON-1 and MON-29. MON-29 was found in the only CL case.
DISCUSSION
The samples in this study are heterogeneous between the different foci. Those from Pyrénées-Orientales and Cévennes include 449 strains out of 712 (63%) (Table 1). These numerous samples resulted from epidemiological field investigations carried out over 20 years by the Montpellier laboratory. These included parasites from all of the hosts, i.e., humans, dogs, and sand flies. All strains had been preserved in the International Leishmania Cryobank of Montpellier (43). The strains from Côte d'Azur were also partly based on field investigations, while in the other foci, strains were isolated from routine diagnostic samples. The Corsica sample is smallest, due to insufficient relationships with local practitioners. Despite this heterogeneity, this study presents an exceptional sample of 712 strains, providing a unique set of data. It gives valuable information on the nature of the parasites in these foci.
In Pyrénées-Orientales, human CL is predominant; a retrospective clinical survey showed that out of 67 cases reported between 1920 and 1996, 52 were CL (78%) and 15 were VL (22%) (our unpublished data). The present human samples, from patients infected in the two valleys of Tech and Têt, confirmed the predominance of CL. In this focus, VL is due to the single zymodeme MON-1, while CL cases show enzymatic polymorphism, with five different zymodemes found. Three of them (MON-11, MON-29, and MON-33) have so far been found only in human CL cases and are called dermotropic variants. MON-34 is a rare zymodeme, occurring in other foci outside of France, in VL as well as CL.
The canine strains were obtained during epidemiological investigations carried out in the valleys where the human cases had been detected (42). The domestic dog is incontestably the reservoir host of human VL and CL due to zymodeme MON-1. For the other zymodemes, the reservoir systems remain unknown.
In Cévennes, VL is predominant. A retrospective clinical survey of the cases reported between 1933 and 1994 showed that 123 cases out of a total of 157 were VL (78%), 31 were CL (20%), and 3 were ML (2%) (1). There is little enzymatic polymorphism; only two zymodemes were found, with MON-1 predominant. This focus, in which dog strains are numerous (116 strains), confirms the role of reservoir host played by the dog in the case of zymodeme MON-1. Entomological surveys carried out in this focus led to the demonstration of P. ariasi and P. perniciosus as vectors of MON-1. The MON-29 zymodeme, already found in Pyrénées-Orientales, was also found infecting humans in Cévennes.
In Provence, a review of the literature for the years 1923 to 1987 (38) showed that VL was predominant, with 713 cases (99.2%), while cases of CL and ML were scarce (2 cases of CL [0.3%] and 4 cases of ML [0.5%]). The enzymatic polymorphism of the studied sample is low, with only two zymodemes. MON-1 was predominant in humans and was also found in the small sample from dogs. Zymodeme MON-108, which differs from MON-1 by a single enzyme (DIA120), was found only in this focus and has never been found elsewhere. The small number of strains of this zymodeme precludes interpretation of its role. Further epidemiological studies are needed.
In Côte d'Azur, VL is also predominant, with 180 VL cases (96.8%) and only 6 CL cases (3.2%) detected between 1975 and 2002 (P. Marty, personal communication). The strains in this focus showed low polymorphism, with only two zymodemes: MON-1 and MON-24. MON-1 is predominant in humans and is the only zymodeme found in dogs. Even though CL cases are scarce, they do present clinical polymorphism (8), as observed in Pyrénées-Orientales (see below). In this focus, two strains were obtained from cats, which remains an exceptional finding. Feline leishmaniasis cases are rarely reported; from 1911 to 1996, 40 cases were published, with most of them being strictly cutaneous forms (reviewed in reference 20). More recently, other sporadic cases have been reported from southern France (27) (including one of the two cases included in our sample), Spain (17), and Italy (28, 30).
Limited data regarding human cases of leishmaniasis are available for Corsica. The two main clinical patterns (VL and CL) exist, but the proportion of each remains unknown. In spite of the small sample (17 strains), at least two zymodemes are present (MON-1 and MON-29), and MON-1 is found in both humans and dogs. The only case of CL reported was due to the dermotropic zymodeme MON-29 (7), which has also been found in two other French areas. The geographical origin of the strains studied shows that leishmaniasis is present throughout Corsica but is predominant in the northeastern part of the island (Fig. 1).
Concerning enzymatic polymorphism, there is a clear differentiation between the Pyrénées-Orientales, with a high polymorphism (five zymodemes) associated with a predominance of CL, and the other foci, with only two zymodemes and where VL is predominant. The three zymodemes MON-11, MON-29, and MON-33 vary from each other by a single enzyme. MON-11 has a distinctive heterozygote-type pattern, NP1, while the others show homozygote-type patterns (29, 35). In some other foci, comparable groups of three zymodemes with similar characteristics have been found: in Morocco, based on PGD and MDH variations (36), and in Sudan, with GOT variation (29). Genetic exchange between strains is the main possible hypothesis to explain the existence of such heterozygote patterns. In 1981, observations of heterozygote patterns in Leishmania led Maazoun et al. to suspect the existence of genetic exchange processes (22). However, genetic exchange has not been definitely demonstrated in Leishmania, despite the description of in vitro cell fusions (19). An alternative hypothesis is that these patterns are the consequence of simultaneous expression of two or more genes within a multigenic family. Systematic sequencing of the Leishmania major genome, currently in progress, has shown that several genes are organized as tandem repeats (26). It can be assumed that the transcription of various genes within a family should be sequentially activated and could produce different proteins either successively or simultaneously. A DNA molecular approach will presumably elucidate this point.
In the south of France, high enzymatic polymorphism is associated with CL, while low enzymatic polymorphism is associated with VL. This is not a strict general rule, as VL foci with high polymorphism occur, for example, in Sudan (33), as do CL foci with low polymorphism, such as in zoonotic CL due to L. major zymodeme MON-25 in Maghreb (23).
In all foci in the south of France, VL in immunocompetent patients is due only to zymodeme MON-1, which is the predominant zymodeme in all other Mediterranean countries (4, 16, 32). The domestic dog is incontestably the reservoir host of MON-1, as demonstrated by the identification of 232 out of 234 canine strains. When investigation of vectors was systematically carried out, as in Cévennes, the vectors found were P. ariasi and P. perniciosus. Thus, in this focus, the life cycle of L. infantum MON-1 has been completed. Such a situation has also occurred in the Catalan focus in Spain, where all of the elements of the life cycle of L. infantum MON-77 have been identified, including dogs and the sand fly P. perniciosus (10).
Despite being the common agent of VL of immunocompetent patients, zymodeme MON-1 can also be responsible for CL cases without later visceralization. This has been documented in four of the five foci. MON-1 CL also occurs in other Mediterranean countries, i.e., Algeria (16, 24), Greece (9), France (42, 46), Italy (13), Spain (31), and Tunisia (2).
CL is very often due to so-called dermotropic variants of L. infantum, such as the zymodemes MON-11, MON-29, and MON-33, which have so far been found only in immunocompetent patients. Special mention should be made of MON-24, which was long considered to be specifically dermotropic in immunocompetent patients (in Algeria [16], France [25], Italy [13], Morocco [45], and Tunisia [2]) but which was later found, albeit rarely, in infantile VL in North Africa (Algeria [5] and Tunisia [3, 4]). MON-34 is an uncommon zymodeme, being equally responsible for VL and CL in immunocompetent patients (32). MON-108 has been found only in Provence, in both humans and dogs; this zymodeme has never been found among more than 1,000 L. infantum strains from the Mediterranean Basin that have been identified.
In Pyrénées-Orientales, where there is high enzymatic polymorphism, the predominant CL is polymorphic clinically, with at least seven clinical forms, including ulcerative lesions with scab, lupoid, impetiginoid, diffusely infiltrative, pseudoepitheliomathous, verrucous, and sporothricoid-nodular forms (42; R. Perello, personal communication). The clinical variation does not correspond to the enzymatic variation.
During immunosuppression following HIV infection, zymodeme MON-1 was predominant (149 strains out of 159), which corresponds to the high frequency of this zymodeme during VL. This agrees with a recent study of 381 isolates from L. infantum-HIV coinfection cases from eight Mediterranean countries, in which MON-1 represented 73% of the sample (34). In these patients, normally dermotropic zymodemes (MON-29, MON-33, and even MON-24) are directly responsible for VL, as has been reported previously (32). In foci where CL is predominant, as in Pyrénées-Orientales, there therefore is a risk of VL in immunosuppressed patients, whatever parasite is present. Eleven cases of VL associated with immunosuppression resulting from organ transplantations occurred in southern France (our unpublished data). All eight resulting isolates that have been identified were MON-1 (included in our sample).
This study allows comparison of polymorphism in southern France with that in the neighboring foci in Italy and Spain. The 10 zymodemes, 9 of which were in humans, reported from Spanish Catalonia (10, 11; M. Gallego, personal communication) include all five zymodemes found in Pyrénées-Orientales. These two areas appear to be within a single epidemiological entity. Among the few strains isolated from sand flies, MON-1 was isolated from P. ariasi and P. perniciosus in both Cévennes and Spanish Catalonia (15, 40), confirming the vector role played by these two species in VL and human CL, while only MON-29 was isolated from P. ariasi in Pyrénées-Orientales. This difference should be taken carefully, as few sand fly strains have been identified, due to technical limitations. In Italy, the focus of Liguria, next to Côte d'Azur, is monomorphic, with the single zymodeme MON-1 reported for human VL and CL and for dogs (12, 14; M. Gramiccia, unpublished data). The zymodeme MON-24, present in Côte d'Azur and not found so far in Liguria, is, however, widely distributed in mainland Italy (Marche and Calabria) as well as in Sardinia and Sicily (6, 12, 14).
Several hypotheses can possibly explain the complexity of the enzymatic patterns encountered. The first is possible genetic exchanges between the parasites (19, 22). Another explanation could be the existence, for some enzymes, of a polymorphism during genetic expression. Particularly, some enzymes could be encoded by a differentially expressed multigenic family or could be subject to posttranscriptional or translational modifications. This last possibility might explain the apparent absence of the dermotropic variant zymodemes in the canine reservoir hosts. This possibility should be illuminated by systematic sequencing of the genes coding for the variant enzymes and by cloning and sequencing of the transcribed RNA.
Conclusion.
While the life cycle of L. infantum MON-1, with 712 strains identified, has been fully demonstrated, the reservoir hosts of the small variant zymodemes, which are scarce in humans, remain unknown, despite epidemiological investigations carried out in Pyrénées-Orientales and Cévennes (35, 42). Further field investigations are needed for a comprehensive understanding of the dynamics of natural life cycles, as shown recently for the MON-77 small variant in Spanish Catalonia (10).
Acknowledgments
We thank Y. Balard, P. Lami, M. Lefebvre, A. Martini, C. Rouquairol, and G. Serres for expert technical assistance. R. Perello, a dermatologist in Perpignan, is acknowledged for providing numerous CL cases from Pyrénées-Orientales. C. Ravel is acknowledged for fruitful discussion, and R. W. Ashford is acknowledged for revision of the manuscript.
The Centre National de Référence des Leishmania receives financial support from the Ministry of Health (Paris, France).
REFERENCES
- 1.Bassenne, I., F. Pratlong, J. Dereure, Y. Balard, and J. P. Dedet. 1996. La leishmaniose humaine en Cévennes: étude rétrospective 1933-1994. Med. Mal. Infect. 26:1164-1168. [Google Scholar]
- 2.Belhadj, S., F. Pratlong, M. Hammami, K. Kallel, J. P. Dedet, and E. Chaker. 2003. Human cutaneous leishmaniasis due to Leishmania infantum in the Sidi Bourouis focus (Northern Tunisia): epidemiological study and isoenzymatic characterization of the parasites. Acta Trop. 85:83-86. [DOI] [PubMed] [Google Scholar]
- 3.Belhadj, S., F. Pratlong, H. Mahjoub, N. H. Toumi, R. Azaïez, J. P. Dedet, and E. Chaker. 2000. Leishmaniose viscérale infantile à Leishmania infantum MON-24: une réalité en Tunisie. Bull. Soc. Pathol. Exot. 93:12-13. [PubMed] [Google Scholar]
- 4.Belhadj, S., F. Pratlong, N. H. Toumi, K. Kallel, H. Mahjoub, H. Babba, R. Azaiez, J. P. Dedet, and E. Chaker. 2002. Visceral leishmaniasis in Tunisia: result of the isoenzymatic characterization of 65 Leishmania infantum strains. Trans. R. Soc. Trop. Med. Hyg. 96:627-630. [DOI] [PubMed] [Google Scholar]
- 5.Benikhlef, R., F. Pratlong, Z. Harrat, N. Seridi, S. Bendali-Braham, M. Belkaid, and J. P. Dedet. 2001. Leishmaniose viscérale infantile causée par Leishmania infantum zymodème MON-24 en Algérie. Bull. Soc. Pathol. Exot. 94:14-16. [PubMed] [Google Scholar]
- 6.Bettini, S., M. Gramiccia, L. Gradoni, P. Biggio, R. Loi, F. Cottoni, M. Pau, and M. C. Atzeni. 1990. Leishmaniasis in Sardinia. IV. Epidemiological appraisal of cutaneous leishmaniasis and biochemical characterization of isolates. J. Trop. Med. Hyg. 93:262-269. [PubMed] [Google Scholar]
- 7.Buffet, P. A., C. Sarfati, M. Rybojad, F. Pratlong. F. Derouin, P. Morel, and J. P. Dedet. 1998. Pemier cas de leishmaniose cutanée à Leishmania infantum en Corse. Med. Trop. 58:375-377. [PubMed] [Google Scholar]
- 8.Del Giudice, P., P. Marty, J. P. Lacour, C. Perrin, F. Pratlong, M. Haas, P. Dellamonica, and Y. Le Fichoux. 1998. Cutaneous leishmaniasis due to Leishmania infantum. Case reports and literature review. Arch. Dermatol. 134:193-198. [DOI] [PubMed] [Google Scholar]
- 9.Frank, C., M. Hadziandoniou, F. Pratlong, A. Garifallou, and J. A. Rioux. 1993. Leishmania tropica and Leishmania infantum responsible for cutaneous leishmaniasis in Greece: sixteen autochthonous cases. Trans. R. Soc. Trop. Med. Hyg. 87:184-185. [DOI] [PubMed] [Google Scholar]
- 10.Gallego, M., F. Pratlong, R. Fisa, C. Riera, J. A. Rioux, J. P. Dedet, and M. Portus. 2001. The life cycle of Leishmania infantum MON-77 in the Priorat (South of Catalonia, Spain) involves humans, dogs and sandflies. Trans. R. Soc. Trop. Med. Hyg. 95:269-271. [DOI] [PubMed] [Google Scholar]
- 11.Gallego, M., F. Pratlong, C. Riera, C. Munoz, E. Ribera, R. Fisa, J. A. Rioux, J. P. Dedet, and M. Portus. 2002. Isoenzymatic identification of Leishmania isolates from repeated clinical human leishmaniasis episodes in Catalonia (Spain). Trans. R. Soc. Trop. Med. Hyg. 96:45-47. [DOI] [PubMed] [Google Scholar]
- 12.Gramiccia, M. 2002. The identification and variability of the parasites causing leishmaniasis in HIV-positive patients in Italy. Ann. Trop. Med. Parasitol. 97(Suppl. 1):65-73. [DOI] [PubMed] [Google Scholar]
- 13.Gramiccia, M., L. Gradoni, and M. Troiani. 1992. HIV-Leishmania co-infection in Italy. Isoenzyme characterization of Leishmania causing visceral leishmaniasis in HIV patients. Trans. R. Soc. Trop. Med. Hyg. 86:161-163. [DOI] [PubMed] [Google Scholar]
- 14.Gramiccia, M., L. Gradoni, and M. Troiani. 1995. Heterogeneity among zymodemes of Leishmania infantum from HIV-positive patients with visceral leishmaniasis in south Italy. FEMS. Microbiol. Lett. 28:33-38. [DOI] [PubMed] [Google Scholar]
- 15.Guilvard, E., M. Gallego, G. Moreno, R. Fisa, P. Rispail, F. Pratlong, E. Martinez-Ortega, J. Gallego, and J. A. Rioux. 1996. Infestation naturelle de Phlebotomus ariasi et Phlebotomus perniciosus (Diptera-Psychodidae) par Leishmania infantum (Kinetoplastida-Trypanosomatidae) en Catalogne (Espagne). Parasite 3:191-192. [Google Scholar]
- 16.Harrat, Z., F. Pratlong, S. Belazzoug, J. Dereure, M. Deniau, J. A. Rioux, M. Belkaid, and J. P. Dedet. 1996. Leishmania infantum and L. major in Algeria. Trans. R. Soc. Trop. Med. Hyg. 90:625-629. [DOI] [PubMed] [Google Scholar]
- 17.Hervas, J., F. Chacon-Manrique de Lara, M. A. Sanchez-Isarria, S. Pellicer, J. A. Castillo, and J. C. Gomez-Villamandos. 1999. Two cases of feline visceral and cutaneous leishmaniosis in Spain. J. Feline Med. Surg. 1:101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labbe, M., I. Targhetta, and P. Ameuille. 1918. Le kala-azar infantile en France. Bull. Acad. Méd. 89:288-290.7999191 [Google Scholar]
- 19.Lanotte, G., and J. A Rioux. 1990. Fusion cellulaire chez les Leishmania (Kinetoplastida, Trypanosomatidae). C. R. Séances Acad. Sci. III 310:285-288. [PubMed] [Google Scholar]
- 20.Laruelle-Magalon, C., and I. Toga. 1996. Un cas de leishmaniose féline. Pratique Méd. Chirurgicale Anim. Compagnie 31:255-261. [Google Scholar]
- 21.Maazoun, R., G. Lanotte, N. Pasteur, J. A. Rioux, M. F. Kennou, and F. Pratlong. 1981. Ecologie des leishmanioses dans le sud de la France. 16. Contribution à l'analyse chimiotaxonomique des parasites de la leishmaniose viscérale méditerranéenne. A propos de 55 souches isolées en Cévenne, Côte-d'Azur, Corse et Tunisie. Ann. Parasitol. Hum. Comp. 56:131-146. [PubMed] [Google Scholar]
- 22.Maazoun, R., G. Lanotte, J. A. Rioux, N. Pasteur, R. Killick-Kendrick, and F. Pratlong. 1981. Signification du polymorphisme enzymatique chez les Leishmanies. A propos de trois souches hétérozygotes de Leishmania infantum Nicolle, 1908, Leishmania cf. tarentolae Wenyon, 1921 et Leishmania aethiopica Bray, Ashford et Bray, 1973. Ann. Parasitol. Hum. Comp. 56:467-475. [PubMed] [Google Scholar]
- 23.Maazoun, R., F. Pratlong, G. Lanotte, and J. A. Rioux. 1986. Le complexe Leishmania major. A propos de l'analyse numérique de 35 souches identifiées par la méthode enzymatique, p. 119-128. In J. A. Rioux (ed.), Leishmania. Taxonomie et phylogenèse. Applications éco-épidémiologiques. IMEEE, Montpellier, France.
- 24.Marty, P., J. P. Lacour, F. Pratlong, C. Perrin, P. Del Giudice, and Y. Le Fichoux. 1998. Leishmaniose cutanée localisée due à Leishmania infantum MON-1 contractée dans le Nord de l'Algérie. Bull. Soc. Pathol. Exot. 91:146-147. [PubMed] [Google Scholar]
- 25.Marty, P., F. Pratlong, B. Marcelet, A. Adda, and Y. Le Fichoux. 1994. Leishmania infantum variant MON-24 isolé d'une lésion cutanée contractée dans la banlieue de Nice (France). Parasite 1:175-176. [DOI] [PubMed] [Google Scholar]
- 26.Myler, P. J., J. Audleman, T. Devos, G. Hixson, P. Kiser, C. Lemley, C. Magness, E. Rickel, E. Sisk, S. Sunkin, S. Swartzell, T. Westlake, P. Bastien, G. F. Fu, A. Ivens, and K. Stuart. 1999. The sequence of Leishmania major chromosome 1 reveals only two polycistronic units of protein coding genes. Proc. Natl. Acad. Sci. USA 96:2902-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozon, C., P. Marty, F. Pratlong, C. Breton, M. Blein, A. Lelievre, and P. Haas. 1998. Disseminated feline leishmaniasis due to Leishmania infantum in southern France. Vet. Parasitol. 75:273-277. [DOI] [PubMed] [Google Scholar]
- 28.Pennisi, M. G. 2002. A high prevalence of feline leishmaniasis in southern Italy, p. 9-48. In R. Killick-Kendrick (ed.), Canine leishmaniasis: moving towards a solution. Proceedings of the Second International Canine Leishmaniasis Forum Seville, Spain. Intervet International, Boxmeer, The Netherlands.
- 29.Piarroux, R., V. Trouvé, F. Pratlong, A. Martini, M. Lambert, and J. A. Rioux. 1994. The use of isoelectric focusing on polyacrylamide gel for the enzymatic analysis of Old World Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 88:475-478. [DOI] [PubMed] [Google Scholar]
- 30.Poli, A., F. Abramo, P. Barsotti, S. Leva, M. Gramiccia, A. Ludovisi, and F. Mancianti. 2002. Feline leishmaniosis due to Leishmania infantum in Italy. Vet. Parasitol. 106:181-191. [DOI] [PubMed] [Google Scholar]
- 31.Portus, M., J. Gallego, J. A. Rioux, F. Pratlong, G. Moreno, R. Fisa, M. Gallego, C. Munoz, C. Riera, F. Sanchez, and T. Serra. 1989. Enzymatic heterogeneity among strains of Leishmania infantum from human visceral and cutaneous leishmaniasis in Catalonia (Spain). Rev. Iber. Parasitol. 49:287-289. [Google Scholar]
- 32.Pratlong, F., J. P. Dedet, P. Marty, M. Portus, M. Deniau, J. Dereure, P. Abranches, J. Reynes, A. Martini, M. Lefebvre, and J. A. Rioux. 1995. Leishmania-human immunodeficiency virus co-infection in the Mediterranean basin: isoenzymatic characterization of 100 isolates of the Leishmania infantum complex. J. Infect. Dis. 172:323-326. [DOI] [PubMed] [Google Scholar]
- 33.Pratlong, F., J. Dereure, B. Bucheton, S. El-Safi, A. Dessein, G. Lanotte, and J. P. Dedet. 2001. Sudan: the possible original focus of visceral leishmaniasis. Parasitology 122:599-605. [DOI] [PubMed] [Google Scholar]
- 34.Pratlong, F., J. Dereure, M. Deniau, P. Marty, F. Faraut-Gambarelli, and J. P. Dedet. 2003. Enzymatic polymorphism during Leishmania/HIV co-infection: a study of 381 strains received between 1986 and 2000 at the international cryobank in Montpellier, France. Ann. Trop. Med. Parasitol. 97(Suppl.)1:47-56. [DOI] [PubMed] [Google Scholar]
- 35.Pratlong, F., A. Martini, M. Lambert, M. Lefebvre, J. P. Dedet, and J. A. Rioux. 1994. Intérêt de la culture et de l'identification isoenzymatique des leishmanies dans le diagnostic et l'épidémiologie des leishmanioses. Méd. Armées 22:61-65. [Google Scholar]
- 36.Pratlong, F., J. A. Rioux, J. Dereure, J. Mahjour, M. Gallego, E. Guilvard, G. Lanotte, J. Perieres, A. Martini, and A. Saddiki. 1991. Leishmania tropica au Maroc. IV. Diversité isozymique intrafocale. Ann. Parasitol. Hum. Comp. 66:100-104. [DOI] [PubMed] [Google Scholar]
- 37.Pringault, E. 1914. Existence de la leishmaniose canine à Marseille. Bull. Soc. Pathol. Exot. 7:41-42. [Google Scholar]
- 38.Quilici, M., S. Dunan, and C. Mary. 1989. La leishmaniose viscérale méditerranéenne dans le Sud-Est de la France. Aspects cliniques et biologiques nouveaux. Sem. Hop. 65:2155-2161. [Google Scholar]
- 39.Ravaut, P. 1920. Deux cas de Bouton d'Orient contractés en Espagne et en France. Premier cas de contagion en France. Bull. Soc. Pathol. Exot. 13:235-238. [Google Scholar]
- 40.Rioux, J. A., E. Guilvard, J. Gallego, G. Moreno, F. Pratlong, M. Portus, P. Rispail, M. Gallego, and P. Bastien. 1986. Phlebotomus ariasi Tonnoir, 1921 et Phlebotomus perniciosus Newstead, 1911, vecteurs du complexe Leishmania infantum dans un même foyer. Infestations par deux zymodèmes syntopiques. A propos d'une enquête en Catalogne (Espagne), p. 439-444. In J. A. Rioux (ed.), Leishmania. Taxonomie et phylogenèse. Applications éco-épidémiologiques. IMEEE, Montpellier, France.
- 41.Rioux, J. A., G. Lanotte, R. Maazoun, R. Perello, and F. Pratlong. 1980. Leishmania infantum Nicolle, 1908 agent du bouton d'Orient autochtone. A propos de l'identification biochimique de deux souches isolées dans les Pyrénées-Orientales. C. R. Séances Acad. Sci. III 291:701-703. [PubMed] [Google Scholar]
- 42.Rioux, J. A., G. Lanotte, F. Pratlong, J. Dereure, D. Jarry, G. Moreno, R. Killick-Kendrick, J. Périères, E. Guilvard, A. Belmonte, and M. Portus. 1985. La leishmaniose cutanée autochtone dans le sud-est de la France. Résultats d'une enquête éco-épidémiologique dans les Pyrénées-Orientales. Med. Mal. Infect. 11:650-656. [Google Scholar]
- 43.Rioux, J. A., G. Lanotte, F. Pratlong, A. Martini, E. Serres, and A. Belmonte. 1986. Centre International de Cryoconservation, d'Identification Enzymatique et d'Etude Taxonomique des Leishmania (Montpellier, France). Structure et fonctionnement, p. 485-502. In J. A. Rioux (ed.), Leishmania. Taxonomie et phylogenèse. Applications éco-épidémiologiques. IMEEE, Montpellier, France.
- 44.Rioux, J. A., G. Lanotte, E. Serres, F. Pratlong, P. Bastien, and J. Perieres. 1990. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann. Parasitol. Hum. Comp. 65:111-125. [DOI] [PubMed] [Google Scholar]
- 45.Rioux, J. A., J. Mahjoub, M. Gallego, J. Dereure, J. Perieres, A. Lahmrani, C. Riera, A. Saddiki, and B. Mouki. 1996. Leishmaniose cutanée urbaine à Leishmania infantum MON-24 au Maroc. Bull. Soc. Fr. Parasitol. 14:2. [Google Scholar]
- 46.Simon, D., P. Marty, Y. Le Fichoux, F. Pratlong, H. Duplay, and M. Gari-Toussaint. 1991. Leishmaniose cutanée autochtone due à Leishmania infantum dans les Alpes-Maritimes. Rev. Eur. Dermatol. 3:479-481. [Google Scholar]